Abstract
Background
There are few reports of glomerulonephritis (GN) with crescents and a rapidly progressive course that lead to a diagnosis of a previously unsuspected B-cell dyscrasia. Case Presentation: We report a case of rapidly progressive GN: the patient showed no evidence of etiology at the time of biopsy and was diagnosed as IgA multiple myeloma (MM) during investigation based on a renal biopsy. He presented diffuse proliferative and exudative GN and marked plasma cell infiltration of the kidney.
Conclusion
The present case raises the possibility that proliferative GN with crescents may be a rare mode of presentation of MM.
Key Words: B-cell dyscrasia, Crescents, Glomerulonephritis, Multiple myeloma, Rapidly progressive glomerulonephritis
Introduction
The literature contains few reports of crescentic glomerulonephritis (GN) with a rapidly progressive course leading to a diagnosis of previously unsuspected B-cell dyscrasia [1, 2, 3].
We present a case of rapidly progressive GN with no evident etiology at the time of renal biopsy. The diagnosis was rapidly progressive GN secondary to multiple myeloma (MM) established only after histopathological examination of the biopsy.
Case Presentation
Six months prior to admission, a 51-year-old man presented with indisposition, weakness and dark urine. Fifteen days earlier, he observed an increase in abdominal volume, a decrease in urinary volume, worsening macrohematuria, as well as lower limb edema and a 7-kg weight loss during the preceding year.
Approximately 15 months earlier, the patient presented with squamous-cell carcinoma of the mouth that was treated surgically. Subsequently, the patient received 35 sessions of radiotherapy. He had been a heavy smoker for 30 years but had stopped 3 years prior to these events; he had also been a moderate-to-severe alcoholic up to 3 years ago.
At the initial physical examination, the patient presented no signs of uremia. His blood pressure was 170/110 mm Hg, heart rate 76 b.p.m. and respiratory rate 16 breaths/min. He had a mitral systolic murmur. There was a cutaneous thickening in the cervical region, which had developed following radiotherapy, and his lymph nodes were not palpable. His abdomen was tense, with moderate ascites.
His initial laboratory blood tests tests showed: hemoglobin, 4.2 g/dl; platelets, 82,000/mm3; white blood cells, 5,600/mm3; prothrombin activity, 100%; iron, 90 mg/dl; ferritin, 243 μg/l; transferrin saturation, 39%; venous pH, 7.35; base excess, −7; creatinine, 583 μmol/l; urea, 51 mmol/l; glucose, 4.66 mmol/l; uric acid, 481.78 μmol/l; sodium, 139 mmol/l; potassium, 4.7 mmol/l; ionic calcium, 1.17 mmol/l; phosphorus, 3.10 mmol/l; lactic dehydrogenase, 41%; alkaline phosphatase, 50 U/l; prostate-specific antigen, 0.4 ng/ml; α-fetoprotein, 2.1 U/ml; IgG, 230 mg/dl; IgM, 16 mg/dl; IgA, 4,330 mg/dl (reference value: 68–423 mg/dl); creatinine clearance, 9 ml/min, and 24-hour proteinuria, 1.5 g/24 h. Urinalysis revealed proteinuria (2.8 g/l), 250,000 leukocytes/mm3 and 24 × 106 erythrocytes/mm3, with erythrocyte dysmorphism (3+); urine culture was negative. The urine Bence-Jones protein test was negative. The following serological tests were nonreactive: anti-HIV, anti-HCV, HbsAg, ANA, anti-DNA, anti-glomerular basement membrane (GBM) antibodies and ANCA. Serum complement levels were normal.
Ultrasonographically, the right and left kidney measured 12.1 × 6.8 × 3.5 and 12.0 × 5.2 × 4.6 cm, respectively, with an increase in echogenicity in the renal parenchyma suggesting chronic nephropathy.
Considering the possibility of rapidly progressive GN, he received single-dose methylprednisolone pulse therapy. He had an ultrasound-guided renal biopsy and was maintained on hemodialysis. Following biopsy, uncontrolled perirenal hemorrhage developed and he was then submitted to nephrectomy.
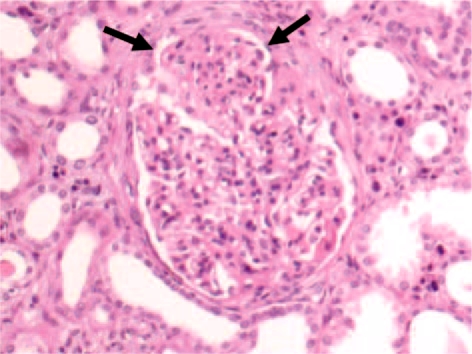
Light-microscopically, the renal biopsy revealed global sclerosis in 1 of 6 glomeruli; in the other glomeruli architecture was preserved: they were voluminous, hypercellular and exudative, with numerous polymorphonuclear leukocytes (fig. 1). Using Masson's trichrome staining, some images were suggestive of ‘humps’, and the GBM presented areas of focal duplication; 50% of the renal cortex showed interstitial fibrosis and focal tubular atrophy, and discrete aggregates of lymphomononuclear cells presented concomitant with plasma cells. Immunofluorescence revealed a glomerular diffuse granular staining with anti-IgG, IgA, C3, and ĸ and λ light chains.
Fig. 1.
Proliferative and exudative acute GN. HE. ×200.
Thus, acute proliferative and exudative GN was diagnosed with evidence of tubulointerstitial disease and arteriolosclerosis.
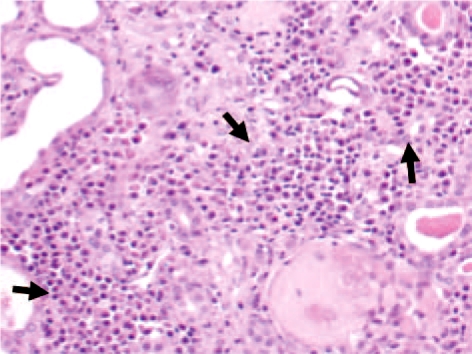
Following nephrectomy, histopathological analysis of the left kidney revealed multifocal MM infiltration with the following findings: interstitial groups of well-differentiated neoplastic plasma cells; presence of a large number of intratubular hyaline casts; multifocal tubular dilatation, and diffuse endocapillary proliferative GN with fuchsinophilic subepithelial deposits, suggesting the presence of ‘humps’ (fig. 2). No evidence of amyloid deposits was observed using Congo red staining. Renal biopsy showed 60% plasma cells, while a bone marrow biopsy revealed approximately 31% atypical plasma cells, confirming the diagnosis of MM.
Fig. 2.
Foci of neoplastic infiltration of plasma cells (MM).
The patient was then submitted to chemotherapy and maintained on dialysis. After 6 months, he died due to an infectious complication with no renal function improvement.
Discussion
This report describes a patient with proliferative and exudative GN with crescents, rapidly progressive renal insufficiency, macrohematuria, nephrotic-range proteinuria, IgA deposits in the kidney and MM with plasma cell infiltration in the kidneys. The involvement of glomeruli in plasma cell dyscrasia has already been described and is well established. In most instances, glomerular lesions correspond to nodular glomerulosclerosis associated with ĸ light chain deposition [4, 5, 6, 7]; GN is an uncommon finding in this condition [7], especially in the presence of glomerular extracapillary proliferative changes [1, 7]. Until recently, crescentic GN was not one of the possible conditions of plasma cell dyscrasia [1, 2]. Glomeruloproliferative lesions with crescent formation are a rare event in MM [1]. Meyrier et al. [2] reported 3 cases of B-cell dyscrasia with presentation corresponding to rapidly progressive GN: 2 cases showed severe cellular proliferation, both endo- and extracapillary, with numerous polymorphonuclear leukocytes within the capillary loops accompanied by severe renal insufficiency; crescents involving few glomeruli were present in the 3rd case. In the present case, dysmorphic hematuria, a finding suggestive of glomerular disease, was observed at presentation and is uncommon in MM; in other published case reports, macroscopic hematuria was present [1, 2, 3, 4, 5, 6, 7].
As previously reported [2, 7], polymorphonuclear leukocytes were numerous in the glomerular tufts and the possibility of a simultaneous postinfectious GN could not be completely ruled out. No previous recent infection was documented in this case. In accord with previous case reports [4, 5, 6, 7], the patient had normal serum complement at presentation.
Evidence for an immune complex pathogenesis in such cases is provided by electron- and immunofluorescence-microscopic studies, which demonstrated IgG-containing immune deposits located along the subepithelial GBM side and absence of amyloid deposits [4, 5, 6, 7]. On the other hand, the relative rarity of GN in MM may be related to the suppression of the synthesis of immunoglobulins other than the M protein that commonly occurs in this disorder [6, 7]. Identification of the pathogenesis of this association is clinically important, since good response to chemotherapy has been observed in previously reported cases of similar GN [7], and consequently treatment of renal involvement is also indicated.
Conclusions
Based on the present and previously reported cases, proliferative GN, with and without crescents, may represent the first manifestation of MM, or MM-induced immune-complex GN might be possible at any time in the course of this disease. Thus, it is important to be aware of this association.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Sirsat R, Deshpande R. Multiple myeloma presenting as proliferative (crescentic) glomerulonephritis. J Postgrad Med. 1994;40:92–93. [PubMed] [Google Scholar]
- 2.Meyrier A, Simon P, Mignon F, Striker L, Ramée M-P. Rapidly progressive (‘crescentic’) glomerulonephritis and monoclonal gammapathies. Nephron. 1984;38:156–162. doi: 10.1159/000183299. [DOI] [PubMed] [Google Scholar]
- 3.Silva FG, Meyrier A, Morel-Maroger L, Pirani CL. Proliferative glomerulonephropathy in multiple myeloma. J Pathol. 1980;130:229–236. doi: 10.1002/path.1711300404. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya S, Fukuno K, Kanemura N, et al. IgG type multiple myeloma and concurrent IgA type monoclonal gammopathy of undetermined significance complicated by necrotizing skin ulcers due to type I cryoglobulinemia. J Clin Exp Hematopathol. 2010;50:71–74. doi: 10.3960/jslrt.50.71. [DOI] [PubMed] [Google Scholar]
- 5.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 6.McLeish KR, Gohara AF, Gillespie C. Mesangial proliferative glomerulonephritis associated with multiple myeloma. Am J Med Sci. 1985;290:114–117. doi: 10.1097/00000441-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Crosthwaite A, Skene A, Mount P. Rapidly progressive glomerulonephritis complicating primary AL amyloidosis and multiple myeloma. Nephrol Dial Transplant. 2010;25:2786–2789. doi: 10.1093/ndt/gfp715. [DOI] [PubMed] [Google Scholar]