Abstract
We have recently reported that Dahl salt-sensitive rats (DS) on high salt diet (HS) have an inappropriate augmentation of intrarenal angiotensinogen. Recent studies also reported that the augmented superoxide anion formation plays important roles in this animal model of hypertension. This study was performed to address the hypothesis that an inappropriate augmentation of intrarenal angiotensinogen by HS is caused by the augmented reactive oxygen species. Male DS (200–220 g) were maintained on low salt diet LS (N = 7) or HS (N = 27) for 4 weeks. The HS group was subdivided into three subgroups to receive null (N = 12), superoxide dismutase mimetic, tempol (3 mmol/l, N = 8), or vasodilator, hydralazine (0.5 mmol/l, N = 7) in drinking water during the period. Systolic BP was significantly increased in the DS + HS group compared to the DS + LS group (184 ± 7 mmHg vs. 107 ± 5 at 4-week). Tempol or hydralazine treatment equivalently attenuated the hypertension (128 ± 3 and 127 ± 5 at 4-week, respectively). Urinary excretion of thiobarbituric acid reactive substances at 4-week was significantly increased in the DS + HS group compared to the DS + LS group (0.66 ± 0.05 µmol/day vs. 0.14 ± 0.01). Tempol treatment prevented this effect (0.24 ± 0.04) but hydralazine treatment only partially prevented the effect (0.40 ± 0.03). Kidney angiotensinogen levels, measured by Western blot analysis, were significantly increased in the DS + HS group compared to the DS + LS group (32 ± 5 densitometric units vs. 21 ± 1). Tempol (14 ± 3) but not hydralazine (32 ± 5) treatment prevented the intrarenal angiotensinogen augmentation. The evidence suggests that the enhanced intrarenal angiotensinogen in DS challenged with HS is associated with the augmented reactive oxygen species.
Keywords: Dahl salt-sensitive rats, High salt diet, Kidney, Angiotensinogen, Oxidative stress
Dahl salt-sensitive rats (DS) have been used as a model of human salt-sensitive hypertension because salt loading exaggerates the development of hypertension in strains that are genetically predisposed to hypertension [1]. Although generally considered to be characterized by a low activity of the circulating renin-angiotensin system (RAS) [1], recent studies indicate that treatment with angiotensin (Ang) converting enzyme inhibitors or Ang II type 1 receptor antagonists reduces cardiac and/ or renal dysfunction in DS fed a high salt diet (HS) [2–8]. These findings suggest that the local RAS may be inappropriately activated and contribute to the development of hypertension in this animal model. We recently reported that DS on HS have an inappropriate augmentation of intrarenal angiotensinogen (AGT) that are not reflected in the plasma levels of RAS components [9]. This enhancement may contribute to the impaired sodium excretion during HS and the development of hypertension in this strain. However, the mechanisms responsible for an inappropriate augmentation of intrarenal AGT by HS remain incompletely understood. Recent studies also reported that an augmented superoxide anion formation plays an important role in this animal model of hypertension [10,11]. This study was performed to address the hypothesis that an inappropriate augmentation of intrarenal AGT by HS is caused by the augmented reactive oxygen species (ROS).
Materials and methods
The experimental protocol was approved by the Animal Care and Use Committees at Tulane University and Kagawa University. Male DS (200–220 g, Seac Yoshitomi, Fukuoka, Japan, N = 34) were selected at random to receive HS (8% NaCl, Oriental Yeast, Osaka, Japan, N = 27) or low salt diet (LS, 0.3% NaCl, Oriental Yeast, N = 7) for 4 weeks. The HS group was subdivided into three subgroups to receive null (N = 12), superoxide dismutase mimetic, tempol (T, 3 mmol/l, N = 8, Sigma, Missouri, USA), or vasodilator, hydralazine (H, 0.5 mmol/l, N = 7, Wako, Tokyo, Japan) in drinking water during the period. The doses of tempol and hydralazine were determined based upon results from a previous study in DS rats [12]. The previous study showed that 3 mmol/l of tempol and 0.5 mmol/l of hydralazine resulted in similar reductions in blood pressure (BP) of DS + HS rats [12].
Systolic BP was measured every week in conscious rats using tailcuff plethysmography. Blood and kidney samples were harvested at the end of the fourth week. Twenty-four-hour urine collection was taken one day before the harvesting. Animals were sacrificed by decapitation, and trunk blood was collected into chilled tubes with protease inhibitors. Plasma was separated and AGT levels were measured by Western blot analysis [9,13–16]. Immediately after removal, one kidney was homogenized in cold methanol and renal Ang II was measured [9,13–16]. The contralateral kidneys were snapped in liquid nitrogen for Western blot analysis. The detailed method of Western blot analysis of plasma AGT, kidney AGT, and kidney β-actin was described previously [9,13–16]. Thiobarbituric acid reactive substances’ (TBARS) concentration was measured as previously described [12,17] using 24-h urine collected one day before the harvesting.
Statistical analysis was performed using a one-way ANOVA with post hoc Scheffe’s F test. All data are presented as means ± SEM. A value of P < 0.05 was considered significant.
Results
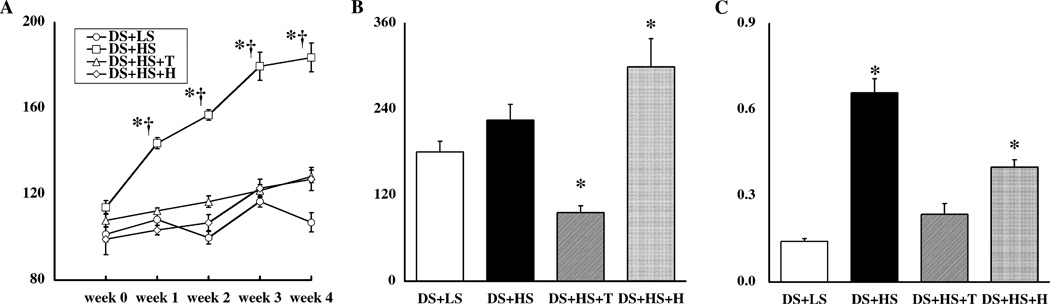
Temporal profile of systolic BP is depicted in Fig. 1A. Systolic BP levels were similar among the four groups at the beginning of protocol. Systolic BP was significantly increased in the DS + HS group compared to the DS + LS group (184 ± 7 mmHg vs. 107 ± 5 at 4-week). Tempol or hydralazine treatment equivalently attenuated the hypertension (128 ± 3 and 127 ± 5 at 4-week, respectively).
Fig. 1.
(A) Temporal profile of systolic BP. Systolic BP levels were similar among the four groups at the beginning of protocol. Systolic BP was significantly increased in the DS + HS group compared to the DS + LS group. Tempol or hydralazine treatment equivalently attenuated the hypertension. (B) Kidney Ang II content was measured by a combined method of solid phase extraction and radioimmunoassay as previously described [9,13–16]. (C) Urinary excretion rates of thiobarbituric acid reactive substances (TBARS) were measured at week 4 as previously described [12,17]. *P < 0.05 compared to Dahl salt-sensitive (DS) rats fed low salt diet (LS) at that time period. †P < 0.05 compared to the corresponding week 0 group. HS, high salt diet; T, tempol treatment; and H, hydralazine treatment.
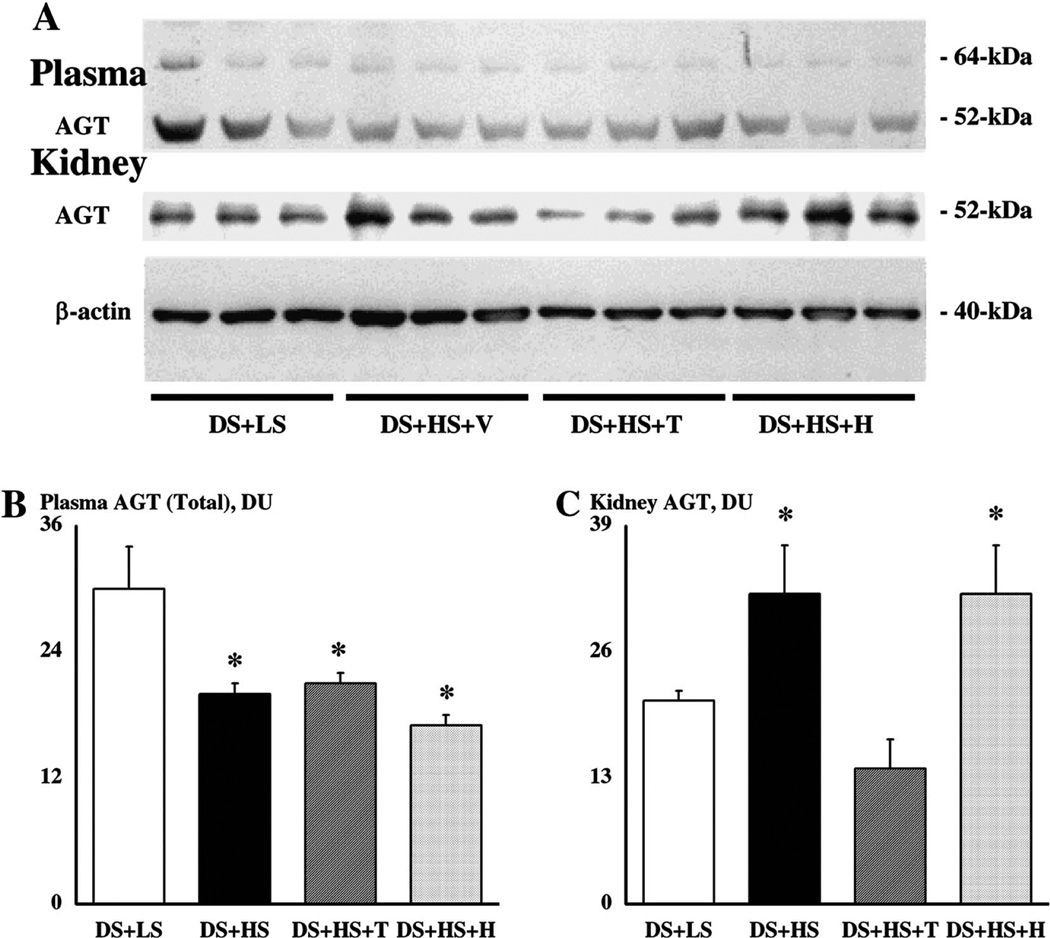
Plasma AGT levels are depicted in Fig. 2A. HS significantly suppressed plasma AGT levels. Tempol or hydralazine treatment did not affect this attenuation (Fig. 2B).
Fig. 2.
(A) A representative Western blot of plasma angiotensinogen (AGT), kidney AGT, and kidney β-actin. (B) Western blot for plasma AGT showed two specific bands at 64-kDa and 52-kDa as previously described [9,13–16]. Densitometric analysis was performed with total densitometric unit (DU) of both bands. (C) Western blot for kidney AGT showed one specific band at 52-kDa as previously described [9,13–16]. *P < 0.05 compared to Dahl salt-sensitive (DS) rats fed low salt diet (LS). HS, high salt diet; T, tempol treatment; and H, hydralazine treatment.
Kidney AGT levels are depicted in Fig. 2A. In contrast to plasma AGT levels, HS significantly increased kidney AGT levels in DS + HS compared to DS + LS. Tempol treatment prevented the augmentation but hydralazine did not affect this enhancement (Fig. 2C). As a control to check for equal loading, membranes were re-probed with an antibody against β-actin (Fig. 2A). Densitometric unit (DU) for β-actin was unaltered among four groups (40 ± 2 DU in DS + LS, 42 ± 3 in DS + HS, 40 ± 2 in DS + HS + T, and 42 ± 3 in DS + HS + H).
Kidney Ang II levels (Fig. 1B) failed to be suppressed in DS + HS group compared to DS + LS group. Tempol treatment significantly decreased kidney Ang II levels. In contrast, hydralazine treatment significantly increased kidney Ang II levels.
Urinary excretion of TBARS at 4 weeks (Fig. 1C) was significantly increased in the DS + HS group compared to the DS + LS group. Tempol treatment prevented this augmentation but hydralazine treatment only partially prevented the enhancement.
Discussion
While we recently reported that DS on HS have an inappropriate augmentation of intrarenal AGT and a failure to suppress intrarenal Ang II which may contribute to the development of hypertension in this strain [9], the mechanisms responsible for an inappropriate augmentation of intrarenal AGT by HS remain incompletely understood. Meanwhile, other groups recently reported that an augmented superoxide anion formation plays an important role in this animal model of hypertension [10,11]. These results prompted us to perform further experiments to evaluate the hypothesis that an inappropriate augmentation of intrarenal AGT by HS is caused by an augmented ROS. As expected, systolic BP was significantly increased by HS in DS and was equally suppressed by tempol and hydralazine. HS has been shown to suppress plasma and intrarenal expression of AGT in Sprague–Dawley [18] and Wistar–Kyoto [19] rats. Similarly, in the present study, plasma AGT levels were also suppressed by HS. However, kidney AGT levels were enhanced by HS and tempol treatment prevented this augmentation but hydralazine did not. It is important to note here that this paradoxical enhancement of intrarenal AGT by HS was observed in DS but not in Dahl salt-resistant rats [9]. Interestingly, urinary excretion of TBARS was changed alike and was elevated by HS. Furthermore, tempol treatment blocked this increase but hydralazine did not. Thus, the present study provides evidence that an inappropriate augmentation of intrarenal AGT by HS is associated with the augmented ROS in DS. Our data were supported by a recent in vitro study. Hsieh et al. [20] demonstrated that the stimulatory action of high glucose on AGT gene expression in immortalized renal proximal tubular cells was mediated at least in part via ROS generation. The models were different, however, this evidence may support that the enhanced intrarenal AGT by HS is mediated by the augmented ROS in DS models. This mechanism may contribute to the development of hypertension in this strain.
In our study, kidney AGT levels in DS were significantly enhanced by HS. Kidney Ang II levels in DS were not statistically increased by HS; however, it is important to emphasize that kidney Ang II levels were not suppressed by HS in DS. One possible explanation for the failure to increase kidney Ang II levels may be linked to the tubular fluid compartmentalization of intrarenal Ang II. Most of the intrarenal AGT mRNA and protein are localized in proximal tubular cells [13,21,22]. Once AGT is synthesized in and secreted from proximal tubular cells, AGT may be metabolized to Ang II by renin or renin-like enzymes [23–25] and Ang converting enzyme [26] present on proximal and/or distal tubular cells. However, there are abundant angiotensinases also present, which may prevent substantial accumulation of Ang II [27]. The increases in intrarenal Ang II may be restricted to the tubular compartment and, therefore, kidney Ang II levels may fail to show significant increases in spite of the enhanced kidney AGT levels in this study.
It seems important to address renal levels of nitric oxide in this study because renal nitric oxide is greatly reduced by ROS. Therefore, it is possible that the enhanced ROS in the present study may reduce renal levels of nitric oxide leading to hypertension observed in DS challenged with HS. However, we did not examine the status of nitric oxide or nitric oxide synthase in the kidney in this study. Because, we already reported that urinary excretion rates of nitric oxide were not increased by HS in DS rats [12,28]. This may suggest that the enhanced ROS-mediated inappropriate augmentation of intrarenal AGT by HS in DS does not involve the nitric oxide or nitric oxide synthase system in the kidney.
We selected hydralazine in this study as an antihypertensive agent without any antioxidant properties [29,30]. However, it seems likely that hydralazine may have some effect as an antioxidant because, in this study, urinary TBARS (Fig. 1C) tended to be suppressed in the DS + HS + H group compared to the DS + HS group. This effect of hydralazine as an antioxidant agent is supported by previous studies [31,32]. However, the important finding in this study was that urinary TBARS in the DS + HS + H group were still significantly higher than those in the DS + LS group, which was associated with significant increases in kidney AGT levels and kidney Ang II levels of the DS + HS + H group compared to the DS + LS group.
We previously reported that renal TBARS contents were significantly increased in DS on HS compared to DS on LS and that tempol treatment prevented this effect but hydralazine treatment only partially prevented the effect [12]. Therefore, we did not examine renal TBARS contents in this study. Instead, we assessed urinary exertion of TBARS as a maker of renal ROS. As similar to the previous study in the kidney [12], urinary TBARS excretion rates in this study were significantly increased in DS on HS compared to DS on LS and tempol treatment prevented this effect but hydralazine treatment only partially prevented the effect (Fig. 1C). A similar observation was reported by other investigators. Meng et al. [10] reported that renal cortical and medullary superoxide anion release was enhanced by HS in DS and that this augmentation was suppressed by tempol treatment. They did not examine hydralazine treatment in their study, however, according to these reports, urinary TBARS, kidney TBARS, and renal superoxide anion release are expected to change parallely.
We used tempol in this study as a superoxide dismutase mimetic similar to our previous study [33]. Consistent with previous reports in hypertensive animals [17,32–34], tempol significantly decreased arterial pressure in DS rats challenged on HS. Therefore, it is possible that the effects of tempol on ROS levels may depend on arterial pressure changes. However, there are many studies demonstrating that tempol reduces superoxide anion levels in vitro [33,35]. In addition, tempol suppresses ROS levels and ameliorates ROS-related tissue injuries in the absence of arterial pressure reduction in vivo [36,37]. Moreover, we have recently demonstrated that tempol prevents ROS generation in aortic and heart tissues induced by acute Ang II infusion in conscious rats [38]. Interestingly, we also observed that the hypertensive response to acute Ang II infusion was not affected by treatment with tempol in conscious rats [38]. These data suggest that the effects of tempol on ROS levels are not solely consequence of arterial pressure changes.
In summary, this study demonstrated that systolic BP in DS was significantly increased by HS. Tempol or hydralazine treatment equivalently attenuated this hypertension. Urinary excretion rates of TBARS were significantly increased in DS + HS compared to DS + LS. Tempol treatment prevented this effect but hydralazine treatment only partially prevented the effect. Kidney AGT levels were significantly increased in DS + HS compared to DS + LS. Tempol treatment prevented AGT augmentation but hydralazine did not. These results may suggest that an inappropriate augmentation of intrarenal AGT by HS is caused by augmented ROS in DS. The evidence supports an important contribution for ROS in the development of hypertension in DS challenged with HS.
Acknowledgments
This work was supported by grants from the Institutional Development Award Program of the National Center for Research Resources, National Institute of Health (P20RR017659, H.K.), the Ministry of Education, Science and Culture of Japan (A.N.), and the Mitsui Life Social Welfare Foundation (Tokyo, Japan, A.N.). The polyclonal antibody against rat AGT was generously provided by Dr. Conrad Sernia (University of Queensland, Australia). The authors thank Dr. L. Gabriel Navar (Tulane University) for his critical review of the manuscript. The authors also acknowledge excellent technical assistance of Ms. My-Linh Rauv (Tulane University) and Ms. Kayoko Miyata (Kagawa University).
References
- 1.Iwai J, Dahl LK, Knudsen KD. Genetic influence on the renin-angiotensin system: low renin activities in hypertension-prone rats. Circ. Res. 1973;32:678–684. doi: 10.1161/01.res.32.6.678. [DOI] [PubMed] [Google Scholar]
- 2.Kodama K, Adachi H, Sonoda J. Beneficial effects of long-term enalapril treatment and low-salt intake on survival rate of Dahl salt-sensitive rats with established hypertension. J. Pharmacol. Exp. Ther. 1997;283:625–629. [PubMed] [Google Scholar]
- 3.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997;96:2407–2413. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka F, Yamauchi T, Kataoka H, Mimura Y, Ogura T, Makino H. Effects of chronic inhibition of ACE and AT1 receptors on glomerular injury in Dahl salt-sensitive rats. Am. J. Physiol. 1998;274:R1797–R1806. doi: 10.1152/ajpregu.1998.274.6.R1797. [DOI] [PubMed] [Google Scholar]
- 5.Sakata Y, Masuyama T, Yamamoto K, Doi R, Mano T, Kuzuya T, Miwa T, Takeda H, Hori M. Renin angiotensin system-dependent hypertrophy as a contributor to heart failure in hypertensive rats: different characteristics from renin angiotensin system-independent hypertrophy. J. Am. Coll. Cardiol. 2001;37:293–299. doi: 10.1016/s0735-1097(00)01064-0. [DOI] [PubMed] [Google Scholar]
- 6.Hayashida W, Kihara Y, Yasaka A, Inagaki K, Iwanaga Y, Sasayama S. Stage-specific differential activation of mitogen-activated protein kinases in hypertrophied and failing rat hearts. J. Mol. Cell. Cardiol. 2001;33:733–744. doi: 10.1006/jmcc.2001.1341. [DOI] [PubMed] [Google Scholar]
- 7.Nishikimi T, Mori Y, Kobayashi N, Tadokoro K, Wang X, Akimoto K, Yoshihara F, Kangawa K, Matsuoka H. Renoprotective effect of chronic adrenomedullin infusion in Dahl salt-sensitive rats. Hypertension. 2002;39:1077–1082. doi: 10.1161/01.hyp.0000018910.74377.93. [DOI] [PubMed] [Google Scholar]
- 8.Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, Saruta T. Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron. 2002;91:710–718. doi: 10.1159/000065035. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 11.Ying WZ, Xia H, Sanders PW. Nitric oxide synthase (NOS2) mutation in Dahl/Rapp rats decreases enzyme stability. Circ. Res. 2001;89:317–322. doi: 10.1161/hh1601.094625. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogenactivated protein kinase activity in Dahl salt-sensitive rats. J. Am. Soc. Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J. Am. Soc. Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiyama A, Kobori H, Fukui T, Zhang G-X, Yao L, Rahman M, Hitomi H, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–760. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sechi LA, Griffin CA, Giacchetti G, Valentin JP, Llorens-Cortes C, Corvol P, Schambelan M. Tissue-specific regulation of type 1 angiotensin II receptor mRNA levels in the rat. Hypertension. 1996;28:403–408. doi: 10.1161/01.hyp.28.3.403. [DOI] [PubMed] [Google Scholar]
- 19.Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J. Clin. Invest. 1986;78:1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 21.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 22.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J. Clin. Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J. Clin. Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 25.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 26.Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–835. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 27.Herzig CM, Schoeppe W, Scherberich JE. Angiotensinase A (aminopeptidase A): properties of chromatographically purified isoforms from human kidney. J. Chromatogr. 1992;625:73–82. doi: 10.1016/0021-9673(92)87223-u. [DOI] [PubMed] [Google Scholar]
- 28.Tomohiro A, Kimura S, He H, Fujisawa Y, Nishiyama A, Kiyomoto K, Aki Y, Tamaki T, Abe Y. Regional blood flow in Dahl-Iwai salt-sensitive rats and the effects of dietary l-arginine supplementation. Am. J. Physiol. 1997;272:R1013–R1019. doi: 10.1152/ajpregu.1997.272.4.R1013. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaka N, de Leon H, Laursen JB, Fukui T, Wilcox JN, De Keulenaer G, Griendling KK, Alexander RW. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation. 1997;96:1923–1929. doi: 10.1161/01.cir.96.6.1923. [DOI] [PubMed] [Google Scholar]
- 30.Chlopkiewicz B. Studies on the mutagenic activity of hydralazine and dihydralazine in Salmonella typhimurium strains differing in expression of antioxidant genes. Toxicol. Lett. 1999;110:203–207. doi: 10.1016/s0378-4274(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 31.Cabell KS, Ma L, Johnson P. Effects of antihypertensive drugs on rat tissue antioxidant enzyme activities and lipid peroxidation levels. Biochem. Pharmacol. 1997;54:133–141. doi: 10.1016/s0006-2952(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 32.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- 34.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- 35.Samuni A, Winkelsberg D, Pinson A, Hahn SM, Mitchell JB, Russo A. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J. Clin. Invest. 1991;87:1526–1530. doi: 10.1172/JCI115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, Poole BD, Bowler RP, Day BJ, Crapo JD, Schrier RW. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am. J. Physiol. Renal Physiol. 2003;284:F532–F537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G-X, Kimura S, Nishiyama A, Shokoji T, Rahman M, Abe Y. ROS during acute phase of Ang II hypertension participates in cardiovascular MAPK activation but not vasoconstriction. Hypertension. 2004;43:117–124. doi: 10.1161/01.HYP.0000105110.12667.F8. [DOI] [PubMed] [Google Scholar]