Abstract
Androgen and androgen receptors (AR) play critical roles in the proliferation of prostate cancer through transcriptional regulation of target genes. Here, we found that androgens upregulated the expression of dynamin-related protein 1 (Drp1), which is involved in the induction of mitochondrial fission (MF), a common event in mitosis and apoptosis. Clinical tissue samples and various prostate cancer cell lines revealed a positive correlation between Drp1 and AR levels. Treatment of androgen-sensitive cells with an AR agonist, R1881, and antagonist, bicalutamide, showed that Drp1 is transcriptionally regulated by androgens, as confirmed by an AR ChIP-seq assay. Live imaging experiments using pAcGFP1-Mito stably transfected LNCaP (mito-green) cells revealed that androgen did not induce significant MF by itself, although Drp1 was upregulated. However, when treated with CGP37157 (CGP), an inhibitor of mitochondrial Ca2+ efflux, these cells exhibited MF, which was further enhanced by pre-treatment with R1881, suggesting that androgen-induced Drp1 facilitated CGP-induced MF. This enhanced MF was correlated with increased apoptosis. Transfection with DN-Drp1 (K38A) rescued cells from increased apoptosis, confirming the role of androgen-induced Drp1 in the observed apoptosis with combination treatment. Further, we found that CGP reduced the expression of Mfn1, a protein that promotes mitochondrial fusion, a process which opposes fission. We suggest that androgen-increased Drp1 enhanced MF leading to apoptosis. The present study demonstrates a novel role for androgens in the regulation of mitochondrial morphology that could potentially be utilized in prostate cancer therapy.
Keywords: androgen, prostate cancer, mitochondria, fission, Drp1, apoptosis, DNML1
Introduction
Prostate cancer is a major cause of death among men in western countries. Androgens, through its binding to the androgen receptor (AR), induce the proliferation of prostate cells and are responsible for the progression of the cancer (1, 2). Androgen ablation is the most commonly used therapy for prostate cancer. However, androgen ablation often leads to progression of the tumor towards androgen-depletion independent (ADI) status, which is more difficult to treat (3, 4). In most ADI prostate cancers, the AR is over-expressed and aberrantly activated despite the elimination of androgens (5).
Mitochondria have gained much attention for its paradoxical roles in cell survival and cell death (6). Mitochondrial morphology can be rapidly altered by mitochondrial fission and fusion in response to the physiological requirements of a cell (7). Mitochondrial fission requires translocation of the cytoplasmic dynamin-related protein 1 (Drp1) to the mitochondria where it interacts with the outer mitochondrial membrane protein, Fission 1 or Fis1 (8–11). Drp1 via its GTPase activity performs the scission of the outer mitochondrial membrane (12, 13). Functional impairment of Drp1 results in aggregates of large, interconnected mitochondria characteristic of unfragmented mitochondria (14). Post-translational modifications of Drp1 such as phosphorylation (14–16), ubiquitination (17), sumoylation (18, 19), and S-nitrosylation (20) affect the function of Drp1. During mitosis, Cdk1/cyclin B mediates Drp1 phosphorylation at Ser-616 (16, 21) to promote mitochondrial fission. On the contrary, cyclic AMP-dependent phosphorylation of Drp1 at Ser-637 reduced mitochondrial fission (14, 15, 22). Thus, Drp1 function seems to be affected by the site of phosphorylation. In contrast to Drp1 function, mitofusin proteins 1 and 2 (Mfn1 and Mfn2, respectively) are essential for GTP-dependent mitochondrial fusion (7). Balance between mitochondrial fission and fusion events are necessary for normal functioning of the cell (23). Although mitochondrial fission (fragmentation) is part of both mitosis and apoptosis, it is not clear why fission leads in one case to cell division (16) and in another to cell death (24–26). Therefore, a better understanding of the regulation of proteins involved in mitochondrial fission and fusion is critical.
The objective of this research was to examine the regulation of Drp1 and its role in mitochondrial fission in prostate cancer cells. Results presented here demonstrate that androgen regulated Drp1 expression at the transcriptional level. Androgen-induced expression of Drp1 and its phosphorylation at Ser-616 correlated with androgen-induced cell proliferation. However, when the cells were treated with CGP37157 (CGP), which is known to affect mitochondrial function by inhibiting a mitochondrial sodium-calcium exchanger to induce mitochondrial calcium overload, androgen-induced Drp1 facilitated a significant increase in mitochondrial fission and apoptosis. Thus, we demonstrate that androgens function as a pro-apoptotic agent when mitochondrial function is disrupted. These results provide a novel alternative to induce apoptosis in prostate cancer cells by taking advantage of AR activation-induced Drp1 expression to sensitize prostate cancer cells to drugs that compromise mitochondrial function.
Materials and Methods
Reagents
The following mammalian expression plasmids were used: pAcGFP1-Mito (mito-green), a green fluorescent label for mitochondria (Clonetech Laboratories, Inc. Mountain View, CA); dominant-negative Drp1 (DN-Drp1, K38A) (a kind gift from Alexander van der Bliek, University of California, Los Angeles, CA). Antibodies used were: anti-Drp1 (BD Biosciences, San Jose, CA); anti-PSA and anti-AR (Santa Cruz Biotechnology Inc., Santa Cruz, CA); anti-Mfn1 (Novus Biologicals Littleton, CO); anti-phospho-Drp1 (Ser-616), anti-COXIV, anti-mouse and anti-rabbit IgG (Cell Signaling, Danvers, MA); anti-Mfn2, anti-β-actin and anti-chicken IgG (Sigma-Aldrich Corporation, St. Louis, MO); anti-GAPDH and anti-Hsp90α (Chemicon International, Temecula, CA). The chemicals used were: charcoal/dextran-stripped FBS (CSS) from Hyclone (Logan, UT); the synthetic androgen methyl trienolone (R1881) from Perkin-Elmer Life Sciences (Boston, MA); bicalutamide and cycloheximide from Sigma-Aldrich Corporation (St. Louis, MO); chloro-benzothiazepin CGP37157 (CGP) from Calbiochem (San Diego, CA); and G418 from Cellgro (Manassas, VA). Scrambled siRNA was from Qiagen (Valencia, CA) and siRNA for AR was from Dharmacon RNAi Technologies (part of Thermo Scientific, Lafayette, CO).
Cell Culture and Treatment
Prostate cancer cell lines LNCaP, DU145, and PC3 were purchased from ATCC (Manassas, VA). LNCaP derived C4-2 cells were a gift from Dr. Leland Chung, Cedars-Sinai Medical Center, Los Angeles, CA. Cells were maintained in RPMI 1640 (Hyclone, Logan, Utah) containing 9% FBS, 0.5% penicillin-streptomycin, and 0.1% fungizone. The CWR-R1 cells (provided by Dr. E. Wilson, University of North Carolina, Chapel Hill, NC) were grown in Richter’s MEM. Non-tumorigenic prostate epithelial cells (P69, a gift from Dr. Leland Chung) were maintained in T-medium (GIBCO-InVitrogen, Carlsbad, CA). VCaP cells (ATCC) were maintained in DMEM-GlutaMax medium (GIBCO-InVitrogen, Carlsbad, CA) containing 10% serum and treated in the same medium containing 5% CSS. Cells were maintained in CSS (5%) containing steroid free medium for 48h and treated with R1881 for 24h or the indicated periods. In combination experiments, bicalutamide was added 8h prior to R1881 treatment for 24h. Cycloheximide (50µg/ml) was added 1h before and during R1881 treatment. CGP (50µM) was added after 24h of R1881 treatment for the indicated periods. Transfection with siRNA was performed using HiPer-Fect reagent (Qiagen, Valencia, CA) for 48h. Stably transfected LNCaP cells expressing GFP in the mitochondria, pAcGFP1-Mito (mito-green), were generated by electroporation using the Electro Square Porator™ ECM-830 (BTX Inc, San Diego, CA), followed by G418 (500µg/ml) selection.
Protein Extraction and Western Blotting
Cell lysates were processed as described previously (27). For phosphorylation studies, phosphatase inhibitor cocktail 1 and 2 were added (Sigma-Aldrich Corporation, St. Louis, MO) to the lysis buffer.
Quantitative Real-Time Reverse Transcription-PCR
Total RNA was extracted using TRIzol (InVitrogen, Carlsbad, CA). The cDNA was synthesized using a Reverse Transcription System (Promega Corporation, Madison, WI) and was amplified by qRT-PCR using Mastercycler ep-realplex2 (Eppendorf, Westbury, NY) with the IQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA). Primers used in this study were: Drp1: 5′-CCAAGGTGCCTGTAGGTGAT-3′ and 5′-CAGCAGTGACAGCGAGGATA-3′; PSA: 5′-CATCAGGAACAAAAGCGTGA-3′and 5′-AGCTGTGGCTGACCTGAAAT-3′; and GAPDH: 5′-ACAGTCAGCCGCATCTTCTT-3′ and 5′-ACGACCAAATCCGTTGACTC-3′. Cycle treshhold (Ct) was normalized using the known Ct from the housekeeping gene GAPDH. To compare the relative levels of gene expression of Drp1 or PSA, ddCt values were calculated using gene expression from the untreated control cells and ddCt values were expressed as the real-fold increase in gene expression.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as previously described (28). ChIP enrichment was tested on one aliquot of the isolated DNA by real-time (quantitative) PCR and the remainder was used for single-end SOLEXA library preparation.
ChIP-seq SOLEXA Library Preparation
Single-end SOLEXA sequencing libraries were prepared as previously described (28).
Sequence Read Analysis
As previously described (28), sequence reads were generated by the Illumina analysis pipeline version 1.3.4 and 1.4.0, and the ChIP-seq data (28) deposited in the GEO (both processed data files and raw data) at the following URLs: http://www.ncbi.nlm.nih.gov/gds?term=GSE28126 (processed data files) and http://www.ncbi.nlm.nih.gov/sra?term=SRA012454.1 (raw data files).
Illumina BeadArrays and Analysis
Illumina beadarrays were performed using standard Illumina protocols as described previously (28). Autocorrelation analysis of Illumina gene expression data was performed as in (28). The Illumina gene expression data (28) have been deposited on GEO at http://www.ncbi.nlm.nih.gov/gds?term=GSE18684.
Mitochondrial Morphology
Stably transfected mito-green LNCaP cells were maintained in androgen-depleted medium for 48h before R1881 treatment for 24h and/or CGP (50µM) for the last 1h. Cells were washed and suspended in HBSS (GIBCO InVitrogen, Grand Island, NY) and at least 200 cells were examined for mitochondrial fragmentation under a fluorescent microscope (Zeiss Axioskop, Carl Zeiss Imaging Inc., Thornwood, NY). Mito-green cells were also grown on cover slips and treated as above. Cells were fixed with 4% paraformaldehyde and then analyzed using LSM 510 confocal microscopy (Carl Zeiss). Using the Z-stack option, signals from different planes were obtained and combined to give a single picture (maximum intensity projection). We considered cells to be undergoing mitochondrial fission when more than 80% of mitochondria in the cell exhibited fragmentation.
Cell Proliferation Assays
Proliferation of LNCaP cells was measured using the Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA). LNCaP cells (5×103 per well) seeded in 96-well microtiter plate were treated with bicalutamide (50µM) and/or R1881 (1nM). CGP (50µM) was added during the last 4h of treatment. Reagent from the kit was added and samples read in a microplate reader. For S-phase analysis, cells were treated with 1nM R1881 and fixed overnight in 70% ethanol, stained with propidium iodide (20µg/ml) and cells in S-phase were measured using a flow cytometry.
Measurement of Apoptosis
Total cell lysates (5µg) were analyzed for apoptosis using an M30 Apoptosense kit (Peviva, DiaPharma Group, West Chester, OH) as described previously (27).
Human Prostate Tissue
Deidentified coded tissue were obtained post-surgery from prostate cancer (n=7) and hormone refractory metastatic prostate cancer patients (n=6) from the University of Michigan SPORE tissue bank. Tissues were lysed in buffer containing 7M urea, 2M thiourea, 100mM DTT, 2% octyl glucoside and EDTA-free protease inhibitor cocktail (Roche) and the proteins were separated by SDS-PAGE, transferred to membranes and immunoblotted for Drp1, PSA (positive control) or GAPDH (loading control).
Statistical Analyses
Data are presented as means ± SEM. We compared group mean values, as appropriate, by Student’s unpaired two-tailed t test or one-way analysis of variance with Tukey’s multiple comparison test (GraphPad Prism). Significant differences were defined at ***P≤0.001, **P≤0.01 and *P≤0.05.
Results
Expression of Drp1 is Correlated with the Expression and Function of Androgen Receptor in the Prostate
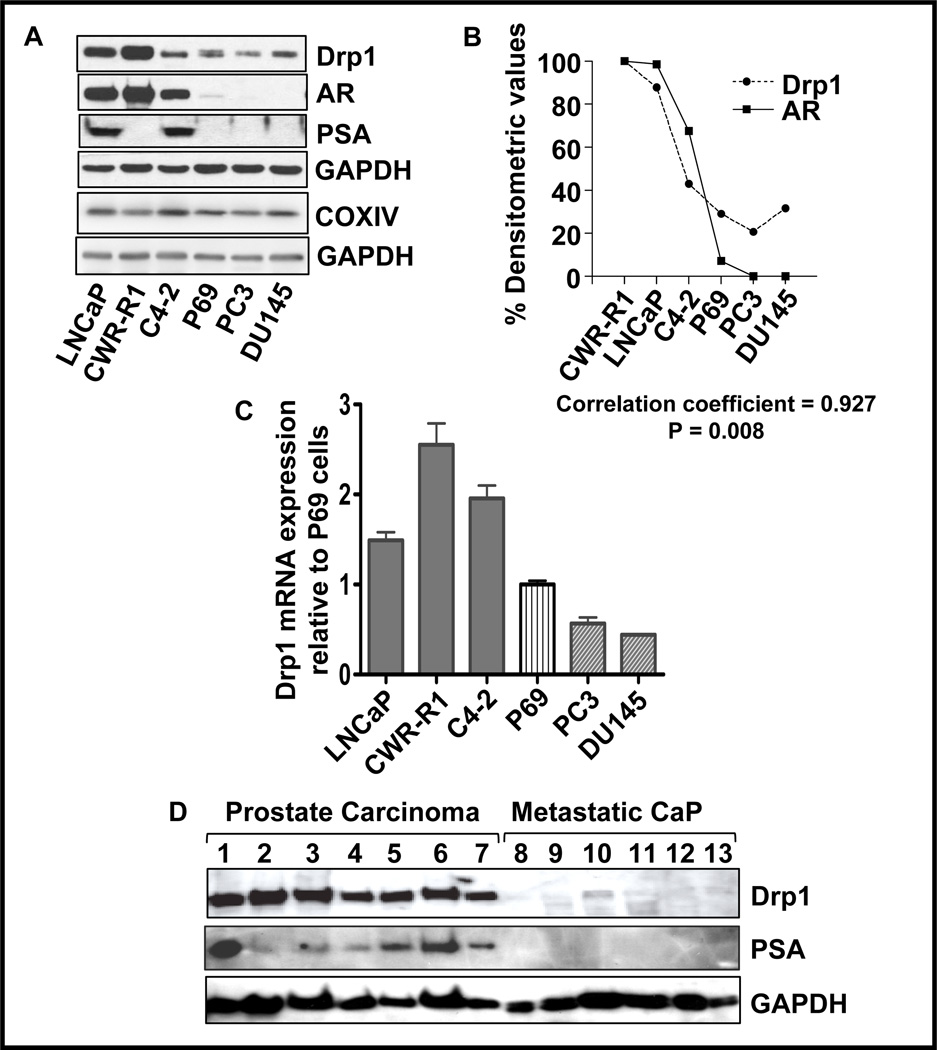
Prostate cells undergo proliferation in response to androgens. Fragmentation or fission of mitochondria is a required step in the process of mitosis and requires the involvement of Drp1, a key regulator of mitochondrial fission. Analysis of prostate cells showed that the expression of Drp1 was highest in androgen-responsive CWR-R1 cells, followed by LNCaP and LNCaP-derived C4-2 cells (Fig. 1A). The lowest levels of Drp1 were found in androgen-independent PC3 and DU145 cells. To assess the mitochondrial content of these cell lines, we also examined the COXIV expression levels (Fig. 1A), which did not differ much in the cell lines. Densitometric analysis of the signals showed a correlation between the expression of Drp1 and of AR (correlation coefficient of 0.927) with a P value of 0.008 (Fig. 1B), suggesting a positive relationship between the expression of these two proteins. Furthermore, the expression of Drp1 mRNA was higher in cells expressing AR compared to AR-null cells (Fig. 1C). Analysis of tissue samples obtained post-surgery from prostate cancer patients showed higher Drp1 levels in tissues where AR is functional, as indicated by the presence of PSA (Fig. 1D). On the other hand, androgen-refractory metastatic tissue showed extremely low levels of Drp1 (Fig. 1D). These results demonstrate a positive correlation between the expression and levels of Drp1 and the expression/function of AR.
Figure 1. Drp1 levels are positively correlated with AR status in prostate cancer cells.
A. Total cell lysates (20µg) from the indicated prostate cancer cells were analyzed by western blots using specific antibodies against Drp1, androgen receptor (AR), PSA, COXIV and GAPDH. B. The above blots were scanned and the densitometric values for Drp1 and AR were normalized to GAPDH and plotted as a percent of the values. The values for the cell line CWR-R1, which express the highest levels of Drp1 and AR were set at 100% and a correlation coefficient was calculated based on these values using GraphPad Prism software. C. Total RNA from prostate cancer cells was isolated, cDNA was prepared and Drp1 mRNA levels were analyzed by qRT-PCR. Drp1 mRNA levels were normalized to GAPDH and the data expressed relative to mRNA levels of normal prostate epithelial cells, P69. D. Drp1 expression in prostate cancer (n=7) and hormone refractory metastatic prostate cancer (n=6) were analyzed by Western blot. Expression of PSA served as an indicator of androgen responsiveness, while GAPDH was used as loading control.
Androgen Regulated Drp1 Expression at the Transcriptional Level
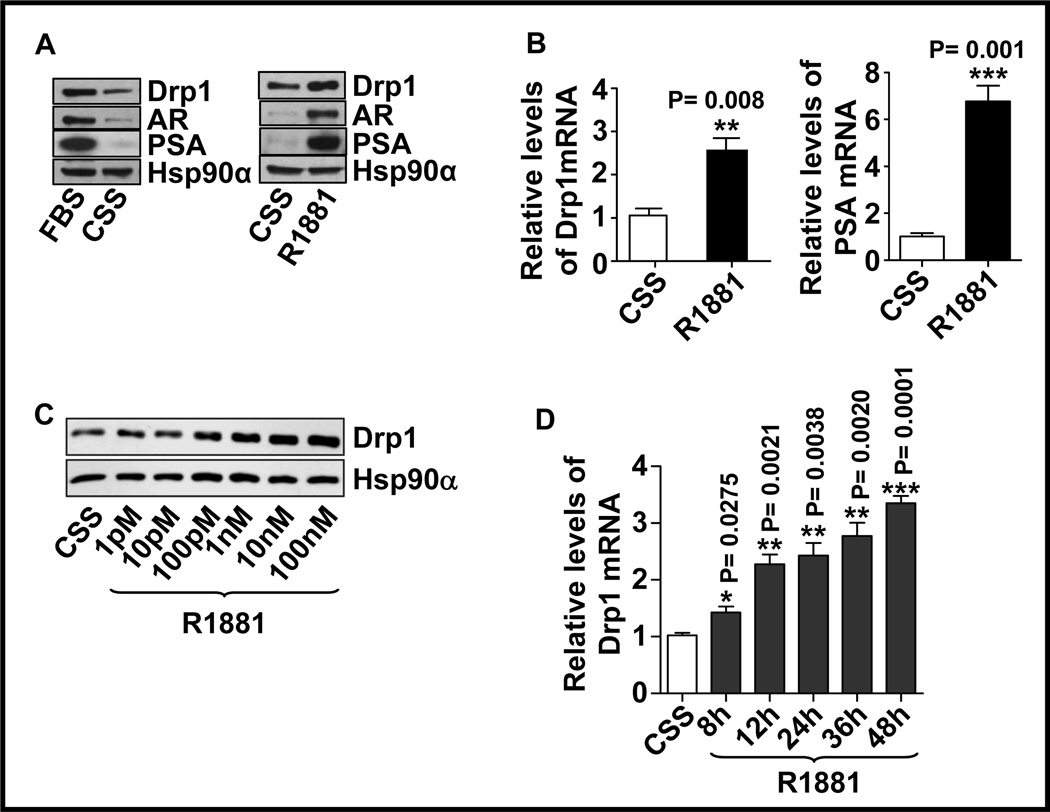
As the levels of Drp1 correlated with the expression of AR, the role of androgens in the regulation of Drp1 expression was investigated. The androgen-responsive LNCaP cells were maintained in androgen-depleted medium, which significantly reduced the levels of AR, Drp1 and PSA proteins (Fig. 2A; left panel). Treating these cells with the synthetic androgen, R1881, restored the protein expression of Drp1 and the positive control PSA (Fig. 2A, right panel), indicating that androgen increased the expression of Drp1 protein. Quantitative RT-PCR analysis demonstrated that treatment of LNCaP cells with R1881 increased the expression of Drp1 mRNA, suggesting that Drp1 is transcriptionally regulated by androgens (Fig. 2B, left panel). As expected, the mRNA levels of the positive control, PSA, was also upregulated in R1881-treated cells (Fig. 2B, right panel). The expression of Drp1 protein increased in a R1881 dose-dependent manner (Fig. 2C). Drp1 mRNA was also upregulated with increasing duration of R1881 treatment (Fig. 2D).
Figure 2. Androgens upregulate Drp1 mRNA and protein expression.
A. In the left panel, cells were grown in medium containing 9% FBS or charcoal/dextran-stripped serum (CSS); and in the right panel, cells were treated with R1881 (10nM) for 24h. Total cell lysates were analyzed by western blot for the levels of Drp1, PSA and AR. Hsp90α was used as a loading control. B. Total RNA was isolated and Drp1 (left) and PSA (right) mRNA were measured by qRT-PCR (**P≤0.01; ***P≤0.001, N=3). C. Cells were treated with increasing concentrations of R1881 for 24h and total cell lysates were analyzed for the levels of Drp1 protein. D. Cells were treated with R1881 for different periods and Drp1 mRNA expression was analyzed by qRT-PCR (P values were compared to the CSS controls, N=3).
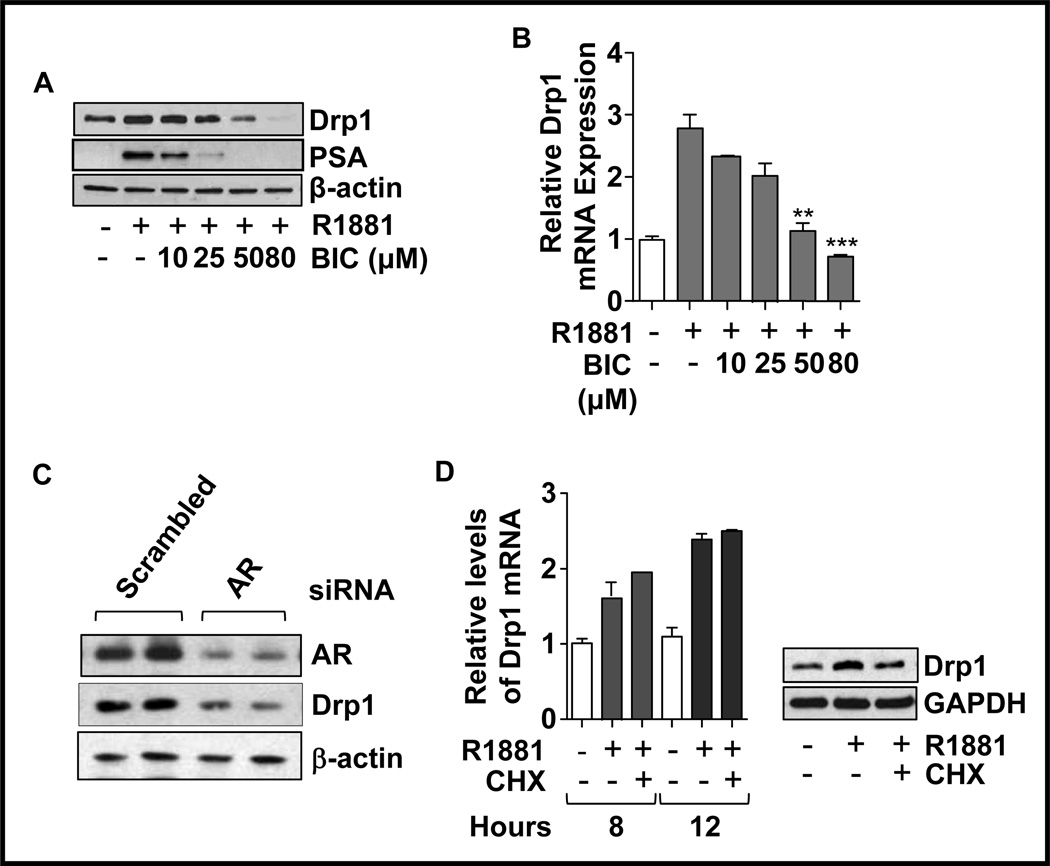
The specificity of the Drp1 response to androgens was confirmed using the anti-androgen, bicalutamide. Bicalutamide inhibited androgen-induced Drp1 upregulation in a dose-dependent manner with a significant decrease at 50µM and a complete abrogation of androgen-induced Drp1 protein expression upon treatment with 80µM bicalutamide (Fig. 3A). Similar analysis of Drp1 mRNA showed decreased expression with increasing concentrations of bicalutamide (Fig. 3B). Furthermore, silencing AR using AR-specific siRNA resulted in Drp1 downregulation in LNCaP cells (Fig. 3C). As the above results confirmed transcriptional regulation of Drp1 by androgens, experiments were conducted to determine whether the androgen-induced upregulation of Drp1 is mediated through an intermediary androgen-responsive protein. The expression of R1881-induced Drp1 mRNA was not altered when cells were treated with cycloheximide (Fig. 3D, left panel), suggesting that the regulation of Drp1 by androgens did not require de novo protein synthesis. Western blots confirmed that Drp1 protein expression did not increase with R1881 in cycloheximide treated cells (Fig. 3D, right panel), indicating the effectiveness of cycloheximide treatment.
Figure 3. Decreasing AR function using the anti-androgen, bicalutamide, or siRNA resulted in decreased Drp1 expression.
A and B. LNCaP cells were treated with varying concentrations of bicalutamide (BIC) in the presence of R1881 (10nM), and total cell lysates were analyzed by western blotting (A) or total RNA was analyzed by qRT-PCR for Drp1 mRNA levels (B). For qRT-PCR results represent the means ± SEM of three separate experiments with *P<0.01, **p<0.001 relative to R1881 treatment in the absence of bicalutamide. C. LNCaP cells were transfected either with siRNA against AR or scrambled siRNA. Total cell lysates were analyzed for the expression of Drp1, AR and β-actin (loading control). D. cells were treated with R1881 (10nM) for 8h or 12h, either in the presence or absence of the protein synthesis inhibitor cycloheximide (CHX; 50µg/ml, 1h before and during R1881 treatment). Drp1 mRNA levels were analyzed by qRT- PCR (left). Total cell lysates were analyzed for the expression of Drp1 (right). The results represent the means ± SEM of three separate experiments.
Presence of the AR Binding Site in the Drp1 Gene
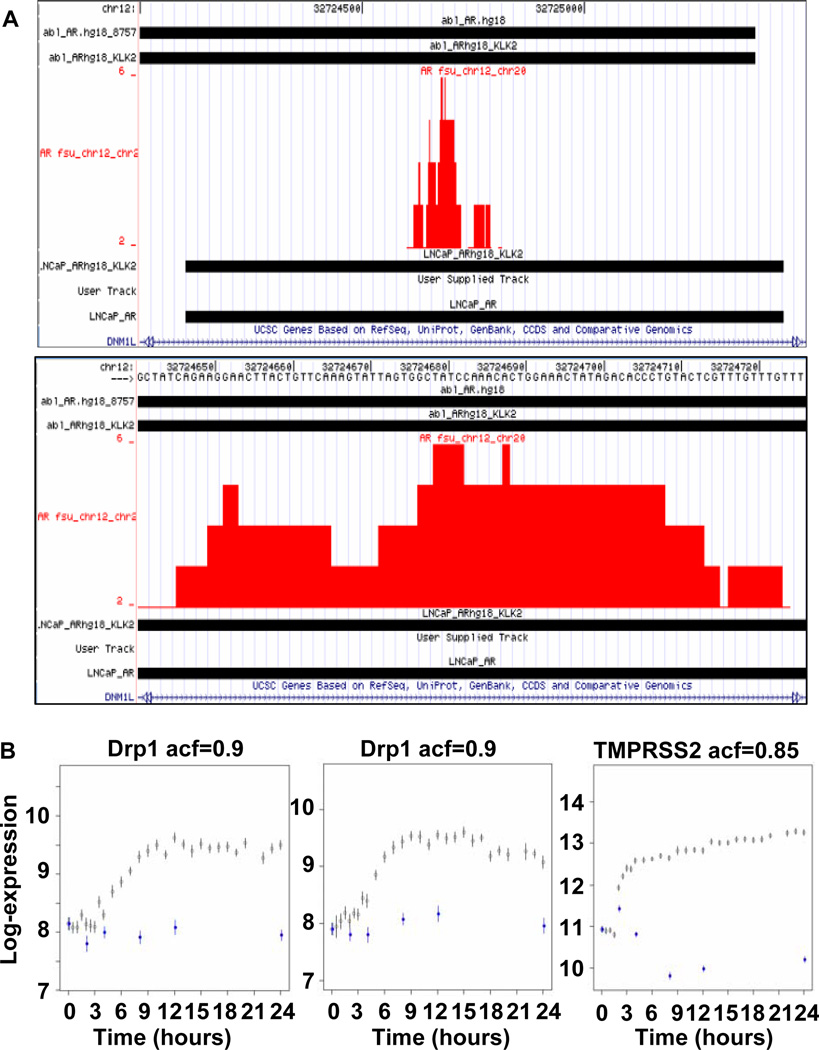
To determine whether AR binds to an androgen response element (ARE) in the Drp1 promoter, we performed chromatin immunoprecipitation (ChIP) analysis. The resultant ChIP screenshot data was uploaded in a .wig format into the UCSC genome browser and visualized against a hg18 reference genome. Analyzed data depicted the AR binding region in the Drp1 gene (Fig. 4A, upper panel). Tracks included RefSeq genes, ENCODE transcription factor binding site data and publicly available AR binding site data (29) generated by the laboratory of Myles Brown in both an androgen-independent (abl_AR) and an androgen-dependent (LNCaP) cell line, as illustrated by black bars near the top of the screenshots. The .wig data appears in red and is generated by ChIP-seq analysis following immunoprecipitation of the AR in the LNCaP cell line as described in Methods. The identified binding site is within Drp1 and overlaps with AR sites in public datasets. Higher resolution of the same site depicted the bases where AR binds to Drp1 gene (Figure 4A, lower panel). The data predict an AR binding site which is downstream of the RNA PolII site at the start of the Drp1 (DNM1L) gene and is located at chr12:32,724,000–32,725,500.
Figure 4. ChIP-seq and Illumina BeadArray expression analyses confirmed the presence of an AR binding site in the Drp1 gene.
A. AR ChIP-seq data analysis showing .wig data of an AR binding site located in the Drp1 gene. As denoted by 'KLK2' in the figure, the sequence in red denotes only AR binding sites that are as enriched or more enriched than the binding site in kallikrein 2 (KLK2), a prostate-associated protease that activates PSA. The data predict an AR binding site that is downstream of the RNA PolII site at the start of the Drp1 (also known as dynamin-like protein 1, or DNM1L) gene, located at chr12:32,724,000–32,725,500. B. LNCaP cells were treated with R1881 for different time periods and isolated mRNA was subjected to Illumina BeadArray analysis. The two plots represent results obtained with two separate probes for Drp1. TMPRSS2 was analyzed as a positive control for an androgen-responsive gene. Open circles represent cells treated with androgen and blue circles with vehicle (EtOH).
We also performed Drp1 expression array plots treating LNCaP cells with R1881 at different time points (Fig. 4B). Each plot represents a distinct BeadArray probe for the gene. Drp1 expression was clearly increased as a result of R1881 treatment in a time-dependent manner. TMPRSS2 was included as a positive control plot as a known androgen-regulated gene in LNCaP cells.
Based on the ChIP-seq analysis and expression beadarray time course, we have confirmed that Drp1 is androgen-regulated via an internal ARE located within a 5’ untranslated region of Drp1.
Regulation of Drp1 in Androgen-Sensitive and Androgen-Refractory cells
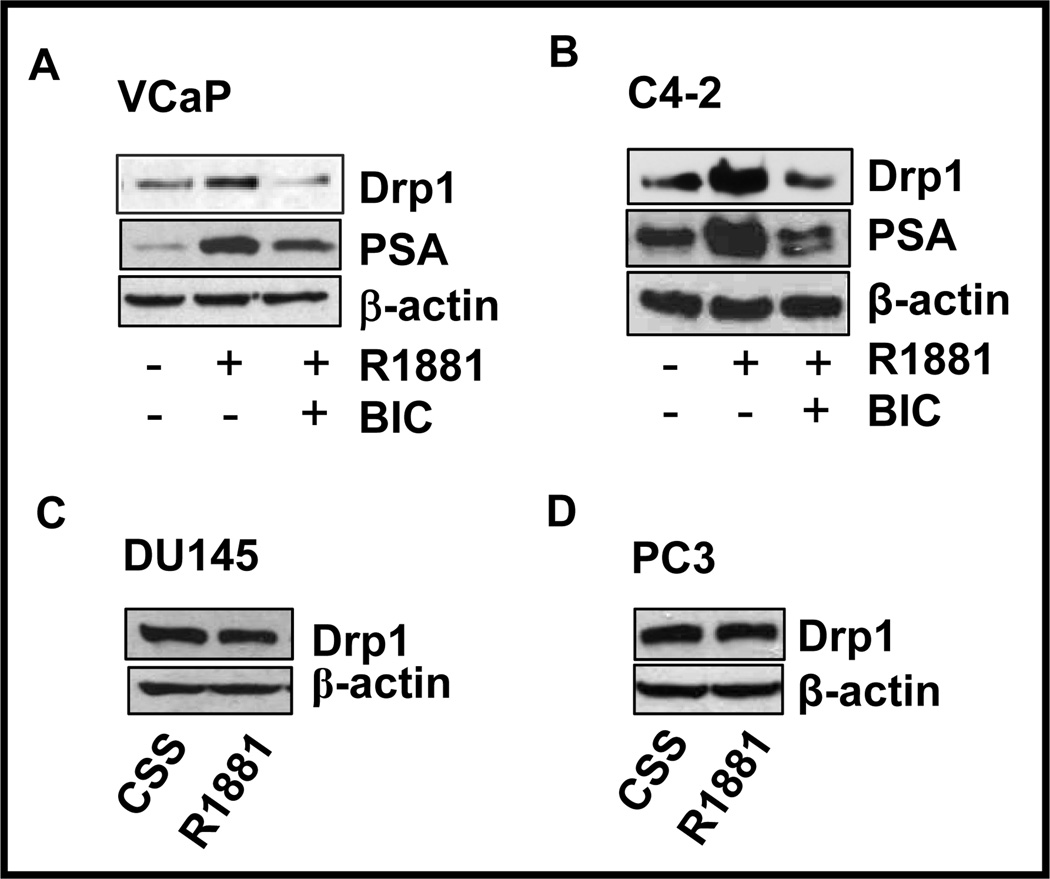
Treatment of another androgen-sensitive prostate cancer cell line, VCaP (30) with R1881 resulted in increased Drp1 expression, which was reduced in the presence of the anti-androgen bicalutamide (Fig. 5A), confirming our results obtained using LNCaP cells. Transition from androgen-dependent to ADI status is a critical problem in prostate cancer therapy. To determine the androgen regulation of Drp1 in ADI cells, C4-2 cells, which are known to have lost their dependence on androgen for proliferation (31, 32), were treated with R1881, which upregulated Drp1 protein (Fig. 5B). Treatment with bicalutamide reduced Drp1 expression, confirming the ability of ADI cells to respond to androgens. Similar experiments in AR-null DU145 and PC3 cells revealed no further increase in Drp1 protein with R1881 treatment, confirming the role of AR in mediating the upregulation of Drp1 (Fig. 5C,D).
Figure 5. Androgens regulate Drp1 in androgen-sensitive and androgen-independent (VCaP and C4-2) but not in androgen-refractory, AR-negative (DU145, PC3) prostate cancer cells.
A. Androgen-sensitive VCaP cells were treated with R1881 and bicalutamide (BIC), and total cell lysates were analyzed by western blot for Drp1 expression. B. Androgen depletion-independent C4-2 cells were treated and analyzed as in (A). C & D. Androgen refractory, AR-negative DU145 (C) and PC3 cells (D) were treated with R1881 (10nM) for 24h. All cells were grown in steroid-depleted medium for 48h before R1881 treatment and analyzed as in (A).
Androgens Facilitated Mitochondrial Fission Induced by CGP
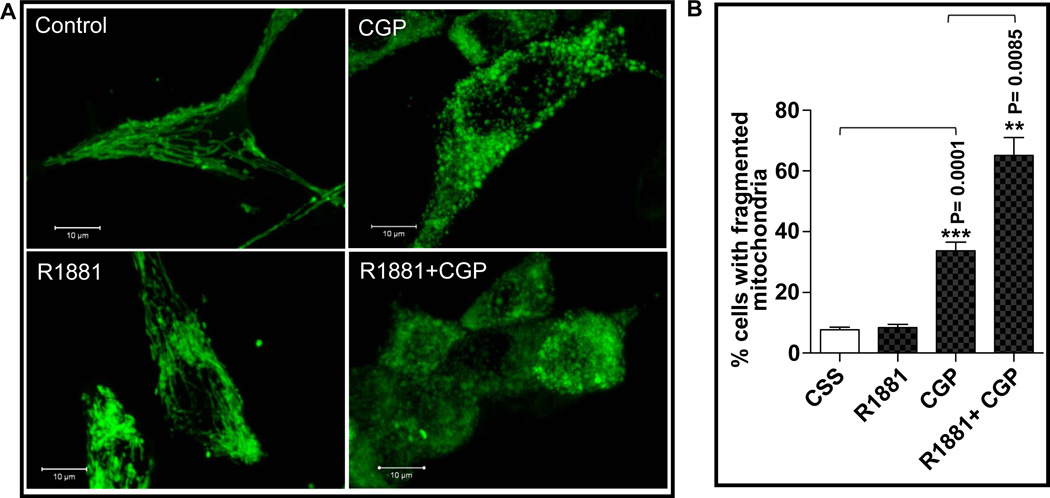
As Drp1 plays a critical role in mitochondrial fission, we hypothesized that its up-regulation by androgens would affect mitochondrial morphology. To verify this hypothesis, LNCaP cells, stably transfected with a construct expressing GFP protein in the mitochondria, were treated with R1881 and mitochondrial fission was scored. Contrary to our expectation, mitochondria in androgen-treated cells remained filamentous as in controls (Fig. 6A), suggesting that the androgen-induced increase in Drp1 was not sufficient to induce significant mitochondrial fission. To confirm the occurrence of mitochondrial fission in these cells, cells were treated with CGP37157 (CGP), an inhibitor of mitochondrial Ca2+ efflux, which induced mitochondrial fission in DU145, another prostate cancer cell line (9). As expected, CGP induced mitochondrial fission resulting in fragmented mitochondria, visualized as punctate structures (Fig. 6A). Surprisingly, CGP in the presence of R1881 resulted in higher number of cells with fragmented mitochondria when compared to CGP treatment alone. These observations were confirmed by quantitative analysis of fragmented mitochondria (Fig. 6B). Therefore, we suggest that androgen-induced upregulation of Drp1 is not sufficient to induce mitochondrial fragmentation, but increased levels of Drp1 readily potentiated mitochondrial fission when an appropriate mitochondrial stimulus is provided.
Figure 6. Androgen facilitated mitochondrial fission induced by CGP.
Stably transfected mito-green LNCaP cells were treated with R1881 (1nM) for 24h with or without CGP (50µM) for the last 1h, the cells were washed with HBSS and live cells were observed in a confocal fluorescent microscope. A. A representative photograph of cells in each treatment group is shown, illustrating the shape of the mitochondria. These pictures are from confocal imaging (Zen software) using ‘Z-stack’ option and signals from all the planes were considered by using ‘Maximum Intensity Projection’ option. B. Cells exhibiting more than 80% punctate (fragmented) mitochondria were counted and presented as a percentage of the total number of cells counted. At least 200 cells were examined in each dish and values represent the means ± SEM from at least 3 separate experiments, with **P≤0.01; *** P≤0.00 relative to the control.
Androgen Affects the phosphorylation of Drp1 at Ser-616
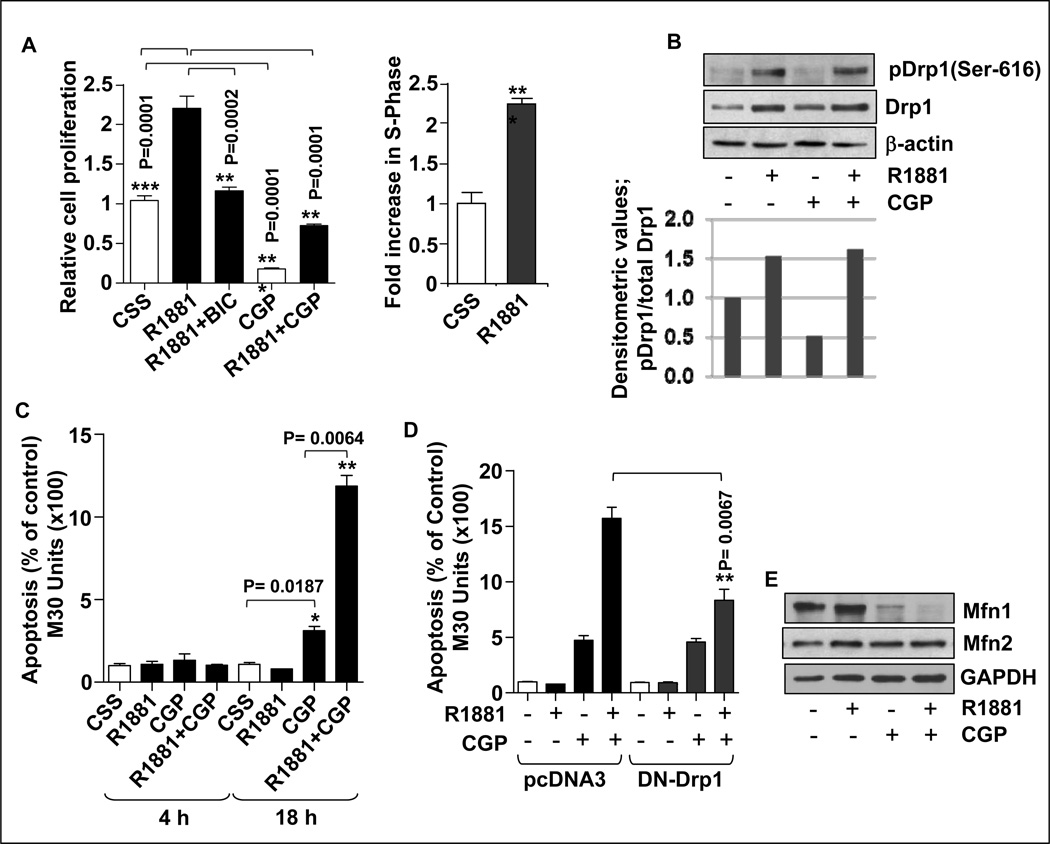
As androgens are known to induce proliferation of prostate cells, the role of Drp1 in cell survival was investigated. As expected R1881 treatment significantly increased the proliferation of LNCaP cells compared to vehicle-treated controls (Fig. 7A, left panel). The specificity of the response was confirmed by the ability of bicalutamide to reduce proliferation. Cell proliferation/viability was lower in CGP-treated cells even when the cells were treated also with R1881 (Fig. 7A, left panel). The effect of R1881 on proliferation was confirmed by flow cytometry, which showed an increased proportion of cells in S-phase (Fig. 7A, right panel). We then investigated whether androgen treatment had any effect on phosphorylation of Drp1 at Ser-616. During mitosis, Drp1 Ser-616 is known to be phosphorylated by Cdk1/cyclin B, a protein that is known to be androgen-responsive (16, 21, 33). Western blots revealed an increase in the phosphorylation of Drp1 at Ser-616 in R1881-treated cells compared to controls (Fig. 7B). Since Drp1 levels increase in response to R1881, we normalized the phospho-Ser-616 Drp1 signal to total Drp1 levels to make sure that the increase in Ser-616 phosphorylation does not merely reflect increased levels of Drp1. The densitometry analysis of the above western blot clearly showed an increase in phosphorylation of Drp1 upon treatment of cells with R1881, establishing that androgen increases Drp1 phosphorylation at Ser-616.
Figure 7. Androgen enhanced CGP-induced apoptosis: mediation by Drp1.
A. Androgen increased cell proliferation. LNCaP cells were treated with R1881 (1nM), bicalutamide (BIC, 50µM) or CGP (50µM) as described above. Cell proliferation was measured using a Quick Cell Proliferation assay kit (BioVision). Data are expressed as means ± SEM, with the brackets indicating the P values (N=5) (left panel). Right Panel: Cells were treated with R1881 (1nM) for 48h, fixed overnight with 70% ethanol, treated with RNAse and then stained with propidium iodide and analyzed using flow cytometry to determine the S-phase of the cell cycle (***P≤0.0002; N=3). B. Androgens increased phosphorylation of Drp1-Ser-616. LNCaP cells were treated with R1881 (1nM) and CGP (50µM for 18h) as described earlier. Cell lysates were subjected to western blot analysis and Drp1 phosphorylation at Ser-616 and total Drp1 determined. The lower panel shows the densitometric values for phospho-Ser-616 Drp1 levels normalized to the levels of total Drp1 in the above blot. C. Androgens increased CGP-induced apoptosis. LNCaP cells were treated with R1881 (1nM) for 24h. Cells were treated with CGP (50µM) for an additional 4h or 18h in the presence or absence of R1881. Cell lysates were used for an apoptosis assay using an M30 apoptosense analyses kit. Results are presented as the percentage of apoptosis compared to the CSS control (*P≤0.05; **P≤0.01; N=3). D. DN-Drp1 reduced androgen-mediated CGP-induced apoptosis. LNCaP cells were transfected with DN-Drp1 and treated with R1881 (1nM) and CGP (50µM for 18h) as described earlier. Cell lysates were analyzed for apoptosis using an M30 apoptosense analysis kit. Results are presented as the percentage of the respective controls (**P≤0.01; N=3). E. CGP induced a decrease in Mfn1. LNCaP cells were treated as in (B) and total cell lysates were analyzed for Mfn1 and Mfn2 levels.
Androgens Enhanced CGP-Induced Apoptosis: Mediation by Drp1
As the known function of Drp1 is in mitochondrial fission concomitant with mitosis or cell death (23, 25), apoptosis was measured under our experimental conditions. CGP treatment for 18h resulted in a 3-fold increase in apoptosis as compared to controls (Fig. 7C). The apoptotic response to CGP was further enhanced (to 11-fold) in the presence of R1881. There was no significant apoptosis observed in the first 4h of CGP treatment, although mitochondrial fission was observed within 1h of CGP treatment, suggesting that mitochondrial fission is an early event compared to apoptosis (Fig. 7C). Transfection of dominant-negative Drp1 (DN-Drp1; K38A) plasmid reduced the apoptotic response in cells treated with both CGP and R1881 (Fig. 7D), indicating that the induction of apoptosis by combination therapy required Drp1 and further confirming that androgen facilitated CGP-induced apoptosis. However, we observed no increase in apoptosis when LNCaP cells overexpressed a wild-type Drp1 (WT-Drp1) plasmid under our treatment conditions (data not shown). This result suggested that the androgen-induced increase in Drp1 is necessary but not sufficient to enhance CGP-induced apoptosis. There may be other factors involved in the CGP-induced apoptotic response in the presence of androgen. Next, we examined the protein expression of Mfn1 and Mfn2, proteins that facilitate mitochondrial fusion, an opposing function relative to Drp1. Western blots showed that Mfn1 expression was not affected by androgen alone. However, CGP treatment in the absence or presence of androgen significantly reduced the levels of Mfn1 (Fig. 7E). Similar analysis of Mfn2 did not show changes in expression with any treatment. These observations suggest that increased expression of Drp1, and perhaps a concomitant decrease in Mfn1, exaggerated CGP-induced mitochondrial fission and apoptosis.
Discussion
Androgen depletion therapy is the mainstay of prostate cancer treatment. However, development of androgen-independent tumors is a major concern in patient care. Therefore, a more effective therapy would be an alternative to androgen ablation that still exhibits efficacy in inducing death of prostate cancer cells. In this manuscript we describe a mechanism to induce apoptosis in prostate cancer cells in the presence of androgens; indeed androgens facilitated the death of these cells.
Mitochondrial fission (fragmentation) is a common event in both cell proliferation and apoptosis. In resting cells, mitochondria are filamentous, while in cells undergoing mitosis or apoptosis they are fragmented appearing as punctate/pinhead-like structures. A key protein involved in mitochondrial fragmentation is Drp1. As androgens induce cell proliferation and are important in prostate cancer progression, we hypothesized that Drp1 may be androgen-responsive. Indeed, our results confirmed a correlation between the expression of Drp1 and the expression and function of AR (PSA expression) in several prostate cell lines at both the mRNA and protein levels. Downregulation of AR using siRNA reduced the expression of Drp1, indicating androgen regulation of Drp1 expression. AR function and Drp1 expression also correlated significantly in androgen-refractory metastatic tissues, indicating that Drp1 is an androgen-regulated gene. An increase in Drp1 mRNA and protein expression in androgen-treated cells and a decrease in Drp1 expression upon treatment with the androgen antagonist, bicalutamide, confirmed the ability of androgen to regulate Drp1 expression at the transcriptional level. Experiments using cycloheximide to inhibit the synthesis of proteins further confirmed that the increase in Drp1 mRNA due to androgen is not a response to other androgen-responsive proteins. Our ChIP-seq data confirmed the presence of an AR binding site in the Drp1 gene. This AR binding region was located almost 1kb downstream of the transcription start site in an untranslated region of Drp1. Illumina expression bead array results confirmed transcriptional regulation of Drp1 by androgens. Thus, these results are significant as this is the first report of the regulation of any gene associated with mitochondrial fission/fusion by androgens.
Initial experiments to explore the mechanisms involved showed an increase in the phosphorylation of Drp1 at Ser-616 in androgen-treated cells. The Drp1 Ser-616 site was selected for investigation as this amino acid is phosphorylated by Cdk1/cyclin B (16, 21). As cyclin B is an androgen-responsive gene (33), it is possible that phosphorylation of Ser-616 by R1881 is mediated through cyclin B resulting in androgen-mediated proliferation of prostate cancer cells. Thus, androgens not only increase the levels of the protein with their effects on transcription of Drp1, but also seem to influence the function of Drp1 via an indirect androgen-responsive protein, ultimately leading to cell proliferation.
Based on its known function in mitochondrial fragmentation and apoptosis (23, 25), we expected that increased expression of Drp1 in androgen-treated cells would lead to mitochondrial fission. Surprisingly, despite the increase in Drp1, mitochondria remained filamentous, characteristic of non-apoptotic cells, which agrees with some published observation that over-expression of Drp1 does not always result in mitochondrial fission (11, 34). However, the lack of mitochondrial fission in androgen-treated cells is not due to incapacity of the cells to undergo mitochondrial fission, as treatment with CGP, either alone or in combination with androgen, induces mitochondrial fission. Over-expression of wild-type Drp1 in LNCaP cells also resulted in no increase in androgen- or combination therapy-induced apoptosis (data not shown), further suggesting that the androgen-induced increase in Drp1 is not sufficient for the induction of apoptosis. Together with our findings that siRNA-mediated knock down of Drp1 inhibited apoptosis induced by combination treatment with androgen and CGP, these results suggest that Drp1 is necessary but not sufficient for apoptosis. Clearly, an additional stimulus, such as mitochondrial calcium overload induced by CGP, is necessary to promote apoptosis in the presence of androgen-increased Drp1. Nevertheless, increased mitochondrial fission with combination therapy correlated with the induction of cell death, suggesting that the availability of higher levels of Drp1 in androgen-treated cells facilitated CGP-induced apoptosis. Thus, androgens seem to function as pro-apoptotic agents when combined with an agent that affects mitochondrial function. These data support earlier observations that androgens are pro-apoptotic under certain conditions, such as upon Bax-mediated cell death (35) or treatment with phorbol 12-myristate 13-acetate (36) or with taxane (37).
In summary, we demonstrate a role of androgens in transcriptional and posttranslational regulation of Drp1, a key protein in the mitochondrial fission machinery. Furthermore, androgen-induced Drp1 facilitated mitochondrial fission and apoptosis in cells treated with CGP. These data suggest a novel pro-apoptotic mechanism of action of androgens and also addresses a fundamental question about the involvement of the mitochondria in proliferation and apoptosis.
Acknowledgements
We thank Arti Jain for her technical support, Asmaa El-Kenawi for her valuable suggestions during preparation of the manuscript, and Dr. Azza El-Remessy and her laboratory members for generously sharing their real-time PCR machine and other equipments.
Grant Support
This research was funded by VA merit awards to MVK and WBB and an award from the American Legion of Georgia to both MVK and WBB. This work is supported in part by the National Cancer Institute grants RO1CA133458-04, 1 R03 CA139489-01 and RCA145444A to AS. AS was also supported by a Georgia Cancer Research Distinguished Scientist award.
Footnotes
Discloser of Potential Conflicts of Interest
No potential conflicts of interest exist.
References
- 1.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. Journal of Cellular Biochemistry. 2006;99(2):333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. Journal of the National Cancer Institute. 2001;93(22):1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 3.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU International. 2005;96 Suppl 2:35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Ok JH, Busby JE, et al. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Research. 2009;69(1):151–160. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBride HM, Neuspiel M, Wasiak S, McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Current Biology. 2006;16(14):R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Human Molecular Genetics 14 Spec No. 2005;2:R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 9.Kaddour-Djebbar I, Choudhary V, Brooks C, et al. Specific mitochondrial calcium overload induces mitochondrial fission in prostate cancer cells. Int J Oncol. 2010;36(6):1437–1444. doi: 10.3892/ijo_00000629. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends in Cell Biology. 2002;12(4):178–184. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon Y, Krueger EW, Oswald BJ, et al. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Molecular & Cellular Biology. 2003;23(15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han XJ, Lu YF, Li SA, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. Journal of Cell Biology. 2008;182(3):573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Molecular Biology of the Cell. 2001;12(9):2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. Journal of Biological Chemistry. 2007;282(30):21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 15.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death.[see comment] EMBO Reports. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taguchi N, Ishihara N, Jofuku A, et al. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. Journal of Biological Chemistry. 2007;282(15):11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura N, Kimura Y, Tokuda M, et al. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Reports. 2006;7(10):1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harder Z, Zunino R, McBride H, Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Current Biology. 2004;14(4):340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Wasiak S, Zunino R, McBride HM, Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. Journal of Cell Biology. 2007;177(3):439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho DH, Nakamura T, Fang J, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CR, Blackstone C. Drp1 phosphorylation and mitochondrial regulation.[comment] EMBO Reports. 2007;8(12):1088–1089. doi: 10.1038/sj.embor.7401118. author reply 9–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO Journal. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks C, Wei Q, Cho SG, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. Journal of Clinical Investigation. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental Cell. 2001;1(4):515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Lazaro M, Bonekamp NA, Galindo MF, et al. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radical Biology & Medicine. 2008;44(11):1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Kaddour-Djebbar I, Lakshmikanthan V, Shirley RB, Ma Y, Lewis RW, Kumar MV. Therapeutic advantage of combining calcium channel blockers and TRAIL in prostate cancer. Mol Cancer Ther. 2006;5(8):1958–1966. doi: 10.1158/1535-7163.MCT-06-0011. [DOI] [PubMed] [Google Scholar]
- 28.Massie CELA, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N, Bon H, Zecchini V, Smith D-M, DeNicola GM, Mathews N, Osborne M, Hadfield J, MacArthur S, Adryan B, Lyons SK, Brindle KM, Griffiths J, Gleave ME, Rennie PS, Neal DE, Mills IG. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. doi: 10.1038/emboj.2011.158. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korenchuk S, Lehr JE, L MC, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15(2):163–168. [PubMed] [Google Scholar]
- 31.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44(2):91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. Jul 1;44(2). [DOI] [PubMed] [Google Scholar]
- 32.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57(3):406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 33.Gregory CW, Johnson RT, Jr, Presnell SC, Mohler JL, French FS. Androgen receptor regulation of G1 cyclin and cyclin-dependent kinase function in the CWR22 human prostate cancer xenograft. J Androl. 2001;22(4):537–548. [PubMed] [Google Scholar]
- 34.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Molecular Biology of the Cell. 1999;10(12):4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Kokontis J, Tang F, et al. Androgen and its receptor promote Bax-mediated apoptosis. Molecular & Cellular Biology. 2006;26(5):1908–1916. doi: 10.1128/MCB.26.5.1908-1916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG. Androgens regulate protein kinase Cdelta transcription and modulate its apoptotic function in prostate cancer cells. Cancer Research. 2006;66(24):11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 37.Hess-Wilson JK, Daly HK, Zagorski WA, Montville CP, Knudsen KE. Mitogenic action of the androgen receptor sensitizes prostate cancer cells to taxane-based cytotoxic insult. Cancer Research. 2006;66(24):11998–12008. doi: 10.1158/0008-5472.CAN-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]