Abstract
Objective
To assess regions and patterns of brain atrophy in patients with Parkinson disease (PD) with normal cognition (PD-NC), mild cognitive impairment (PD-MCI), and dementia-level cognitive deficits (PDD).
Design
Images were quantified using a region-of-interest approach and voxel-based morphometry analysis. We used a high-dimensional pattern classification approach to delineate brain regions that collectively formed the Spatial Pattern of Abnormalities for Recognition of PDD.
Setting
The Parkinson’s Disease and Movement Disorders Center at the University of Pennsylvania.
Subjects
Eighty-four PD patients (61 PD-NC, 12 PD-MCI, and 11 PDD) and 23 healthy control subjects (HCs) underwent magnetic resonance imaging of the brain.
Results
The PD-NC patients did not demonstrate significant brain atrophy compared with HCs. Compared with PD-NC patients, PD-MCI patients had hippocampal atrophy (β=−0.37; P=.001), and PDD patients demonstrated hippocampal (β=−0.32; P=.004) and additional medial temporal lobe atrophy (β=−0.36; P=.003). The PD-MCI patients had a different pattern of atrophy compared with PD-NC patients (P=.04) and a similar pattern to that of PDD patients (P=.81), characterized by hippocampal, prefrontal cortex gray and white matter, occipital lobe gray and white matter, and parietal lobe white matter atrophy. In nondemented PD patients, there was a correlation between memory-encoding performance and hippocampal volume.
Conclusions
Hippocampal atrophy is a biomarker of initial cognitive decline in PD, including impaired memory encoding and storage, suggesting heterogeneity in the neural substrate of memory impairment. Use of a pattern classification approach may allow identification of diffuse regions of cortical gray and white matter atrophy early in the course of cognitive decline.
Patients with Parkinson disease (PD) are at an increased risk of developing dementia (PDD), with cumulative prevalence rates of up to 80%.1 Approximately 25% of nondemented PD patients meet neuropsychological criteria for mild cognitive impairment (PD-MCI),2 which converts to PDD in many cases,3 and even mild cognitive deficits in PD are associated with functional impairments4 and worse quality of life.5 Biomarkers of PD-MCI would improve diagnostic accuracy, identify those at highest risk of developing PDD, and predict response to cognitive-enhancing treatments.6
There has been extensive biomarker research concerning PDD. For structural imaging, the most consistent findings have been parietal-temporal lobe and prefrontal cortex atrophy compared with healthy control subjects (HCs) and PD patients without dementia.7-9 The neural substrate of MCI in PD is not well known, and studies of nondemented PD patients often include a mixture of PD-MCI patients and those with normal cognition (PD-NC).10
A pattern classification approach has been developed11,12 that integrates structural measurements from the entire brain and determines the brain regions that collectively form a Spatial Pattern of Abnormality for Recognition of Early Alzheimer’s Disease (SPARE-AD score). The MCI patients with higher SPARE-AD scores demonstrated greater decline in MiniMental State Examination scores during long-term follow-up11 and increased rates of conversion to AD.12 To our knowledge, such a structural pattern of atrophy has not been developed for PDD.
Given the limited research on neurodegenerative biomarkers at the initial stage of cognitive decline in PD, we report (1) structural imaging findings from a cohort of PD patients with a range of cogni-tive abilities and (2) preliminary findings from the generation and application of a high-dimensional pattern classification method to identify PDD. We hypothesized that PD-MCI patients would (1) demonstrate hippocampal and prefrontal cortex atrophy and (2) have a spatial pattern of atrophy similar to that of PDD patients.
METHODS
PARTICIPANTS
Data were obtained as part of the University of Pennsylvania Center of Excellence for Research on Neurodegenerative Diseases, which evaluated a convenience sample of individuals at risk for late-life dementia (AD and PD) with a range of neuro-pathologically linked biomarkers. The diagnosis of PD was based on British Brain Bank criteria,13 and movement disorders specialists (including A.D.S. and J.E.D.) administered the motor subscale (part III) of the Unified Parkinson’s Disease Rating Scale,14 determined Hoehn and Yahr stage,14 and provided a clinical impression of PDD (yes/no).
Eighty-four PD patients and 23 HCs underwent structural magnetic resonance imaging and neuropsychological testing within 6 months of each other. Levodopa and dopamine agonist dosages are presented as levodopa equivalent daily dosage.15 Demographic and clinical characteristics are presented in the Table.
Table. Characteristics of the Study Samplea.
| PD Groups |
||||
|---|---|---|---|---|
| Variable | HCs (n=23) |
PD-NC (n=61) |
PD-MCI (n=12) |
PDD (n=11) |
| Clinical and demographic | ||||
| Age, y | 71.5 (9.2) | 69.3 (5.7) | 75.7 (7.7)b | 73.1 (7.2) |
| Male sex, No. (%) | 5 (22) | 39 (64)c | 10 (83) | 7 (64) |
| Education, y | 15.8 (2.9) | 16.0 (2.8) | 15.2 (1.6) | 14.4 (2.7) |
| PD duration, y | NA | 7.1 (3.8) | 9.3 (6.6) | 9.0 (7.1) |
| Hoehn and Yahr stage, median (IQR) | NA | 2.0 (2.0-2.5) | 2.0 (2.0-3.0) | 3.0 (2.5-3.0)d |
| Levodopa dosage, mg/de | NA | 424 (368) | 543 (441) | 610 (209) |
| Dopamine agonist dosage, mg/de | NA | 101 (164) | 75 (145) | 45 (101) |
| UPDRS motor score | NA | 20.3 (8.9) | 23.6 (11.1) | 29.5 (12.7) |
| GDS-15 scoref | 0.7 (1.4) | 2.2 (2.3)g | 3.3 (3.1) | 4.0 (3.1) |
| Neuropsychological assessment | ||||
| DRS-2 | ||||
| Total score | 141.5 (2.5) | 139.4 (3.0)h | 127.8 (3.5) | 108.5 (17.1)i |
| Total standardized score | 13.2 (2.1) | 11.5 (2.0)j | 6.8 (0.9) | 3.3 (1.2)k |
| Memory subscale | NA | 23.7 (1.3) | 20.5 (2.2) | 16.4 (4.7) |
| Attention subscale | NA | 36.2 (1.7) | 34.9 (1.9) | 33.1 (3.4)l |
| Conceptualization subscale | NA | 37.3 (1.6) | 35.3 (2.1) | 31.8 (3.6)l,m |
| Initiation/perseveration subscale | NA | 36.2 (1.7) | 31.3 (3.9) | 22.8 (6.9)n |
| Construction subscale | NA | 5.9 (0.3) | 5.9 (0.3) | 4.4 (2.2)o |
| HVLT-Rp | ||||
| Immediate recall | NA | 21.0 (4.8) | 13.4 (4.7) | 11.7 (7.0)l,q |
| Delayed recall | NA | 6.6 (3.0) | 3.8 (2.5) | 1.6 (2.1)l,r |
| Recognition discrimination | NA | 10.0 (1.5) | 8.3 (1.8) | 6.3 (4.4)l |
Abbreviations: DRS-2, Dementia Rating Scale–2; GDS-15,15-Item Geriatric Depression Scale16 (a commonly used version as opposed to the original 30-item GDS); HCs, healthy controls; HVLT-R, Hopkins Verbal Learning Test–Revised; IQR, interquartile range; NA, not applicable; PD, Parkinson disease; PDD, PD with dementia-level cognitive deficits; PD-MCI, PD with mild cognitive impairment; PD-NC, PD with normal cognition; UPDRS, Unified Parkinson’s Disease Rating Scale.
Comparisons are between PD-NC and HC groups and among PD-NC, PD-MCI, and PDD groups. Unless otherwise indicated, data are expressed as mean (SD)
P= .01, PD-NC vs PD-MCI group (Tukey test).
P= .001, PD-NCvs HC group ().
P= .001, PDDvs PD-NC group (Tukey test)
Includes 83 PD patients.
Includes 102 PD patients and HCs.
P= .001, HCvs PD-NC group (t67.1 = −3.6)
P= .005, PD-NC vs HC group (t82 = 2.9).
P< .001 among all PD groups (F2,81 = 105.6)
P= .001, PD-NC vs HC group (t82 = 3.3).
P< .001 among all PD groups (F2,81 = 114.9)
P< .001, PDD vs PD-NC group (Tukey test).
P = .03, PDDvs PD-MCI group (Tukeytest)
P< .001 among all PD groups (F2,81 = 88.0).
P< .001, PDD vs PD-MCI group and PDD vs PD-NC group (Tukey test)
Includes 60 PD patients.
P= .001, PD-NCvs PD-MCI group (Tukey test)
P= .03, PD-NC vs PD-MCI group (Tukey test).
The institutional review board at the University of Pennsylvania approved this research, and written informed consent was obtained from all study participants.
NEUROPSYCHOLOGICAL TESTING AND COGNITIVE CLASSIFICATION
The Dementia Rating Scale–2 (DRS-2)17 has been validated as an assessment instrument for PDD,18 including discriminat-ing PD-MCI from PDD.19 The DRS-2 total score is constructed from the following 5 subscores: attention, initiation, construction, conceptualization, and memory. Because formal assessments of self-reported cognitive decline and functional impairment were not performed, cognitive categorization of patients was solely on the basis of their DRS-2 performance, and the terms PD-NC, PD-MCI, and PDD were retained for descriptive purposes. Cognitive categories were defined on the basis of the following recommended age-standardized DRS-2 scores17: (1) for PD-NC, a DRS-2 score of greater than 8, which corresponds to greater than the 28th percentile (n=61); (2) for PD-MCI, a DRS-2 score of 6 to 8 inclusive, which corresponds to the 6th through 28th percentiles (n=12); and (3) for PDD, a DRS-2 score of less than 6, which corresponds to less than the 6th percentile (n=11). A subset of PD patients (n=60) underwent assessment with the Hopkins Verbal Learning Test–Revised (HVLT-R),20 and HVLT-R subscale scores (immediate free recall, delayed free recall, and recognition discrimination) are reported.
The agreement between DRS-2 categorization and the clinicians’ clinical impression of dementia diagnosis was high, with 60 (98%) PD-NC and 10 (83%) PD-MCI patients assigned a clinical impression of no dementia, and 9 (82%) PDD patients assigned a clinical impression of dementia.
STRUCTURAL IMAGING AND ANALYSES
Image Acquisition
The data sets included standard T1-weighted magnetic resonance images acquired sagittally using volumetric 3-dimensional magnetization prepared rapid gradient echo with 1.25×1.25-mm in-plane spatial resolution and 1.2-mm-thick sagittal sections (flip angle, 8°; echo time, 3.55 milliseconds; repetition time, 3000 milliseconds; and imaging frequency, 63.64 Hz) performed on 1.5-T scanners.
Image Analysis
We based the Center of Excellence for Research on Neurodegenerative Diseases magnetic resonance imaging analysis on an image-processing protocol developed at the Section of Biomedical Image Analysis of the Department of Radiology at the University of Pennsylvania.21 Global volumes were obtained via an automated segmentation technique that labels the brain into white matter (WM), gray matter (GM), cerebrospinal fluid, and ventricles after a sequence of preprocessing steps that remove extracranial material and align each scan with the anterior commissure–posterior commissure plane. Quantification of regional brain volumes is performed through an elastic atlas warping algorithm that coregisters a template of brain anatomy with each scan.22 The template has 97 regions of interest (ROIs) based on the Montreal Neurological Institute template, which are transferred to individual scans, after which regional volumetric and functional measurements can be obtained. These ROIs were then collapsed into 14 larger ROIs, including primarily left and right lobar GM and WM volumes, hippocampi, and ventricles (eTable 1; http://www.archneurol.com). To limit the number of variables presented, we calculated the average of the right and left volumes for each ROI. We performed ROI analyses with statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm/software/spm5).
To further characterize local atrophy in the brain, we performed a voxel-based morphometry (VBM) analysis (Regional Analysis of Volumes Examined in Normalized Space [RAVENS]).23 This approach computes GM, WM, and ventricle tissue density maps separately in a common coordinate system after spatial normalization. The RAVENS approach bears similarities with the optimized VBM approach except that it uses a high-dimensional image-warping algorithm (termed HAMMER [hierarchical attribute matching mechanism for elastic registration]).22,23 It uses tissue-preserving transformations, which ensure that image warping absolutely preserves the amount of GM, WM, and cerebrospinal fluid tissue present in an individual’s scan. Voxel dimensions were 2.0×2.0×2.0 mm.
Statistical Analysis and Pattern Classification
We compared PD-NC and HC groups using linear regression models with age, sex, education, and intracranial volume (ICV) as covariates. For cognitive comparisons within the PD groups, χ2 tests, t tests, nonparametric tests to compare medians, and analysis of variance with post hoc analyses (Tukey tests) were used for between-group comparisons on clinical, demographic, and ROI variables. For ROI variables that were significant on bivariate analysis, an additional linear regression model was run with age, sex, educational level, ICV, and disease severity (Hoehn and Yahr stage) as covariates. To examine the association between brain atrophy and neuropsychological test performance, raw DRS-2 scores were analyzed using a Pearson partial correlation controlling for age, sex, educational level, ICV, and disease severity. Normality assumptions were checked whenever required by the tests. All statistical tests were 2-sided with statistical significance set at the .05 level. Analyses were conducted with commercially available software (PASW Statistics, version 18.0; SPSS, Inc, Chicago, Illinois).
Other imaging comparisons were performed via voxelbased statistical analysis of RAVENS maps that were downsampled, normalized by ICV, and smoothed using an 8-mm full-width at half-maximum smoothing kernel. Group comparisons involved voxel-by-voxel t tests applied by AFNI software (available at http://afni.nimh.nih.gov/). Comparison for multiple corrections used the false discovery rate method.24
Because VBM analysis is not suitable for deriving diagnostic biomarkers on an individual patient basis, we used an individual-patient, high-dimensional pattern approach to classify individual scans belonging to PD-NC or PDD patients.25-27 This approach considers all brain regions jointly and identifies a minimal set of regions in which volumes jointly and maximally differentiate between the 2 groups under consideration on an individual scan basis. The leave-one-out cross-validation tests this classification scheme on data sets not used for training to obtain a relatively unbiased estimate of the generalization power of the classifier to new patients. The pattern classification method provides a structural phenotypic score, herein called the SPARE-PDD score. For a classifier constructed from the PD-NC and PDD groups, a positive SPARE-PDD score implies PDD-like brain structure, and a PDD-like brain structure implies a positive SPARE-PDD score. For the SPARE-PDD score, we plotted the receiver operating characteristic curve, with the area under the curve determining its discriminant validity for detecting PDD, and we calculated the sensitivity, specificity, and negative and positive predictive values (NPVs and PPVs) for different cutoff points. The classifier that was determined to maximally distinguish between PD-NC and PDD patients was subsequently applied to the PD-MCI group. The software used to generate SPARE-PDD scores is available through the Section of Biomedical Image Analysis at the University of Pennsylvania at http://www.rad.upenn.edu/sbia.
RESULTS
PD-NC PATIENTS COMPARED WITH HCS
The ROI volumes for all subject groups are presented in eTable 1. After controlling for age, sex, education, and ICV, there were no between-group differences in regional brain volumes for PD-NC patients and HCs (eTable 2). When we examined the HCs only, there was no association between total DRS-2 score and any regional brain volumes (data not shown).
ATROPHY IN PD-MCI PATIENTS
Within PD, there were cognitive group–level differences in hippocampal (F2,81=14.91; P<.001) and medial temporal lobe (F2,81=6.79; P=.002) volumes. The PD-MCI (P=.001) and PDD patients (P<.001) had smaller hippocampal volumes compared with PD-NC patients, with no difference between PD-MCI and PDD patients (P=.79). The PDD patients, but not PD-MCI patients, also had medial temporal lobe atrophy compared with PD-NC patients (P=.006). There were no between-group differences for other brain regions.
Using linear regression analyses to control for possible confounding variables, we continued to find smaller hippocampal volumes in the PD-MCI (β=−0.37; P=.001) and PDD (β=−0.32; P=.004) patients compared with PD-NC patients. Likewise, PDD patients continued to have a smaller medial temporal lobe volume compared with PD-NC patients (β=−0.36; P=.003).
VBM ANALYSES
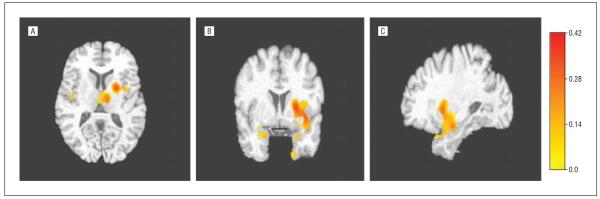
In complementary VBM analyses not based on ROIs, controlling for age and using an uncorrected P=.01, PD-MCI patients had greater atrophy in the hippocampus, insula, and putamen compared with PD-NC patients (areas in yellow-red in Figure 1). When using a more stringent P=.05 corrected for multiple comparisons, PD-MCI patients demonstrated greater head of the hippocampus, inferior globus pallidus, and superior-posterior amygdala atrophy (areas in yellow-red in Figure 2).
Figure 1.
Hippocampus, insula, and putamen atrophy in patients with Parkinson disease (PD) and mild cognitive impairment compared with PD patients with normal cognition (uncorrected P=.01). A, Axial view. B, Coronal view. C. Sagittal view. The color scale indicates the amount of atrophy (in cubic millimeters) per cubic millimeter of tissue in the reference image at that voxel, adjusted by the intracranial volume.
Figure 2.
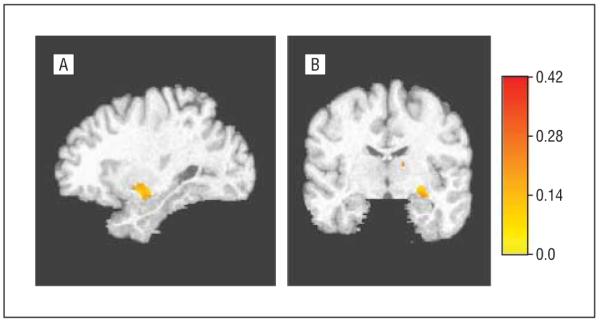
Hippocampus, amygdala, and globus pallidus atrophy in patients with Parkinson disease (PD) and mild cognitive impairment compared with PD patients with normal cognition (corrected P=.05). A, Sagittal view. B, Coronal view. The color scale indicates the amount of atrophy (in cubic millimeters) per cubic millimeter of tissue in the reference image at that voxel, adjusted by the intracranial volume.
NEUROPSYCHOLOGICAL TESTING AND HIPPOCAMPAL ATROPHY IN NONDEMENTED PD PATIENTS
When we examined all PD patients, there were positive correlations between hippocampal size and total DRS-2 score (r=0.44; P<.001), as well as the attention (r=0.31; P=.005), initiation (r=0.40; P<.001), and memory (r=0.41; P<.001) subscale scores. When we examined nondemented PD patients (ie, the PD-NC and PD-MCI groups), there were positive correlations between hippocampal size and total DRS-2 score (r=0.38; P=.008) and DRS-2 memory subscale score (r=0.31; P=.04) but no correlation with any of the other DRS-2 subscale scores.
In the subset of PD patients undergoing assessment with the HVLT (n=60), there was a positive correlation between hippocampal size and recognition discrimination performance (r=0.27; P=.05) but no correlation with immediate or delayed free recall. When we examined only nondemented PD patients (n=51), the correlation between hippocampal size and recognition discrimination remained (r=0.41; P=.005).
GENERATION OF SPARE-PDD
The SPARE-PDD score had an area under the receiver operating charactheristic curve of 0.79 for differentiating PDD from PD-NC patients (eFigure). At the optimal SPARE-PDD cutoff score (ie, the point of maximum combined sensitivity and specificity), the psychometric properties for identifying PDD were sensitivity of 91% and specificity of 61%. At this cutoff point, the NPV was 97% and the PPV was 29%.
When we applied the SPARE-PDD score to PD-MCI patients, there were overall differences between the cognitive subgroups (F2,81=6.92; P=.002). We noted a difference between the PD-NC and PD-MCI groups in mean SPARE-PDD score (P=.04), with the PD-MCI group mean (SD) score (0.40 [0.87]) falling between those of the PD-NC (−0.13 [0.64]) and PDD (0.58 [0.69]) groups (Figure 3). There was no difference between the PD-MCI and PDD groups (P=.81). In addition, there was no difference between the PD-NC and HC groups (t82=−0.67; P=.51).
Figure 3.
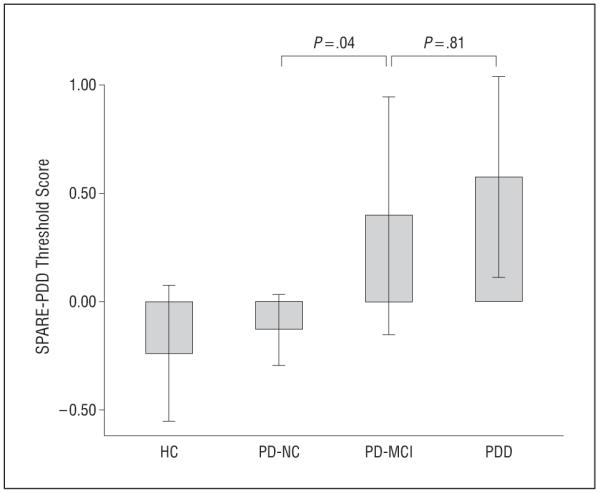
Scores for the Spatial Pattern of Abnormality for Recognition of Parkinson disease with dementia-level cognitive deficits (SPARE-PDD) instrument by cognitive subgroup. Data are presented as mean threshold scores; whiskers represent 95% CIs. HC indicates healthy controls; MCI, mild cognitive impairment; and NC, normal cognition.
After examining the data maps for the features used most frequently by the classifier, we identified the following regions, all smaller in the PDD compared with PD-NC patients: (1) for GM: hippocampus, medial and lateral prefrontal cortex, medial orbitofrontal cortex, occipital lobe, and pericentral sulcus; and (2) for WM: internal and external capsules, inferior temporal lobe and gyrus, medial orbitofrontal cortex, occipital lobe, and precuneus.
COMMENT
The relationship between the neurodegenerative process and cognitive decline in PD remains unclear. Regarding neuropathologic features, PDD is associated with diffuse Lewy body disease manifestations, including in the transentorhinal and entorhinal cortices, hippocampus, other limbic cortex regions, and neocortex.28,29 In addition, more than half of PD patients have AD-related neuropathologic changes on autopsy,30,31 including in the hippocampus.29,32 Thus, the neurodegeneration that contributes to cognitive decline in PD, particularly in regions predisposed to AD pathologic changes (eg, the hippocampus), is likely due to a complex interaction of different diseases.33
Patients with PDD are reported to have decreased hippocampal, temporal, and parietal lobes and decreased prefrontal cortex volumes compared with HCs and nondemented PD patients.7-9,34-37 Nondemented PD patients are reported to have varying degrees of atrophy compared with HCs,8,36,38,39 with mixed evidence of a correlation between atrophy and neuropsychological test performance or conversion to PDD.7,40-42 However, nondemented PD patients are a heterogeneous group that includes PD-NC and PD-MCI patients, thus blurring any distinctions between the 2 groups. In addition, there are no consensus diagnostic criteria for PD-MCI; therefore, MCI populations can vary widely in terms of cognitive abilities. One study of PD patients with amnestic MCI reported GM atrophy in the precuneus and the left prefrontal and left primary motor cortices compared with HCs,43 and other studies44,45 reported mixed results for the presence of atrophy at the stage of PD-MCI.
After separating nondemented PD patients into PD-NC and PD-MCI groups purely on the basis of cognitive performance, we found that PD-NC patients had regional brain volumes similar to those of HCs. This suggests that significant regional brain atrophy does not occur in PD in the absence of comorbid cognitive impairment.
Patients with only MCI demonstrated hippocampal atrophy using ROI and VBM analyses and basal ganglia (putamen and globus pallidus), amygdala, and insula atrophy using VBM analyses. The putamen and globus pallidus are associated with learning, executive abilities, and attention in PD patients,46,47 and the amygdala has been shown to subserve memory and attention.48 At the stage of PDD, we found additional medial temporal lobe atrophy (ie, in the entorhinal cortex, perirhinal cortex, and posterior parahippocampal gyrus). This suggests that hippocampal neurodegeneration is associated with the initial stages of cognitive decline in PD, with more severe cognitive impairment associated with additional atrophy in medial temporal lobe structures.
The positive correlation between memory performance specifically (ie, memory subscales of the DRS-2 and the HVLT-R) and hippocampal volume in nondemented PD patients extends the aforementioned results. On the HVLT-R, the correlation was with recognition discrimination ability, which requires memory encoding and storage abilities subserved by medial temporal lobe structures and has been shown to be impaired in a subset of nondemented PD patients.49,50 There was no correlation between hippocampal volume and free recall performance, which in part reflects retrieval abilities that depend on frontostriatal circuitry.
Our preliminary results using a pattern classification approach to identify patterns of atrophy associated with dementia in PD suggest that it is possible to differentiate PDD and PD-NC patients with high sensitivity and NPV (ie, good for screening), although the specificity and PPV were suboptimal (ie, not adequate for diagnos-ing). However, the relatively low specificity (ie, high false-positive rate) may indicate patients who are at increased risk of future cognitive decline. We anticipate that, with a larger sample of PDD patients and additional clinical characterization (ie, application of formal diagnostic criteria to support neuropsychological test results), the psychometric properties of the pattern classifier will improve.
The PD-MCI patients demonstrated a pattern of atrophy different than that of the PD-NC patients and similar to although less severe than that of the PDD patients. This suggests that a diffuse pattern of GM and WM brain atrophy can be detected at an early stage of cognitive decline in PD if sensitive imaging analyses are used. Because the use of traditional imaging methods in PD patients has produced mixed results regarding the correlation between atrophy and current and future cognitive performance,40,41 additional research is needed to determine whether the pattern classifier for PDD is able to predict cognitive decline in nondemented PD patients, as has been reported for individuals with MCI in the general population.27
Our study has a number of limitations. First, the classification of patients into cognitive groups was based solely on neuropsychological test results because formal MCI and PDD diagnostic criteria were not applied. However, there currently are no commonly accepted diagnostic criteria for PD-MCI, and the validity of self-reporting of nonmotor symptoms in PD has been called into question.51 Second, the DRS-2 was the primary neuropsychological test; consequently, sensitivity may have been suboptimal. In addition, the recommended standardized DRS-2 cutoff scores for MCI and PDD have not been validated in PD, and the mean standardized DRS-2 scores for all 3 cognitive groups were lower than those reported in previous research using formal diagnostic criteria for PD-MCI and PDD.52 Third, the PD sample was predominantly male, and there was a sex imbalance between HCs and PD-NC patients. Finally, the sample sizes of PDD and PD-MCI patients were relatively small, which may affect the reproducibility of our findings.
With growing recognition of PD-MCI as common and clinically significant, it will be important to develop consensus diagnostic criteria, validate assessment instruments for use in clinical care and research, and test treatments for their symptomatic and disease-modifying effects. Validating biomarkers of neurodegeneration associated with MCI and distinguishing PD-MCI from the early stages of dementia with Lewy bodies will inform our understanding of the development, course, profile, and neuropathophysiologic features of the initial stage of cognitive decline in PD. Emerging evidence implicates hippocampal involvement early in the course of cognitive—and specifically memory—decline in PD.
Acknowledgments
Funding/Support: This study was supported by health research grant SAP4100027296 from the Department of Health of the Commonwealth of Pennsylvania from the Tobacco Master Settlement Agreement under Act 2001-77; by P50 NS053488 from the National Institute of Neurological Disorders and Stroke (Penn Udall Center); and by grants R01-14971 and P30-AG10124 from the National Institute on Aging.
Role of the Sponsors: The funding sponsors only provided monetary support and did not participate in the design and conduct of the study; in the collection, management, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Study concept and design: Weintraub, Davatzikos, Siderowf, Duda, Wolk, and Clark. Acquisition of data: Koka, Davatzikos, Duda, and Moberg. Analysis and interpretation of data: Weintraub, Doshi, Davatzikos, Wolk, and Xie. Drafting of the manuscript: Weintraub, Koka, and Davatzikos. Critical revision of the manuscript for important intellectual content: Doshi, Davatzikos, Siderowf, Duda, Wolk, Moberg, Xie, and Clark. Statistical analysis: Weintraub, Davatzikos, and Xie. Obtained funding: Davatzikos and Clark. Administrative, technical, and material support: Weintraub, Doshi, Koka, Davatzikos, Siderowf, Wolk, and Moberg. Study supervision: Weintraub and Davatzikos.
Financial Disclosure: None reported.
Online-Only Material: The eTables and eFigure are available at http://www.archneurol.com.
REFERENCES
- 1.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Bronnick K, Williams-Gray CH, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25(9):1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Parkinson Study Group DATATOP Investigators. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Mov Disord. 2008;23(5):653–659. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Ravina B. Biomarkers of PD progression: is CSF the answer? Neurology. 2010;75(12):1036–1037. doi: 10.1212/WNL.0b013e3181f6f272. [DOI] [PubMed] [Google Scholar]
- 7.Jokinen P, Brück A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15(2):88–93. doi: 10.1016/j.parkreldis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Lyoo CH, Ryu YH, Lee MS. Topographical distribution of cerebral cortical thinning in patients with mild Parkinson’s disease without dementia. Mov Disord. 2010;25(4):496–499. doi: 10.1002/mds.22975. [DOI] [PubMed] [Google Scholar]
- 9.Kenny ER, Burton EJ, O’Brien JT. A volumetric magnetic resonance imaging study of entorhinal cortex volume in dementia with Lewy bodies: a comparison with Alzheimer’s disease and Parkinson’s disease with and without dementia. Dement Geriatr Cogn Disord. 2008;26(3):218–225. doi: 10.1159/000153432. [DOI] [PubMed] [Google Scholar]
- 10.Ibarretxe-Bilbao N, Tolosa E, Junque C, Marti MJ. MRI and cognitive impairment in Parkinson’s disease. Mov Disord. 2009;24(suppl 2):S748–S753. doi: 10.1002/mds.22670. [DOI] [PubMed] [Google Scholar]
- 11.Davatzikos C, Bhatt P, Shaw LM, Batmanghelich N, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification [published online June 29, 2010] Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.05.023. doi:10.1016/j.neurobiolaging .2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage. 2009;44(4):1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahn S, Elton RL. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 15.Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287(4):455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press; New York, NY: 1986. pp. 165–173. [Google Scholar]
- 17.Jurica PJ, Leitten SL, Mattis S. Dementia Rating Scale–2 (DRS-2): Professional Manual. Psychological Assessment Resources; Odessa, FL: 2001. [Google Scholar]
- 18.Llebaria G, Pagonabarraga J, Kulisevsky J, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson’s disease. Mov Disord. 2008;23(11):1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- 19.Martin RC, Okonkwo OC, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson’s disease patients without dementia. Mov Disord. 2008;23(13):1867–1874. doi: 10.1002/mds.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test–Revised. Psychological Assessment Resources; Odessa, FL: 2001. [Google Scholar]
- 21.Goldszal AF, Davatzikos C, Pham D, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22(5):827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21(11):1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 23.Shen DB, Davatzikos C. Very high-resolution morphometry using mass-preserving deformations and HAMMER elastic registration. Neuroimage. 2003;18(1):28–41. doi: 10.1006/nimg.2002.1301. [DOI] [PubMed] [Google Scholar]
- 24.Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J Stat Planning Inference. 1999 December;82:171–196. [Google Scholar]
- 25.Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiol Aging. 2008;29(4):514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y, Shen D, Gur RC, Gur RE, Davatzikos C. COMPARE: classification of morphological patterns using adaptive regional elements. IEEE Trans Med Imaging. 2007;26(1):93–105. doi: 10.1109/TMI.2006.886812. [DOI] [PubMed] [Google Scholar]
- 27.Fan Y, Batmanghelich N, Clark CM, Davatzikos C. Alzheimer’s Disease Neuroimaging Initiative. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39(4):1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H, Rüb U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64(8):1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 29.Kalaitzakis ME, Pearce RK. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 2009;118(5):587–598. doi: 10.1007/s00401-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman AN. Point of view: dementia in Parkinson’s disease. Parkinsonism Relat Disord. 1997;3(3):151–158. doi: 10.1016/s1353-8020(97)00017-5. [DOI] [PubMed] [Google Scholar]
- 31.Sabbagh MN, Adler CH, Lahti TJ, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23(3):295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59(1):102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 33.Kurosinski P, Guggisberg M, Götz J. Alzheimer’s and Parkinson’s disease—overlapping or synergistic pathologies? Trends Mol Med. 2002;8(1):3–5. doi: 10.1016/s1471-4914(01)02246-8. [DOI] [PubMed] [Google Scholar]
- 34.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 35.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003;18(7):784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 36.Tam CWC, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- 37.Junqué C, Ramírez-Ruiz B, Tolosa E, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord. 2005;20(5):540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 38.Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song SK, Lee JE, Park H-J, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson’s disease according to cognitive status. Mov Disord. 2011;26(2):289–296. doi: 10.1002/mds.23477. doi:10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- 40.Martin WR, Wieler M, Gee M, Camicioli R. Temporal lobe changes in early, untreated Parkinson’s disease. Mov Disord. 2009;24(13):1949–1954. doi: 10.1002/mds.22680. [DOI] [PubMed] [Google Scholar]
- 41.Aybek S, Lazeyras F, Gronchi-Perrin A, Burkhard PR, Villemure JG, Vingerhoets FJ. Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(7):521–524. doi: 10.1016/j.parkreldis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Dalaker TO, Zivadinov R, Larsen JP, et al. Gray matter correlations of cognition in incident Parkinson’s disease. Mov Disord. 2010;25(5):629–633. doi: 10.1002/mds.22867. [DOI] [PubMed] [Google Scholar]
- 43.Lee JE, Park H-J, Song SK, Sohn YH, Lee JD, Lee PH. Neuroanatomic basis of amnestic MCI differs in patients with and without Parkinson disease. Neurology. 2010;75(22):2009–2016. doi: 10.1212/WNL.0b013e3181ff96bf. [DOI] [PubMed] [Google Scholar]
- 44.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apostolova LG, Beyer M, Green AE, et al. Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord. 2010;25(6):687–695. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Beilen M, Leenders KL. Putamen FDOPA uptake and its relationship tot [sic] cognitive functioning in PD. J Neurol Sci. 2006;248(1-2):68–71. doi: 10.1016/j.jns.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Scott RB, Harrison J, Boulton C, et al. Global attentional-executive sequelae following surgical lesions to globus pallidus interna. Brain. 2002;125(pt 3):562–574. doi: 10.1093/brain/awf046. [DOI] [PubMed] [Google Scholar]
- 48.Schaefer A, Gray JR. A role for the human amygdala in higher cognition. Rev Neurosci. 2007;18(5):355–363. doi: 10.1515/revneuro.2007.18.5.355. [DOI] [PubMed] [Google Scholar]
- 49.Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17(4):195–200. [PubMed] [Google Scholar]
- 50.Brønnick K, Alves G, Aarsland D, Tysnes O-B, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease: the retrieval deficit hypothesis revisited. Neuropsychology. 2011;25(1):114–124. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- 51.McKinlay A, Grace RC, Dalrymple-Alford JC, Anderson TJ, Fink J, Roger D. Neuropsychiatric problems in Parkinson’s disease: comparisons between self and caregiver report. Aging Ment Health. 2008;12(5):647–653. doi: 10.1080/13607860802343225. [DOI] [PubMed] [Google Scholar]
- 52.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]