Abstract
High-fiber diets are associated with improved lipid profiles. However, pre- and postmenopausal women respond differently to fiber intake, suggesting that endogenous estradiol mediates the effect. The authors' objective was to determine the direct effect of fiber intake on lipoprotein cholesterol levels independent of estradiol among premenopausal women. The BioCycle Study, a prospective cohort study conducted at the State University of New York at Buffalo from 2005 to 2007, followed 259 healthy women for up to 2 complete menstrual cycles. Serum lipoprotein and hormone levels were measured at 16 visits timed using fertility monitors. Fiber intake was assessed by 8 24-hour recalls. Marginal structural models with inverse probability weights for both lipoprotein and estradiol levels were used to estimate controlled direct effects of the highest category of fiber intake (≥22 g/day vs. <22 g/day) while accounting for age, body mass index, total energy, vitamin E intake, physical activity, luteinizing hormone, follicle-stimulating hormone, and progesterone. Reductions were observed in total and low density lipoprotein cholesterol in women with higher fiber intakes. Direct effects were greater than total effects. These analyses suggested that estradiol mediates at least part of the association between fiber and cholesterol among premenopausal women. More research is needed to elucidate the biologic mechanisms driving these associations.
Keywords: cholesterol, dietary fiber, estradiol, lipoproteins, menstrual cycle
High-fiber diets (generally >25 g/day for women) are recommended because of their many associated health benefits (1, 2). In particular, evidence from randomized controlled trials, observational studies, and animal models demonstrates that dietary fiber lowers levels of total cholesterol and low density lipoprotein (LDL) cholesterol (1–5), which are common risk factors for cardiovascular disease (1–3, 6, 7). Overall decreases in total cholesterol are usually attributed to a reduction in LDL cholesterol, since high density lipoprotein (HDL) cholesterol and triglycerides have not shown similar effects.
The mechanisms involved in the relation of reduced serum cholesterol levels to increased fiber intake remain inconclusive, though animal models have provided some insight. The major mechanism is thought to work through bile acid metabolism (8). However, increased fiber intake does not always lead to an increased fecal output of bile acids, suggesting that the reduction in cholesterol may work through another mechanism (9, 10). Alternatively, dietary fiber may alter serum sex hormone concentrations, which could affect lipid metabolism (9). In fact, high fiber intake in women has been associated with lower levels of estradiol (11–19). There is also evidence that pre- and postmenopausal women respond differently to fiber intake (20, 21), since premenopausal women have been found to have smaller reductions in lipoprotein cholesterol levels in response to fiber intake than postmenopausal women (20).
We hypothesized that estradiol could mediate fiber's effect on lipoprotein cholesterol in premenopausal women. To date, there has been little research on how much of the observed effect of fiber on lipoprotein cholesterol levels is direct and not mediated by estradiol. Therefore, our objective in this study was to evaluate the effect of dietary fiber intake on lipoprotein cholesterol levels independent of estradiol among healthy, regularly menstruating women. Because leading dietary and public health associations continue to endorse high-fiber diets, a better understanding of the direct and indirect effects of fiber intake on lipoprotein cholesterol levels is essential. This knowledge could provide further insight regarding possible mechanisms, as well as valuable knowledge for interpreting studies of fiber intake among women of reproductive age.
MATERIALS AND METHODS
Study sample
The BioCycle Study was a prospective cohort study of 259 women recruited from healthy premenopausal volunteers aged 18–44 years during 2005–2007 from western New York State to study the effects of reproductive hormones on oxidative stress (22). Nine women were followed for 1 menstrual cycle as part of a pilot study, and an additional 250 women were recruited and followed for 2 complete cycles (23). Exclusion criteria included pregnancy in the last 6 months; current use of oral contraceptives, other medications (including lipid-lowering drugs), and/or aspirin; and diagnosis of certain chronic conditions. Women with a self-reported body mass index (BMI; weight (kg)/height (m)2) less than 18 or greater than 35 at screening were excluded, as were women planning to restrict their diet for weight loss or medical reasons. Full details on inclusion and exclusion criteria have been reported elsewhere (23). The Health Sciences Institutional Review Board of the State University of New York at Buffalo approved the study, and all participants provided written informed consent.
Data collection
The study involved 5–8 clinic visits per menstrual cycle (94% of all women completed at least 7 visits per cycle) for up to 2 cycles, with visits timed using fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts) so that biospecimen collection occurred during specific phases of the menstrual cycle (24). Visits corresponded to biologically relevant windows, including menstruation, the middle and late follicular phase, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) surges, ovulation, and the early, middle, and late luteal phase.
Dietary assessment
Dietary intake was assessed using the 24-hour dietary recall method (25–27). Recalls were conducted 4 times per menstrual cycle, for a total of up to 8 recalls, by trained and certified research staff using Nutrition Data System for Research software, version 2005 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota). This program computed the nutrients (i.e., total energy, vitamin E) and nonnutrients (i.e., dietary fiber) consumed for each day of intake. All women completed at least 2 recalls per cycle, and 87% completed 4 recalls per cycle.
Hormone assessment
Levels of estradiol, progesterone, LH, and FSH were measured in fasting serum samples collected at each visit (28). Estradiol was measured by radioimmunoassay. Progesterone, LH, and FSH were measured using a solid-phase competitive chemiluminescent enzymatic immunoassay by Specialty Laboratories, Inc. (Valencia, California) on the DPC Immulite2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, Illinois). All samples were analyzed at the Kaleida Center for Laboratory Medicine (Buffalo, New York). All samples from a participant's menstrual cycle were analyzed together in 1 batch to control for interassay differences. Across the study period, the interassay coefficients of variation were reported as <10% for estradiol, <5% for LH and FSH, and <14% for progesterone.
Lipoprotein assessment
A complete lipid profile was performed for each cycle visit, including analysis of total cholesterol, HDL cholesterol, and triglycerides, measured using a Beckman LX20 automated chemistry analyzer at the Kaleida Center for Laboratory Medicine (Buffalo, New York) (<5% coefficient of variation). LDL cholesterol was determined indirectly using the Friedewald formula (29).
Covariate assessment
Participants were asked to complete questionnaires on lifestyle (smoking status), physical activity (International Physical Activity Questionnaire long form 2002) (30), and reproductive history. High, moderate, and low physical activity categories were formed on the basis of standard International Physical Activity Questionnaire cutpoints. Physical and anthropometric measures were carried out according to standardized protocols and included height and weight, which were used to calculate BMI. All covariates assessed had at least a 95% response rate.
Controlled direct effects
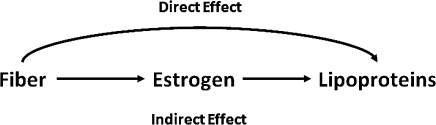
Figure 1 displays the direct effect of fiber on lipoprotein cholesterol and the indirect effect of fiber on lipoprotein cholesterol working through estradiol (31). This simplified diagram could be extended to represent the longitudinal structure of the data and the hypothesized confounders of the fiber-lipoprotein (age, BMI, energy intake, and physical activity), fiber-estradiol (age, BMI, energy intake, and vitamin E intake) (12), and estradiol-lipoprotein (age, BMI, and reproductive hormones) associations. We were interested in estimating controlled direct effects, which are the direct effects observed when setting estradiol to a given level (e.g., 45 pg/mL), which essentially controls for the effects of fiber on estradiol. The proposed methods conceive of hypothetical interventions on the mediator (estradiol) that change its value, so that controlled direct effects can be conceptualized (with fixed values of the mediator). In theory, there are as many direct effects as there are levels of estradiol, but in practice, meaningful and realistically modifiable levels should be used. We considered the controlled direct effects where estradiol was set to the mean level among premenopausal women on different formulations of oral contraceptives (30–110 pg/mL, equivalent to intervening and giving women oral contraceptives), while accounting for the hypothesized confounders (32–35). The levels of estradiol considered in this analysis were all within the range of values observed among the women in this study.
Figure 1.
Direct and indirect effects of fiber intake on lipoprotein cholesterol levels.
Statistical analysis
Descriptive statistics were computed for all study variables, and Fisher's exact tests and repeated-measures analysis of variance were used to test for associations between demographic variables and fiber intake by cycle, while taking multiple cycles per woman into account. Median hormone levels across the cycle (estradiol, LH, FSH, and luteal progesterone) and baseline (second day of menses during the first menstrual cycle) lipoprotein cholesterol levels (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) were compared across levels of fiber intake, and P values were calculated using repeated-measures analysis of variance with Bonferroni-adjusted comparisons on the log-transformed values (36). Hormone and lipoprotein values were log-transformed for normality in statistical models. Predicted mean levels of lipoproteins were calculated for each visit by fiber intake after adjustment for age, BMI, and total energy intake, using linear mixed models with random intercepts (see Appendix).
The average daily fiber intake per cycle was calculated, since there were no significant differences in dietary fiber intake across phases of the cycle (12). Average fiber intake per cycle was categorized into multiple groups of equal size (e.g., 3 groups (tertiles), 4 groups (quartiles), 5 groups (quintiles), and 8, 10 (deciles), 12, and 15 groups), and the groups were compared using linear mixed models, adjusting for age, BMI, and energy intake, as well as using linear spline models, to determine whether there was evidence of a threshold effect of fiber intake on lipoprotein cholesterol levels.
Marginal structural models (MSMs) with inverse probability weights were applied to estimate total effects and controlled direct effects of fiber intake on lipoprotein cholesterol levels (37). Weighted generalized linear mixed-effects models with random intercepts were used to estimate the parameters of the MSM, allowing lipoprotein levels to vary over time, and treating total fiber intake per cycle as a dichotomous variable (≥22 g/day vs. <22 g/day). Intakes of insoluble and soluble fiber above the 75th percentile were also evaluated (11.4 g/day and 4.4 g/day, respectively). The weighted generalized linear mixed-effects models utilize all available data and do not rely on the complete case approach for handling missing data. For estimation of total effects, stabilized inverse probability weights for dichotomous fiber intake per cycle were obtained by logistic regression (adjusting for total energy intake, age, BMI, and physical activity) and applied to generalized linear mixed-effects models. For estimation of controlled direct effects, weighted generalized linear mixed-effects models were used to estimate the counterfactual level of lipoprotein cholesterol (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) at each time point modeled as a linear function of fiber intake, estradiol, and the interaction between fiber and estradiol (see Appendix) (37).
Stabilized weights were obtained by estimating 2 sets of weights, 1 for dichotomous fiber intake using logistic regression and 1 for continuous estradiol levels by linear regression, replacing the probabilities with values from a normal probability density function (38). Weights were calculated for fiber intake per cycle (2 total) and for estradiol at each cycle visit (16 total), and the weights were multiplied together to form a single weight for each individual at each cycle visit. Models used to calculate the weights included age, BMI, energy intake, vitamin E intake, physical activity, LH, FSH, and progesterone levels, as well as past measurements of fiber and estradiol. Inverse probability weighting is used to consistently estimate the parameters of the MSM under the assumptions of positivity, no unmeasured confounding, and correct model specification (37–40).
To assess the impact of a possible unmeasured confounding factor (high plasma volume) of the estradiol-cholesterol relation on estimates of direct effects, we performed a sensitivity analysis for direct effects (see Appendix) (41). Plasma volume was considered as a potential unmeasured confounder, since increases in total cholesterol during the follicular phase could be due, at least in part, to the reduction in plasma volume observed during this phase of the menstrual cycle (42–44). Hematocrit is a common measure of plasma volume, but data on this factor were not collected in this study. The sensitivity analysis estimated the difference in the prevalence of high plasma volume when comparing high and low fiber intakes for various potential effect sizes of the plasma volume–lipoprotein cholesterol association.
Further, we evaluated the effects of meeting the Dietary Reference Intake standard for fiber intake on lipoprotein cholesterol levels, with the Dietary Reference Intake being based on the Institute of Medicine's recommendation of 14 g of fiber per day per 1,000 kcal (45). We calculated each woman's estimated fiber requirements on the basis of her estimated total energy intake from the 24-hour recall (e.g., for a woman consuming 1,500 kcal/day, her estimated requirement would be 21 g/day) and her estimated energy requirements according to her age, weight, height, and physical activity level, using the Institute of Medicine's formula for adult females (45). Fiber intake was categorized as at or above the estimated requirement or below the estimated requirement for each woman. SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina), was used for all statistical analyses.
RESULTS
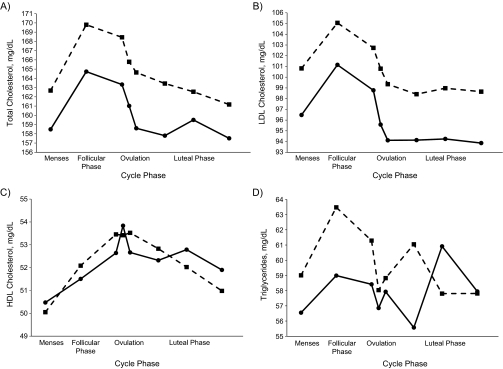
The women in the BioCycle Study were, on average, aged 27.3 years (range, 18–44) and consisted mainly of single, nulliparous, normal-weight, highly physically active white women with some postsecondary education. The average fiber intake among the women in this study was 13.6 g/day, with approximately 17% being derived from fruits, 36% from vegetables, and 41% from grains. Fiber intake (≥22 g/day vs. <22 g/day) varied significantly according to BMI and race/ethnicity, with heavier and minority women tending to consume less fiber (Table 1). Average estradiol level across the menstrual cycle, average luteal-phase progesterone level, and baseline total cholesterol, LDL cholesterol, and triglyceride levels were lower among women consuming at least 22 g of fiber per day. Women consuming fiber at levels of 22 g/day or more also had lower predicted mean total cholesterol and LDL cholesterol levels across the menstrual cycle after adjustment for age, BMI, and total energy intake (Figure 2). Triglyceride levels were lower during menses, during the follicular phase, and around the time of expected ovulation. No consistent pattern was observed for HDL cholesterol. The final sample used for the analysis included all 259 women, and information from missing visits comprised only 4.2% of the total observations (173 missing out of a possible 4,072).
Table 1.
Characteristics of Participants (n = 259 Women) in the BioCycle Study According to Dietary Fiber Intake per Menstrual Cycle (n = 509 Cycles), Buffalo, New York, 2005–2007
| Fiber Intake, g/day |
P Valuea | ||||||||||||
| Total |
<22 |
≥22 |
|||||||||||
| No. of Cycles | % | Mean (SD) | Median (IQR) | No. of Cycles | % | Mean (SD) | Median (IQR) | No. of Cycles | % | Mean (SD) | Median (IQR) | ||
| No. of cyclesb | 509 | 468 | 41 | ||||||||||
| Demographic characteristics | |||||||||||||
| Age, years | 27.4 (8.2) | 27.4 (8.3) | 27.0 (8.0) | 0.73 | |||||||||
| Body mass indexc | 24.1 (3.9) | 24.2 (3.8) | 22.8 (4.3) | 0.05 | |||||||||
| Race/ethnicity | 0.04 | ||||||||||||
| White | 302 | 59.3 | 269 | 57.5 | 33 | 80.5 | |||||||
| Black | 101 | 19.8 | 98 | 20.9 | 3 | 7.3 | |||||||
| Other | 106 | 20.8 | 101 | 21.6 | 5 | 12.2 | |||||||
| High school education or less | 65 | 12.8 | 63 | 13.5 | 2 | 4.9 | 0.29 | ||||||
| Married | 131 | 25.7 | 118 | 25.2 | 13 | 31.7 | 0.49 | ||||||
| Nulliparous | 367 | 73.6 | 336 | 73.4 | 31 | 75.6 | 0.81 | ||||||
| Current smoker | 20 | 3.9 | 20 | 4.3 | 0 | 0.0 | 0.98 | ||||||
| Physical activity level | 0.51 | ||||||||||||
| Low | 48 | 9.5 | 44 | 9.4 | 4 | 9.8 | |||||||
| Moderate | 182 | 36.0 | 163 | 34.8 | 19 | 46.3 | |||||||
| High | 275 | 54.5 | 261 | 55.8 | 18 | 43.9 | |||||||
| Past oral contraceptive use | 275 | 54.7 | 248 | 53.7 | 27 | 65.9 | 0.24 | ||||||
| Reproductive hormone levels | |||||||||||||
| Estradiol, pg/mL | 82.0 (10.0) | 84.0 (108.0) | 62.0 (75) | 0.003 | |||||||||
| Luteal progesterone, ng/mL | 7.0 (9.25) | 7.2 (9.4) | 4.8 (8.1) | 0.04 | |||||||||
| Luteinizing hormone, ng/mL | 5.7 (6.0) | 5.7 (6.0) | 5.6 (5.8) | 0.21 | |||||||||
| Follicle-stimulating hormone, mIU/mL | 5.6 (4.0) | 5.6 (4.1) | 5.5 (3.6) | 0.83 | |||||||||
| Baseline lipoprotein cholesterol levels, mg/dL | |||||||||||||
| Total cholesterol | 160.0 (30.0) | 160.5 (32.0) | 147.0 (37.0) | 0.01 | |||||||||
| High density lipoprotein cholesterol | 49.0 (17.0) | 48.0 (17.0) | 50.0 (16.0) | 0.86 | |||||||||
| Low density lipoprotein cholesterol | 98.0 (29.0) | 98.5 (29.0) | 91.0 (35.0) | 0.01 | |||||||||
| Triglycerides | 53.0 (33.0) | 53.0 (33.0) | 49.0 (28.0) | 0.08 | |||||||||
| Dietary intake | |||||||||||||
| Total energy, kcal/day | 1,608.1 (405.0) | 1,578.7 (390.0) | 1,943.3 (426.9) | <0.01 | |||||||||
| Total fiber, g/day | 13.6 (6.0) | 12.3 (4.0) | 28.1 (5.6) | <0.0001 | |||||||||
| Total fiber per 1,000 kcal, g/day | 8.6 (3.3) | 8.0 (2.6) | 15.0 (3.8) | <0.0001 | |||||||||
| Fruit fiber, g/day | 2.3 (1.9) | 2.1 (1.8) | 4.6 (2.2) | <0.0001 | |||||||||
| Vegetable fiber, g/day | 4.9 (3.0) | 4.5 (2.3) | 9.9 (4.6) | <0.0001 | |||||||||
| Grain fiber, g/day | 5.6 (3.1) | 5.1 (2.4) | 10.7 (5.1) | <0.0001 | |||||||||
| Insoluble fiber, g/day | 9.6 (4.7) | 8.6 (3.1) | 21.0 (4.6) | <0.0001 | |||||||||
| Soluble fiber, g/day | 3.8 (1.4) | 3.5 (1.1) | 6.8 (1.4) | <0.0001 | |||||||||
Abbreviations: IQR, interquartile range; SD, standard deviation.
Two-sided P values were calculated for continuous variables using repeated-measures analysis of variance and for categorical variables using Fisher's exact test. All comparisons took repeated measures and correlations between cycles into account.
A total of 250 women in the BioCycle Study were followed for 2 menstrual cycles and 9 women were followed for 1 menstrual cycle, for a total of 509 cycles. A total of 233 women had fiber intakes <22 g/day for both cycles; 11 women had fiber intake ≥22 g/day in only 1 cycle; and 15 women had fiber intakes ≥22 g/day in both cycles.
Weight (kg)/height (m)2.
Figure 2.
Predicted mean levels of A) total cholesterol, B) low density lipoprotein (LDL) cholesterol, C) high density lipoprotein (HDL) cholesterol, and D) triglycerides across the menstrual cycle according to fiber intake (squares, <22 g/day; circles, ≥22 g/day) among women in the BioCycle Study, Buffalo, New York, 2005–2007. The predicted mean values were based on linear mixed-effects models with random intercepts, adjusted for age, body mass index, and total energy intake, and correspond to total effects. (P values for the overall difference between high and low fiber intakes across the menstrual cycle were 0.01 for total cholesterol, 0.005 for LDL cholesterol, 0.7 for HDL cholesterol, and 0.5 for triglycerides).
Significant associations between high fiber intake and total and LDL cholesterol levels were only observed when the cutpoint for the highest category was above 21.8 g/day or when knots for the spline models were greater than 20 g/day, pointing to a possible threshold effect. In particular, the highest fiber category was significantly different from all categories of lower intake, with no differences in lipoprotein cholesterol levels between the lower categories. Thus, fiber intake was categorized according to whether a woman consumed at least 22 g/day.
In models which estimated total effects, high total fiber intake (≥22 g/day) and high soluble fiber intake (≥4.4 g/day) were associated with decreased total and LDL cholesterol levels, with no significant effects on HDL cholesterol or triglycerides (Table 2). We observed significant controlled direct effects of high fiber intakes on total and LDL cholesterol levels. That is, high fiber intakes were associated with lower total and LDL cholesterol levels, not through estradiol, when LDL cholesterol was set to levels of women on oral contraceptives (Table 3). The controlled direct effects of high fiber consumption (≥22 g/day) were associated with decreases in total cholesterol of approximately 7.5 mg/dL, on average, whereas the total effects were associated with decreases of approximately 5.5 mg/dL, on average. Thus, total effects, which include the mediating effects of estradiol, were smaller than the direct effects that do not operate through estradiol. Controlled direct effects on total cholesterol were also reduced at higher estradiol levels (P for interaction = 0.3) (direct effects on triglyceride levels were also reduced (P for interaction = 0.03)), whereas effects on LDL cholesterol were consistent at each level of estradiol evaluated (P for interaction = 0.9).
Table 2.
Estimated Total Effect of Fiber Intake on Log Lipoprotein Levels (mg/dL) Among Women Participating in the BioCycle Study, Buffalo, New York, 2005–2007
| Total Effecta | 95% Confidence Interval | |
| Total cholesterol | ||
| Total fiber intake ≥22 g/day | ||
| Model 1b | −0.035 | −0.063, −0.007 |
| Model 2c | −0.036 | −0.063, −0.008 |
| Insoluble fiber intake ≥11.4 g/day | ||
| Model 1 | 0.007 | −0.006, 0.020 |
| Model 2 | 0.007 | −0.006, 0.019 |
| Soluble fiber intake ≥4.4 g/day | ||
| Model 1 | −0.012 | −0.024, 0.0001 |
| Model 2 | −0.012 | −0.024, −0.0001 |
| High density lipoprotein cholesterol | ||
| Total fiber intake ≥22 g/day | ||
| Model 1 | −0.003 | −0.041, 0.034 |
| Model 2 | −0.006 | −0.044, 0.031 |
| Insoluble fiber intake ≥11.4 g/day | ||
| Model 1 | 0.002 | −0.015, 0.020 |
| Model 2 | 0.002 | −0.015, 0.020 |
| Soluble fiber intake ≥4.4 g/day | ||
| Model 1 | −0.001 | −0.018, 0.015 |
| Model 2 | −0.003 | −0.190, 0.013 |
| Low density lipoprotein cholesterol | ||
| Total fiber intake ≥22 g/day | ||
| Model 1 | −0.058 | −0.097, −0.018 |
| Model 2 | −0.056 | −0.097, −0.019 |
| Insoluble fiber intake ≥11.4 g/day | ||
| Model 1 | 0.004 | −0.015, 0.023 |
| Model 2 | 0.004 | −0.015, 0.022 |
| Soluble fiber intake ≥4.4 g/day | ||
| Model 1 | −0.021 | −0.038, −0.004 |
| Model 2 | −0.020 | −0.037, −0.004 |
| Triglycerides | ||
| Total fiber intake ≥22 g/day | ||
| Model 1 | −0.038 | −0.120, 0.044 |
| Model 2 | −0.032 | −0.113, 0.050 |
| Insoluble fiber intake ≥11.4 g/day | ||
| Model 1 | 0.053 | 0.014, 0.093 |
| Model 2 | 0.053 | 0.014, 0.093 |
| Soluble fiber intake ≥4.4 g/day | ||
| Model 1 | 0.010 | −0.027, 0.047 |
| Model 2 | 0.010 | −0.027, 0.047 |
The total effects presented here include both the direct effects (not mediated through estradiol; Table 3) and the indirect effects (mediated through estradiol).
Results were adjusted for total energy intake.
Results were adjusted for total energy intake, age, body mass index, physical activity, and menstrual cycle phase.
Table 3.
Results From Marginal Structural Models for Estimating the Controlled Direct Effect of Fiber Intake (≥22 g/day vs. <22 g/day) on Log Lipoprotein Levels (mg/dL) Among Women Participating in the BioCycle Study, Buffalo, New York, 2005–2007
| Lipoprotein and Estradiol Level, pg/mL | Estimate of Controlled Direct Effecta | 95% Confidence Interval | P for Interactionb |
| Total cholesterol | 0.3 | ||
| 30 | −0.048 | −0.081, −0.014 | |
| 45 | −0.045 | −0.076, −0.013 | |
| 110 | −0.039 | −0.070, −0.006 | |
| High density lipoprotein cholesterol | 0.9 | ||
| 30 | −0.013 | −0.059, 0.032 | |
| 45 | −0.012 | −0.056, 0.033 | |
| 110 | −0.009 | −0.053, 0.035 | |
| Low density lipoprotein cholesterol | 0.7 | ||
| 30 | −0.062 | −0.111, −0.015 | |
| 45 | −0.062 | −0.111, −0.018 | |
| 110 | −0.064 | −0.110, −0.020 | |
| Triglycerides | 0.03 | ||
| 30 | −0.078 | −0.184, 0.027 | |
| 45 | −0.053 | −0.151, 0.044 | |
| 110 | 0.004 | −0.092, 0.099 |
Estimates were adjusted for age, body mass index, physical activity, total energy intake, vitamin E intake, luteinizing hormone, follicle-stimulating hormone, and progesterone levels through the use of inverse probability weights. Direct effects are the effects of fiber on lipoprotein cholesterol levels that are not mediated through estradiol. The total effects shown in Table 2 include the mediating effects. The direct effects shown here are larger than the total effects shown in Table 2, suggesting that estradiol mediates at least part of the association.
P value for the interaction between fiber and estradiol.
A sensitivity analysis showed that the controlled direct effects we observed for total and LDL cholesterol are unlikely to be explained by unmeasured confounding by plasma volume of the estradiol-cholesterol association (Table 4) (41). Even for a large change in total cholesterol between persons with high and low plasma volumes (15-mg/dL difference), the difference in prevalence of high plasma volume between high and lower fiber intakes, conditional on estradiol levels set at 30 pg/mL, would have to be −0.54 in order to completely explain away the observed controlled direct effect. A degree of confounding this extreme seems implausible, and similar results were observed at other estradiol levels and for LDL cholesterol.
Table 4.
Results of Sensitivity Analysis for Unmeasured Confounding of the Estradiol-Cholesterol Association by Plasma Volume Among Women Participating in the BioCycle Study, Buffalo, New York, 2005–2007
| Effect of High Plasma Volume on Lipoprotein Cholesterol Level, mg/dLa | Difference in Prevalence of High Plasma Volume for High Fiber Intake (≥22 g/day) vs. Low Fiber Intake (<22 g/day)b |
|||||
| Total Cholesterol |
Low Density Lipoprotein Cholesterol |
|||||
| Estradiol Level 30 pg/mL | Estradiol Level 45 pg/mL | Estradiol Level 110 pg/mL | Estradiol Level 30 pg/mL | Estradiol Level 45 pg/mL | Estradiol Level 110 pg/mL | |
| 1 | −7.70c | −7.22 | −6.26 | −6.23 | −6.33 | −6.53 |
| 5 | −1.56 | −1.46 | −1.27 | −1.27 | −1.29 | −1.33 |
| 6 | −1.30 | −1.22 | −1.06 | −1.06 | −1.08 | −1.12 |
| 7 | −1.12 | −1.05 | −0.91 | −0.92 | −0.93 | −0.96 |
| 8 | −0.98 | −0.92 | −0.80 | −0.81 | −0.82 | −0.84 |
| 9 | −0.88 | −0.82 | −0.71 | −0.72 | −0.73 | −0.75 |
| 10 | −0.79 | −0.74 | −0.64 | −0.65 | −0.66 | −0.68 |
| 11 | −0.72 | −0.68 | −0.59 | −0.59 | −0.60 | −0.62 |
| 12 | −0.66 | −0.62 | −0.54 | −0.55 | −0.56 | −0.57 |
| 13 | −0.61 | −0.58 | −0.50 | −0.51 | −0.52 | −0.53 |
| 14 | −0.57 | −0.54 | −0.46 | −0.47 | −0.48 | −0.50 |
| 15 | −0.54 | −0.50 | −0.44 | −0.44 | −0.45 | −0.47 |
| 20 | −0.41 | −0.38 | −0.33 | −0.34 | −0.35 | −0.36 |
The effect of high plasma volume on lipoprotein cholesterol levels is conditional on fiber intake, estradiol levels, and covariates.
Differences in the prevalence of high plasma volume comparing high fiber intake with low fiber intake are conditional on the levels of estradiol and covariates.
The absolute value of difference in prevalence cannot be greater than 1. Therefore, for small effects of high plasma volume on lipoprotein cholesterol, there is no prevalence difference that would eliminate the observed effects.
We did not observe significant reductions in lipoprotein cholesterol levels in response to meeting the estimated percentage requirements of fiber intake based on either a woman's estimated total energy intake from the 24-hour recall or her estimated energy requirements according to her age, weight, and height (data not shown). We observed a wide range of energy intakes in this population (515–3,717 kcal), corresponding to estimated fiber requirements of 7–52 g/day. Estimated energy requirements based on the Institute of Medicine's formula averaged 2,334 kcal/day, with a range of 1,663–3,357 kcal/day, corresponding to estimated fiber requirements of 23–47 g/day (45, 46).
DISCUSSION
We found that fiber consumption at or above 22 g/day was associated with lower total cholesterol and LDL cholesterol levels, independent of measured endogenous estradiol level, when estradiol was set at levels corresponding to oral contraceptive use. The controlled direct effects of high fiber intake were in fact larger than the total effects, since the effect of fiber on cholesterol through estradiol has been shown to increase cholesterol levels. The fact that direct effects were larger than total effects suggests that estradiol mediates the effect of fiber on lipoproteins; high-fiber diets may also have reduced effects among premenopausal women. The observed direct effects of fiber on total and LDL cholesterol provide further insights regarding possible biologic mechanisms of fiber on lipoprotein metabolism, suggesting that fiber has a direct effect on lowering lipoprotein cholesterol levels, in addition to its effect that operates through estradiol.
The observed associations between high fiber intake and reduced total and LDL cholesterol levels are in line with several randomized controlled trials and observational studies that found fiber intake to be associated with a less atherogenic lipid profile (11–19). In the randomized controlled trials, women typically consumed more than 20 g/day of fiber. In observational studies, typically the highest quintile of fiber intake was associated with lower total cholesterol levels, with cutpoints similar to the threshold we observed around 22 g/day (47). The apparent threshold possibly could be explained by the low fiber intakes in this population. However, we observed no reductions in lipoprotein cholesterol levels for women just meeting the estimated percentage requirements. Thus, it seems that the direct effects of fiber intake on lowering of lipoprotein cholesterol are due to high levels of intake and not to consuming a certain percentage of fiber from the diet.
The controlled direct effects of fiber on total cholesterol were greater than the total effects and were slightly reduced at high levels of estradiol. Our findings are similar to those of studies comparing pre- and postmenopausal women that have observed reduced responses to fiber supplementation among premenopausal women (20, 21). In a study of 8 premenopausal women and 11 postmenopausal women with hypercholesterolemia, fiber supplementation (15 g/day) significantly decreased total cholesterol levels from baseline among postmenopausal women but not premenopausal women (20). However, in a small crossover trial among healthy persons, Vega-Lopez et al. (21) found that total cholesterol decreased by 7% during the fiber supplementation period among men but only by 5% and 4% among pre- and postmenopausal women, respectively. Interestingly, they found that triglyceride levels increased among women but decreased in men after fiber supplementation, highlighting sex differences in response to fiber intake. While this crossover trial differed from our study (i.e., a fiber supplementation trial, an older and more obese study population, and higher average fiber intakes before supplementation), together these findings suggest potential differences in the impact of fiber intake on lipoprotein cholesterol that could be due to estradiol levels.
The decreased effect of fiber intake at high estradiol levels and the magnitude of the total and direct effects are also in line with biologic evidence. High fiber intake has been associated with lower levels of estradiol (11–19), presumably because of a reduction in β-glucuronidase activity in feces in response to fiber intake, which subsequently leads to a decline in the reabsorption of estradiol in the colon (47). Because increased intake of exogenous estradiol tends to have beneficial effects on the lipid profile in older women (48–50), the reduction in estradiol in response to fiber intake would presumably lead to an increase in lipoprotein cholesterol levels. In fact, we observed that direct effects were larger in magnitude than the total effects, suggesting that the direct effect of fiber intake was opposite of the effect mediated through estradiol.
To our knowledge, this is the first study to evaluate the controlled direct effects of high fiber intake on lipoprotein cholesterol levels. MSMs were used to estimate the controlled direct effects because they offer several important advantages over standard approaches. The most important advantages of this approach are that they can control for time-varying confounders affected by prior exposure and they can accommodate interactions between fiber and estradiol. First, MSMs adjust for time-modified confounding caused by changing reproductive hormone levels during the menstrual cycle. Second, based on our knowledge of the effects of fiber on both estradiol and lipoproteins, the absence of an interaction seems biologically implausible. When such an interaction is present, the total effect cannot be partitioned into direct and indirect effects using standard approaches, and effect decomposition requires that additional assumptions be met when using MSMs (37, 51). Had we analyzed the association using the standard approach (comparing the effect estimate adjusted for potential confounders with an estimate adjusted for the same confounders plus the hypothesized mediating variable) (51–54), we would not have observed the varying effect of fiber by estradiol level.
Our analysis was restricted by the assumptions of MSMs, which limits the interpretation of our results (37–40). In estimating controlled direct effects, we hypothesized interventions to set estradiol levels to a certain value. Although intervention is possible through the use of oral contraceptives, it is not necessarily practical. In addition, MSMs are based on several strong assumptions—specifically no unmeasured confounding, which is hard to verify but is assumed in standard analysis methods as well (37–40). Despite the fact that we had standardized assessments of a wide variety of participant and dietary characteristics, which increased our ability to adjust for potential confounders, unmeasured confounding is possible. However, based on our sensitivity analysis, it is unlikely that these results could be explained by unmeasured confounding. Adjustment for other dietary factors (i.e., total and saturated fat, alcohol consumption) did not appreciably alter our estimates.
With regard to the assumption of positivity, given that lipoprotein cholesterol levels and estradiol were considered as continuous variables, practical violations could have occurred. However, we observed a positive probability of fiber intake at each level of the confounders when they were categorized into meaningful categories. As a practical assessment of positivity, we evaluated the distribution of the weights to ensure that there were no extreme values, and the mean was close to 1 (39). The distribution of the weight models was not indicative of nonpositivity. We also compared different model specifications for the weight models, as well as the final MSM. The inferences did not change on the basis of the model specification, and the mean of the weights was very close to 1 (a necessary condition for correct model specification (39)), thus supporting this assumption. Although one might be interested in estimating natural direct effects—direct effects where the level of estradiol is allowed to vary—these effects are generally not identifiable when time-dependent confounding is present (37).
We restricted our study sample to healthy, regularly menstruating women in order to exclude potential confounders by design, but such restrictions could also limit the generalizability of our findings. While assessments of fiber intake are subject to measurement error, our use of multiple validated 24-hour recalls reduced the potential for a large degree of misclassification (25–27). Differences in weekday versus weekend consumption are not likely to have been a significant source of misclassification. While the average fiber intake among women in this study was 13.6 g/day (standard deviation, 6.0), which is substantially lower than the current recommendations, this level of intake is comparable to the average fiber intake in the United States (13.8 g/day for reproductive-age women). We were also limited by observing only a small number of women consuming at least 22 g/day. While fiber intakes were low, there was no evidence of a drop-off of intakes across the cycle, and to our knowledge the women were not engaged in strenuous physical training. We were unable to directly address other possible pathways, since we did not have information on bile acid levels or other potential mechanisms.
In conclusion, we observed reductions in total cholesterol and LDL cholesterol that were not mediated through estradiol among women consuming at least 22 g/day of fiber, when setting estradiol at specified levels corresponding to oral contraceptive use. The lipid-lowering effects of fiber intake were observed only at high levels, indicating the importance of consuming a high amount of fiber. The controlled direct effects of fiber intake were greater than the total effects, suggesting that estradiol may mediate the association between fiber and lipoproteins by slightly diminishing the effect. These results support recommendations of a high-fiber diet, however, with reduced effects to be expected among premenopausal women due to higher estradiol levels. These findings regarding direct effects provide further insight into possible biologic mechanisms and support the hypothesis of a direct effect that might work through alternative pathways such as bile acid metabolism. More research is needed to elucidate these mechanisms among premenopausal women.
Acknowledgments
Author affiliations: Epidemiology Branch, Division of Epidemiology, Statistics and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Maryland (Sunni L. Mumford, Enrique F. Schisterman, Audrey J. Gaskins); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Sunni L. Mumford, Anna Maria Siega-Riz); Department of Nutrition, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Anna Maria Siega-Riz); Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, New York (Jean Wactawski-Wende); and Departments of Epidemiology and Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Tyler J. VanderWeele).
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Tyler J. VanderWeele was supported by National Institutes of Health grant 1R03HD060696-01.
The authors are indebted to all of the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the State University of New York at Buffalo for their respective roles in the study, their dedication and effort, and their assistance in study implementation. The authors thank Dr. Andrew Olshan, Dr. Julie Daniels, Dr. Anne Steiner, Dr. Mary Hediger, Dr. Jean Wactawski-Wende, Dr. Maurizio Trevisan, Dr. Edwina Yeung, Anna Pollack, and Dr. Cuilin Zhang and the rest of the BioCycle Working Group for their helpful suggestions.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- FSH
follicle-stimulating hormone
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LH
luteinizing hormone
- MSM
marginal structural model
APPENDIX
Model Specification and Sensitivity Analysis for Direct Effects
Model specification
Predicted mean levels of lipoproteins were calculated for each visit by fiber intake after adjustment for age, body mass index, and total energy intake, using linear mixed models with random intercepts. An example of the linear mixed model is given in equation 1, where i indexes patient (1, …, n), j represents cycle (1, 2), k represents visit (1, …, 8), Y refers to the lipid parameter of interest, A refers to dichotomous fiber intake per cycle, C represents the confounding factors age, body mass index, and total energy intake, and “Visit” represents cycle phase.
| (1) |
For estimation of controlled direct effects, weighted generalized linear mixed-effects models were used to estimate the counterfactual level of lipoprotein cholesterol (total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and triglycerides) at each time point modeled as a linear function of fiber intake (A), estradiol (M), and the interaction between fiber and estradiol (A × M) (37). The linear mixed-effects model shown in equation 2 was used to estimate the parameters of the marginal structural model, where C includes the baseline covariates used for stabilization of the weights. The model is weighted by wijk, with stabilized weights obtained by estimating 2 sets of weights, one for fiber intake and one for estradiol levels.
| (2) |
Sensitivity analysis
We adapt results for sensitivity analysis for direct effects with 1 measurement for each variable (41) to the time-varying setting we are considering here. Let Yt denote serum lipid cholesterol level at time t, let Mt denote estradiol level at time t, and let At denote a high-fiber diet versus a low-fiber diet. Let Lt denote the measured covariates at time t. Note that in this study At is assumed to be fixed over time (though Lt, Mt, and Yt vary). Finally, let Ut denote an unmeasured binary confounding variable such as plasma volume levels (high vs. low), which we hypothesize affects both estradiol levels Mt and cholesterol levels Yt but does not affect high fiber intake versus low fiber intake At.
The repeated-measures marginal structural model assumed that current cholesterol Yt depended on fiber intake but only on the most recent estradiol levels (rather than the entire history). Suppose now also that current cholesterol Yt depends only on recent plasma levels (rather than the entire history). Let Yt(a,m) denote the counterfactual cholesterol level at time t if fiber had been set to level a and recent estradiol to level m. The marginal structural model is a model for Yt(a,m). Suppose that the effects of A and M on Y were unconfounded conditional on (Lt ,Ut) but not on Lt alone. We are then interested in the difference between what would be estimated as the controlled direct effect (with the mediator fixed at level m) if adjustment were made for (Lt, Ut) versus adjustment for just Lt. Under the above assumptions, along with the assumption that the unmeasured confounder Ut does not interact on the additive scale with A in its effects on cholesterol, this difference reduces to γδ, where γ denotes the effect of high versus low plasma levels on cholesterol on the additive scale and δ denotes the difference in the prevalence of U comparing high fiber (A = 1) with low fiber (A = 0), conditional on estradiol being set to level m; by assuming that current cholesterol depends only on the most recent levels of estradiol and plasma, the sensitivity analysis for the time-varying settings reduces to that in which there is a single time period for each variable.
References
- 1.Anderson JW, Baird P, Davis RH, Jr, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown L, Rosner B, Willett WW, et al. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez ML. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12(1):35–40. doi: 10.1097/00041433-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kritchevsky D. Dietary fibre and lipid metabolism. Int J Obes. 1987;11(suppl 1):33–43. [PubMed] [Google Scholar]
- 5.Lipsky H, Gloger M, Frishman WH. Dietary fiber for reducing blood cholesterol. J Clin Pharmacol. 1990;30(8):699–703. doi: 10.1002/j.1552-4604.1990.tb03629.x. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Kumanyika SK, Lemaitre RN, et al. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. 2003;289(13):1659–1666. doi: 10.1001/jama.289.13.1659. [DOI] [PubMed] [Google Scholar]
- 7.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins DJ, Kendall CW, Vuksan V. Viscous fibers, health claims, and strategies to reduce cardiovascular disease risk. Am J Clin Nutr. 2000;71(2):401–402. doi: 10.1093/ajcn/71.2.401. [DOI] [PubMed] [Google Scholar]
- 9.Kay RM. Effects of dietary fibre on serum lipid levels and fecal bile acid excretion. Can Med Assoc J. 1980;123(12):1213–1217. [PMC free article] [PubMed] [Google Scholar]
- 10.Marlett JA, Hosig KB, Vollendorf NW, et al. Mechanism of serum cholesterol reduction by oat bran. Hepatology. 1994;20(6):1450–1457. doi: 10.1002/hep.1840200612. [DOI] [PubMed] [Google Scholar]
- 11.Bagga D, Ashley JM, Geffrey SP, et al. Effects of a very low fat, high fiber diet on serum hormones and menstrual function. Implications for breast cancer prevention. Cancer. 1995;76(12):2491–2496. doi: 10.1002/1097-0142(19951215)76:12<2491::aid-cncr2820761213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Gaskins AJ, Mumford SL, Zhang C, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90(4):1061–1069. doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gann PH, Chatterton RT, Gapstur SM, et al. The effects of a low-fat/high-fiber diet on sex hormone levels and menstrual cycling in premenopausal women: a 12-month randomized trial (the Diet and Hormone Study) Cancer. 2003;98(9):1870–1879. doi: 10.1002/cncr.11735. [DOI] [PubMed] [Google Scholar]
- 14.Goldin BR, Woods MN, Spiegelman DL, et al. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer. 1994;74(suppl 3):1125–1131. doi: 10.1002/1097-0142(19940801)74:3+<1125::aid-cncr2820741521>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Kaneda N, Nagata C, Kabuto M, et al. Fat and fiber intakes in relation to serum estrogen concentration in premenopausal Japanese women. Nutr Cancer. 1997;27(3):279–283. doi: 10.1080/01635589709514538. [DOI] [PubMed] [Google Scholar]
- 16.Rose DP, Goldman M, Connolly JM, et al. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. 1991;54(3):520–525. doi: 10.1093/ajcn/54.3.520. [DOI] [PubMed] [Google Scholar]
- 17.Aubertin-Leheudre M, Gorbach S, Woods M, et al. Fat/fiber intakes and sex hormones in healthy premenopausal women in USA. J Steroid Biochem Mol Biol. 2008;112(1–3):32–39. doi: 10.1016/j.jsbmb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods MN, Barnett JB, Spiegelman D, et al. Hormone levels during dietary changes in premenopausal African-American women. J Natl Cancer Inst. 1996;88(19):1369–1374. doi: 10.1093/jnci/88.19.1369. [DOI] [PubMed] [Google Scholar]
- 19.Wu AH, Pike MC, Stram DO. Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst. 1999;91(6):529–534. doi: 10.1093/jnci/91.6.529. [DOI] [PubMed] [Google Scholar]
- 20.Ganji V, Kuo J. Serum lipid responses to psyllium fiber: differences between pre- and post-menopausal, hypercholesterolemic women. Nutr J. 2008;7:22. doi: 10.1186/1475-2891-7-22. (doi: 10.1186/1475-2891-7-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega-López S, Vidal-Quintanar RL, Fernandez ML. Sex and hormonal status influence plasma lipid responses to psyllium. Am J Clin Nutr. 2001;74(4):435–441. doi: 10.1093/ajcn/74.4.435. [DOI] [PubMed] [Google Scholar]
- 22.Schisterman EF, Gaskins AJ, Mumford SL, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol. 2010;172(4):430–439. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howards PP, Schisterman EF, Wactawski-Wende J, et al. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 26.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 27.Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32(6):1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- 28.Browne RW, Bloom MS, Schisterman EF, et al. Analytical and biological variation of biomarkers of oxidative stress during the menstrual cycle. Biomarkers. 2008;13(2):160–183. doi: 10.1080/13547500701775563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 30.Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 31.Petersen ML, Sinisi SE, van der Laan MJ. Estimation of direct causal effects. Epidemiology. 2006;17(3):276–284. doi: 10.1097/01.ede.0000208475.99429.2d. [DOI] [PubMed] [Google Scholar]
- 32.Rible RD, Taylor D, Wilson ML, et al. Follicular development in a 7-day versus 4-day hormone-free interval with an oral contraceptive containing 20 mcg ethinyl estradiol and 1 mg norethindrone acetate. Contraception. 2009;79(3):182–188. doi: 10.1016/j.contraception.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108(14):1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 34.Birtch RL, Olatunbosun OA, Pierson RA. Ovarian follicular dynamics during conventional vs. continuous oral contraceptive use. Contraception. 2006;73(3):235–243. doi: 10.1016/j.contraception.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Vandever MA, Kuehl TJ, Sulak PJ, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77(3):162–170. doi: 10.1016/j.contraception.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(6973):170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20(1):18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 38.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3(2):143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 41.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21(4):540–551. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adlercreutz H, Tallqvist G. Variations in the serum total cholesterol and hematocrit values in normal women during the menstrual cycle. Scand J Clin Lab Invest. 1959;11(1):1–9. doi: 10.3109/00365515909060400. [DOI] [PubMed] [Google Scholar]
- 43.Cullinane EM, Yurgalevitch SM, Saritelli AL, et al. Variations in plasma volume affect total and low-density lipoprotein cholesterol concentrations during the menstrual cycle. Metabolism. 1995;44(8):965–971. doi: 10.1016/0026-0495(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 44.Pahwa MB, Seth S, Seth RK. Lipid profile in various phases of menstrual cycle and its relationship with percentage plasma volume changes. Clin Chim Acta. 1998;273(2):201–207. doi: 10.1016/s0009-8981(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 45.Institute of Medicine of the National Academies . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 46.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Goldin BR, Adlercreutz H, Gorbach SL, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982;307(25):1542–1547. doi: 10.1056/NEJM198212163072502. [DOI] [PubMed] [Google Scholar]
- 48.Knopp RH, Paramsothy P, Retzlaff BM, et al. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep. 2006;8(6):452–459. doi: 10.1007/s11886-006-0104-0. [DOI] [PubMed] [Google Scholar]
- 49.LaRosa JC. Women, lipoproteins and cardiovascular disease risk. Can J Cardiol. 1990;6(suppl B):23B–29B. [PubMed] [Google Scholar]
- 50.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman JS, MacLehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1(1):4. doi: 10.1186/1742-5573-1-4. (doi: 10.1186/1742-5573-1-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. 200–202, 545–546. [Google Scholar]
- 53.Susser M. Causal Thinking in the Health Sciences: Concepts and Strategies in Epidemiology. New York, NY: Oxford University Press; 1973. pp. 121–124. [Google Scholar]
- 54.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Gaithersburg, MD: Aspen Publishers; 2000. pp. 184–187. [Google Scholar]