Abstract
Gingival epithelium produces β-defensins, small cationic peptides, as part of its contribution to the innate host defense against the bacterial challenge that is constantly present in the oral cavity. Besides their functions in healthy gingival tissues, β-defensins are involved in the initiation and progression, as well as restriction of periodontal tissue destruction, by acting as antimicrobial, chemotactic, and anti-inflammatory agents. In this article, we review the common knowledge about β-defensins, coming from in vivo and in vitro monolayer studies, and present new aspects, based on the experience on three-dimensional organotypic culture models, to the important role of gingival β-defensins in homeostasis of the periodontium.
Keywords: bacteria, beta-defensins, epithelium, periodontium
Human gingival epithelium constitutes a stratified squamous epithelium, which protects underlying soft and hard tissues of the periodontium. Based on its location and composition, gingival epithelium is divided into oral gingival, oral sulcular, and junctional epithelia. In regard to its highly specified cell-to-cell junctions and high renewal capabilities, gingival epithelium was for long time considered a strong but passive barrier against the bacterial challenge that is constantly present in the oral cavity. With improved understanding of the periodontal disease pathogenesis, it has been recognized that the epithelium is not only a mechanical barrier but, by secreting chemokines and proinflammatory cytokines, it also plays a significant role in the initiation and progression of the inflammation-mediated destruction of periodontal tissues. Furthermore, the identification of a new group of oxygen-independent antimicrobial peptides in gingival epithelial cells brought a new aspect to the role of the epithelium in the host defense against oral bacteria (1). These cysteine-rich antimicrobial peptides of the epithelium, assumed to contribute to the innate host defense, are called as β-defensins.
Defensins were among the first antimicrobial peptides to be described in mammalians. In humans, defensins consist of two subfamilies, α- and β-defensins. While α-defensins are produced by polymorphonuclear leukocytes and intestinal paneth cells, β-defensins are produced by epithelial cells. The latter group of defensins was first identified in tracheal epithelial cells two decades ago (2, 3). Human β-defensins are expressed by various epithelial cell types, including the epithelium of the airways, kidney, skin, cornea, and gingiva (4, 5). Four different human β-defensins (hBD 1–4) have been found and functionally characterized, however, based on genomic targeting, the number of β-defensins has been suggested to be over 20 (6, 7). Of the four β-defensins known so far, hBD 1–3 are expressed and secreted in the human oral cavity (1, 8, 9).
In the review, we introduce new perspectives on various functions of gingival β-defensins, namely hBD 1–3, by combining the current knowledge about defensins and the experience on in vitro three-dimensional organotypic culture models, including our own dento-epithelial model. The topic covers the biological activity of β-defensins through their functional properties, expression, localization, and interaction with other protective mechanisms (Table 1). The structure and gene distribution of β-defensins are out of the present scope, so the readers are advised to consult with well-written reviews on these topics (10, 11).
Table 1.
Main properties of the hBD 1–3
| Localization in gingiva | Biological activity | Induction by periodontal bacteria | Suppression by periodontal bacteria | Antibacterial activity against periodontal bacteria | Degradation by bacterial/host proteases | Saline sensitivity | |
|---|---|---|---|---|---|---|---|
| hBD-1 | mRNA in spinous layers, peptide in granular layers (1, 53, 57) | N/A | P. intermedia (37) P. gingivalis (33) | N/A | P. intermedia (17) P. gingivalis (17) T. forsythia (20) F. nucleatum (17) A. actinomycetem-comitans (17) | P. gingivalis (62) | High (16, 17) |
| hBD-2 | mRNA in spinous layers, peptide in upperspinous and granular layers (1, 53, 57, 59) | Increase in proinflammatory cytokine expression, keratinocyte proliferation and migration (47, 51, 52) | P. intermedia (29, 37) F. nucleatum (29–33, 37, 56) P. gingivalis (37, 38) A. actinomycetem-comitans (4) | T. denticola (26, 37) | P. intermedia (17) P. gingivalis (17) T. forsythia (20) F. nucleatum (17) A. actinomycetem-comitans (17) | P. gingivalis (62) Cathepsins (64) | Moderate (16, 17) |
| hBD-3 | mRNA and peptide in upperspinous and granular layers (53, 59) | Increase in proinflammatory cytokine expression, keratinocyte proliferation and migration (45, 51, 52) Immunosuppressive activity (50) Promotes periodontal regeneration (69) Chemotactic effect (46, 47) | P. intermedia (37) F. nucleatum (29, 30, 33, 37, 56) P. gingivalis (29, 33) | P. gingivalis (37) T. forsythia (27, 37) T. denticola (27, 37) | P. intermedia (17, 20) T. forsythia (20) P. gingivalis (17, 37) F. nucleatum (17) A. actinomycetem-comitans (17) | P. gingivalis (25) Cathepsins (64) | Low (16, 17) |
N/A, not applicable.
Antibacterial effect of β-defensins: mechanisms and limitations
Microbicidal activities against gram-positive and gram-negative bacteria, fungi, and some parasites are common characteristics of all β-defensins examined in in vitro conditions (12). Like α-defensins, human β-defensins have been thought to exert their antibacterial effects by permeabilizing the bacterial cellular membrane (13). Pore formation on the membrane stimulates the leakage of small molecules from the bacterial cell that eventually leads to its death. However, these membrane disruption characteristics are not universal, but vary depending on the defensin type and bacterial species (10, 11). The bacterial membrane-selective characteristic of β-defensins mainly depends on the electrostatic interaction between the cationic structure of β-defensins and negatively charged bacterial membranes. Since the negative charge on bacterial cell membranes is much higher than that on mammalian cell membranes, there is a selective binding between β-defensins and bacterial membranes (14). In the literature, other explanations for this phenomenon have been presented; for example, according to the novel hypothesis of Schmidt and coworkers on bacterial membranes, the amino acid composition and design of cationic antimicrobial peptides are the main determinants in the preference of β-defensins (15).
β-defensins are broad-spectrum antibacterial peptides, however, their antibacterial effect is considerably salt-dependent. This means that β-defensins show their highest activities in conditions with low ion concentrations and their antibacterial activities are significantly impaired by the presence of ions, such as Na+, Mg2+, and Ca2+ (16). Among the three β-defensins found in gingiva, hBD-3 has the lowest sensitivity to salts, being insensitive up to 200 mM Na+ concentrations, most probably due to its high positive charge (+11), whereas hBD-1 has the highest sensitivity, being insensitive up to 100 mM Na+ concentrations. The inhibitory effect of salt ions on antibacterial properties of β-defensins does not necessarily be directed to their protein structure, but merely to their interaction with bacteria (17). In the oral cavity, β-defensins are constantly exposed to ions in saliva, which contains 8–60 mM of NaCl. The salivary NaCl concentration may be much less than what is needed to inactivate β-defensins, however, NaCl is not the only ion present in saliva. Indeed, the orchestrated effect of salivary ions may affect the function of β-defensins, even if they do not totally impair their activity. Therefore, it is plausible that β-defensins are inactive against oral bacteria outside the tissues, like in saliva. On the other hand, there is evidence that β-defensins in in vivo conditions are less sensitive to salts than in in vitro conditions (18). Since the interaction between the host and bacteria is a coordinated action with multiple players, the decreased β-defensin response against bacteria may be compensated with other antibacterial proteins. Further studies are necessary to clarify the role of β-defensins against bacteria within and outside the tissues.
Beyond their antibacterial effects, β-defensins are able to neutralize the lipopolysaccharide (LPS) activity of gram-negative bacteria (19). Very limited data exist on periodontitis-associated organisms in this context. In an experimental study, where the human acute monocytic leukemia cell line (THP-1) was incubated with fluorescently labeled LPS of Tannerella forsythia, it was demonstrated that all tested defensin types, hBD-1, hBD-2 and hBD-3, inhibited the LPS activity by affecting the binding of LPS to monocytes (20). This LPS-neutralizing effect of hBDs was independent from their antibacterial activities in a similar way as shown for LL-37, a member of the cathelicidin family of antimicrobial peptides (21).
Bacterial resistance against β-defensins
In response to the challenge by β-defensins, bacteria exert resistance by forming a capsule, modifying their cell envelope molecules, forming biofilms or cleaving defensins (22–24). Porphyromonas gingivalis, a well-known periodontal pathogen, degrades hBD-3 with its gingipains that leads to the inactivation of the peptide (25). Also some other periodontal bacteria are able to protect themselves from β-defensins, most probably, by inhibiting the defensin expression pathways. For instance, Treponema denticola inhibits the secretion of hBD-2 and hBD-3 by suppressing the expression of tumor necrosis factor (TNF)-α and toll-like receptor 2 (TLR 2) (26, 27). Hence, the ability of these periodontitis-associated organisms to overcome the β-defensin challenge seems to be associated with their virulence (17, 28).
Recognition of periodontal bacteria in stimulation of β-defensin secretion
To understand the process of β-defensin expression, the recognition of periodontal bacteria by gingival epithelia is of interest. TLRs provide the first line in the recognition of gram-positive and gram-negative bacteria, hence, to study the role of bacterial recognition in β-defensin expression, TLR-deficient cell line models are preferred. Using epithelial cell lines with silenced TLR2, it has been demonstrated that Fusobacterium nucleatum and P. gingivalis are not able to induce hBD-2 expression, whereas the non-silenced cell line expresses hBD-2 against these anaerobic bacteria (29). This corroborates with the findings of another study where knockdown of TLR2 RNA reduced the F. nucleatum-induced hBD-2 and hBD-3 upregulation with 93 and 96%, respectively (30). The relation between TLR2 and hBDs has been demonstrated using other periodontal bacteria and bacterial components as well. Recently, it was shown that knockdown of TLR2 in immortalized gingival cells suppresses hBD-3 expression in response to T. denticola (26, 27). Unlike the activation of β-defensins by whole bacteria, LPS of gram-negative bacteria, the main targets of TLRs, act as relatively poor stimulants of β-defensins (28). It is plausible that, besides the TLR-related β-defensin secretion, other mechanisms are involved in the β-defensin induction. An example of an alternative pathway is the stimulation of hBD-2 secretion from human gingival epithelial cells by Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans; this periodontal pathogen triggers hBD-2 secretion with its outer membrane protein (OMP) 100, and the interaction of OMP 100 with fibronectin activates the α5b1 integrin signaling that ends up with the secretion of hBD-2 via the MAP kinase pathway (4).
Periodontal bacteria and secretion of β-defensins
RNA expression and secretion of hBD-2 and hBD-3, unlike those of hBD-1, from epithelial cells are not constituent but dependent on infectious or inflammatory stimuli (9–12). Moreover, epithelial cells do not express these two β-defensins against all bacteria. In culture conditions, commensal bacteria, rather than major periodontopathogens, are excellent stimulants of hBD-2 from gingival epithelia. According to Krisanaprakornkit et al. (31), the incubation of gingival epithelial cells with F. nucleatum, unlike that with P. gingivalis, effectively triggers the hBD-2 mRNA expression. Later, it was demonstrated that the ability of F. nucleatum to induce β-defensin secretion is related to its unique cell wall protein, Fusobacterium-associated defensin inducer (FAD)-1 and that isolated FAD-1 stimulates hBD-2 secretion via TLR2 (32). An interesting observation was that P. gingivalis, after being transfected with FAD-1, becomes able to induce hBD-2 from oral epithelial cells (32).
The ability of P. gingivalis to evade the host recognition and hBD-2 challenge can be regarded as part of its high virulence (31, 33). The missing hBD-2 response against P. gingivalis has been explained by its rather unusual LPS structure, which may impair the cellular recognition of P. gingivalis by epithelial cells and inhibit hBD-2 expression (23). The selective character of the β-defensin response has been confirmed in several other in vitro studies. Vankeerberghen et al. (33) examined the stimulation of hBD 1–4 using gingival epithelial cells incubated with commensal and pathogenic bacteria. According to their results, F. nucleatum increases the secretions of hBD-2 and hBD-3 by epithelia, but not those of hBD-1 and hBD-4. On the other hand, P. gingivalis had no stimulating effect on hBD-2 and hBD-4 secretions, while hBD-1 secretion was evident after 14 h of incubation, being similar to the A. actinomycetemcomitans-induced hBD-1 secretion. Incubation of epithelial cells with P. gingivalis induced transiently hBD-3 expression but, after 3 h, the expression returned to the level at the baseline (33). This time-dependent change in β-defensin secretion can be attributed to the virulence of P. gingivalis, which invades gingival epithelial cell monolayers within 90 minutes and then continues to replicate intracellularly (34). During the invasion, P. gingivalis inhibits the proliferation and migration of epithelial cells (35). In this way, pathogenic P. gingivalis modulates the behavior of host cells for its own benefits, which may also include the impairment of hBD secretion. Since the invasion also requires bacterial proteinases (34), the initially secreted hBDs most probably are degraded by over-expressed P. gingivalis proteases.
Interestingly, the stimulation of hBD-3 expression varies significantly within the strains of the same pathogenic bacterium. For example, the incubation of epithelial cells with A. actinomycetemcomitans ATCC 29523 did not change the hBD-3 expression profile of host cells, while the strain ATCC 33384 enhanced hBD-3 expression significantly after 0.5 h of incubation (33). The latter hBD-3 expression-inducing strain (serotype c) is more frequently detected in periodontally healthy than in periodontitis subjects, while A. actinomycetemcomitans ATCC 29523 (serotype a), which hinders hBD-3 expression, is more prevalent in aggressive periodontitis patients than in periodontally healthy subjects (36). Therefore, it can be postulated that the capability of stimulating β-defensin expressions is strain-dependent and, in particular, dependent on the bacterium's association with disease (30, 33). As already stated, there seems to be a strong correlation between the virulence of a periodontal pathogen and its ability to minimize the induction of β-defensin expression and secretion.
In contrast to the current understanding, there are some reports in the literature on pathogenic bacteria that induce β-defensins from epithelia (37, 38). The difference between bacterial strains tested or selected epithelial cell lines may have an impact on the conflicting results. It is known that β-defensins, which are secreted by many epithelial cell types, differ in their regulation and expression characteristics. For example, in the respiratory tract, where the epithelium is constantly exposed to the variety of microorganisms, β-defensin genes are activated by all types of bacteria and TLR agonists and induced primarily through an NF-κB-mediated pathway (39). However, in the gingival epithelium, an environment similar to the respiratory tract in regard to the constant exposure to microorganisms, β-defensin genes are activated by only a subset of bacteria and TLR ligands and induced via different pathways (39). Therefore, even though the secretion of β-defensins is a common characteristic of human epithelial cell types, the difference in the cellular origin and regulation of the response should be taken into account before generalizing the research outcomes. The discrepancy between studies may also be due to differences in study designs, including incubation inocula.
Periodontal bacteria and activation of β-defensins: a hypothesis through hBD-1
To colonize and survive in the oral cavity, periodontal pathogens develop mechanisms to evade the defensin challenge, while periodontal health-associated bacteria stimulate and activate gingival β-defensins. To understand this complex relationship, the impact of bacteria on the β-defensive response of the gingiva needs to be discussed.
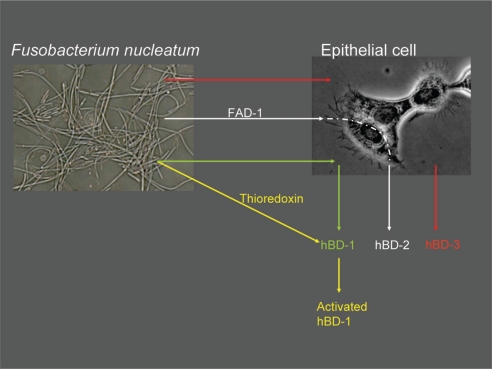
β-defensins are regarded as being part of the non-specific host response; in other words, they play a major role in the innate protection by the epithelium. Therefore, one would expect to see an enhanced β-defensin production and activity when epithelial cells are exposed to bacteria. Gingival hBD-1 secretion fits well with this innate response hypothesis, since gingiva is constantly in contact with numerous bacteria and hBD-1 is secreted constitutively in the epithelium (40). There are hundreds of bacterial species in the oral cavity, where hBD-1 may take part in the suppression and control of this constant bacterial exposure. Although hBD-1 is considered to control the oral bacterial ecosystem at some level, in vitro studies have demonstrated only a limited antimicrobial effect of hBD-1 on oral bacteria. The antibacterial activity of hBD-1 against periodontal pathogens is much lower than that of hBD-2 and hBD-3 (17). However, as recently demonstrated by Schroeder et al. (41), hBD-1 requires post-translational changes to become an active antimicrobial agent. According to their results, the weak antibacterial effect of hBD-1 against gram-positive anaerobes and Candida albicans can be significantly enhanced after hBD-1 is reduced by the thioredoxin system (41). Thioredoxin, which is a multifunctional oxidoreductase, is mainly released by dendritic cells upon contact with antigen-presenting T-cells (42, 43). This new ground-breaking information indicates that the constitutive hBD-1 secretion is a ready-to-be-used system, and its antibacterial functions can be enhanced by post-translation modifications. As a second hypothesis, oral bacteria may contribute to the hBD-1 activity through the thioredoxin system, at least in extracellular regions. F. nucleatum, an opportunistic pathogen, can increase its thioredoxin production when exposed to oxidative stress (44). This may, at least hypothetically, point to oral bacteria in the activation of secreted hBD-1 (Fig. 1). No data on β-defensins other than hBD-1 exist in this context. Further studies are needed to clarify this relationship.
Fig. 1.
Possible mechanisms of Fusobacterium nucleatum-induced β-defensin secretion and activation. F. nucleatum stimulates hBD-2 and hBD-3 secretions from epithelial cells. hBD-2 secretion induced by F. nucleatum is dependent on an outer membrane protein of the bacterium, namely Fusobacterium nucleatum-associated beta-defensin inducer (FAD)-1. F. nucleatum may, at least hypothetically, play a role in the activation of hBD-1 via its thioredoxin reductase, an enzyme which increases intracellularly as a response to oxidative stress.
β-defensins in regulation of innate and immune responses
While hBD-1 is constitutively secreted in the epithelium, hBD-2 and hBD-3 secretions are stimulation-dependent. This stimulation does not necessarily come only from bacteria, since proinflammatory cytokines are also able to induce β-defensin secretion. In human keratinocytes, proinflammatory cytokines, such as TNF-α, interferon (IFN)-γ, interleukin (IL)-1β, IL-17, and IL-22, stimulate hBD-2 secretion, while anti-inflammatory cytokines, IL-4 and IL-10, suppress its production (45). In in vitro conditions, TNF-α induces expressions of hBD-2, hBD-3, and hBD-4 from gingival epithelial cells without an effect on hBD-1 expression (33). TNF-α is not the only inducer of hBD secretion from gingival epithelia; hBD-2 secretion by gingival keratinocytes can be induced by IL-1β and hBD-1 and hBD-3 secretions by IFN-γ (28).
With the expanded knowledge about periodontal diseases, it seems that the relation between β-defensins and cytokines/chemokines is bilateral. Similarities between chemokines and β-defensins have been described at a functional level; chemokines present antimicrobial effects and β-defensins can act as chemoattractants (16). In addition to the induction of proinflammatory cytokine secretions, β-defensins impair inflammatory pathways, and bring blood cells, in particular, to the site of infection by acting as chemotactic agents (10, 45). Mast cells and macrophages have been found to migrate towards hBD 1–4, indicating a chemotactic function of β-defensins (46). Recently, Yin et al. (47) demonstrated that although all β-defensins contribute to the immune response, there are marked differences between their chemotactic effects. According to their study, hBD-2 from gingival epithelial cells significantly induces IL-6 secretion from dendritic cells, while hBD-3 induces monocyte chemotactic protein (MCP)-1 from the same cell type. β-defensins may have immuno-modulatory effects through connective tissue cells; in in vitro conditions, hBD-2 and hBD-3 trigger fibroblast proliferation (48) and, more specifically, hBD-3 stimulates cyclooxygenase-2 expression and prostaglandin E2 synthesis of fibroblasts (49).
The role of β-defensins in the immune response is not only through aggravating it, but also suppressing the response. hBD-3 inhibits the proinflammatory response by inhibiting the secretion of TNF-α from human myelomonocytic cells (50), while hBD-2 inhibits the IL-17 production by T-cells (45). In LPS-treated mice, injected hBD-3 significantly suppresses the TNF-α production. Anti-inflammatory effects of hBD-3 are independent on the production of IL-10, a well known anti-inflammatory cytokine (50).
β-defensins in gingival wound healing
Besides their antibacterial and immune-regulatory functions, β-defensins contribute to the healing process of wounds. Wound healing has four phases: inflammation, re-epithelization, granulation tissue formation, and tissue remodeling. The re-epithelization phase requires migration, differentiation, and proliferation of epithelial cells (51). In addition, hBD-2 and hBD-3, but not hBD-1, increase keratinocyte migration and proliferation (52). As demonstrated in in vitro conditions, hBD-2 promotes intestinal wound healing (53). It can be anticipated that β-defensins contribute to and play an active role also in gingival wound healing, however, there is no scientific evidence available on their contribution so far.
Localization of β-defensins in gingiva and in organotypic epithelium models
Although hBD 1–3 are rather similar concerning their structures and functions, they differ from each other in their localizations of expression and secretion. To start with, β-defensins are not detected in the junctional epithelium, an area which is often affected with inflammation, but they are secreted and present in the oral and sulcular epithelium. β-defensin expression in epithelial cells is highly connected to the cellular differentiation. Involucrin, a strong marker of cell differentiation, has similar localization patterns with hBD-1 and hBD-2 in gingiva (1). In gingival tissues, mRNAs of hBD-1 and hBD-2 are expressed in the spinous layer, while the peptides are present in the granular layers of the gingiva (1). On the other hand, hBD-3 localizes in the basal layers of the gingival epithelium (54).
Localization and stimulation patterns of hBD-1, hBD-2, and hBD-3 are very similar in vivo and in vitro; in three-dimensional organotypic epithelium models, hBD-1 and hBD-2 are secreted from the superficial layers of the model, while hBD-3 is localized on the basal cell layers (1, 55, 56). Similar to findings in monolayer studies, hBD-2 and hBD-3 secretions are barely visible in non-infected control models, whereas their secretions clearly increase after being infected with F. nucleatum biofilms (56).
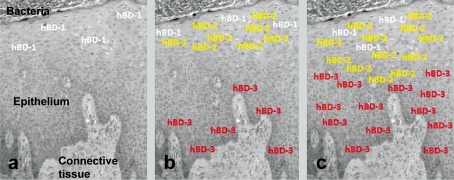
In the context of defensins, the major contribution of in vitro organotypic models is much more than confirming the localization patterns of β-defensins; the model enables to follow the secretion patterns of β-defensins in response to bacterial exposure. In our recent study (56), hBD-2 and hBD-3 secretions were followed in an organotypic dento-epithelial culture model. After 5 h of infection with F. nucleatum biofilms, the secretions of hBD-2 and hBD-3 were significantly increased in the superficial and basal layers of the model, respectively. After 24 h of infection, hBD-2 extended from the superficial layers to the basal layers of the epithelium, while hBD-3 extended towards superficial layers (56). The re-localization patterns of hBDs in in vitro organotypic dento-epithelial culture model are in line with their patterns in gingival tissues, where hBD-2 extends towards the basal layers and hBD-3 towards the superficial layers of the epithelium (54, 57) (Fig. 2).
Fig. 2.
The re-localization character of β-defensins in gingiva during the periodontal disease pathogenesis. (a) hBD-1 is constitutively expressed in the superficial layers of healthy gingiva, (b) when stimulated by infectious agents (e.g. gram-negative bacteria or their lipopolysaccharides), hBD-2 is secreted in the superficial layers, while hBD-3 is secreted in the basal layers of the epithelium, (c) with the progression of the disease, hBD-2 secretion extends towards the basal layers, while hBD-3 secretion extends towards the superficial layers of the epithelium.
β-defensins in periodontal health and disease
Based on in vitro studies with monolayer and multilayer cell culture designs, infection and inflammation influence the secretions of hBD-2 and hBD-3, while hBD-1 is secreted constitutively. Furthermore, especially in multilayer models, the levels of hBD-2 and hBD-3 secretions correlate to the incubation time with bacteria (56). However, there are apparent discrepancies in the infection-induced character of hBD-2 and hBD-3 secretions between in vitro and in vivo conditions. In analyses of gingival biopsies from periodontitis subjects and from their healthy controls, mRNAs of hBD-1, hBD-2, and hBD-3 were found at lower levels in inflamed gingival tissues than in healthy ones (8, 58–62). Protein levels, on the other hand, varied, being either slightly higher or equal, or in some cases lower, in periodontitis subjects than in their periodontally healthy counterparts (57, 62, 63). Decreased β-defensin expression has been shown at the induction phase of an experimental gingivitis model (60). These observations by different research groups raise attention, however, no clear answer has been given to define why the expression and secretion of β-defensins are suppressed during periodontitis. Concomitantly, several hypotheses have been presented to explain the discrepancy. Degradation of β-defensins by bacteria- or host-derived enzymes and genetic polymorphisms, leading to suppressed expression of defensins, are among the common explanations, which are discussed in detail below.
The first hypothesis is that, in periodontitis, the secreted β-defensins are degraded by proteolytic enzymes produced by periodontal pathogens and the host (63). Periodontal pathogens are able to eliminate the β-defensin challenge in different ways, including the degradation of these host peptides. For example, trypsin-like proteases and gingipains of P. gingivalis degrade hBD-1, hBD-2, and hBD-3 (25, 62, 63). In addition to bacterial proteases, proteolytic enzymes of the host may contribute to β-defensin degradation. Cysteine proteinase family enzymes, cathepsin B and L, which are produced predominantly by macrophages, increase in gingival tissues with the initiation and progression of periodontitis. These enzymes can degrade and inactivate hBD-2 and hBD-3 in in vitro conditions (64).
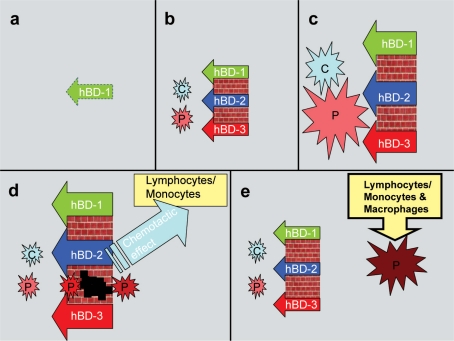
According to the second hypothesis, the replacement of innate response with immune response during the periodontal disease process is responsible for the decreased β-defensin secretion (61). In the gingival epithelium, β-defensins form the first line of defense, being prepared to awaken the innate response to increasing bacterial exposure. From an initial gingival lesion towards the formation of an established lesion, antibacterial functions of β-defensins are replaced by immune cells (61). Colonization of bacteria enhances the secretions of hBD 1–3, which in turn inhibit the bacterial growth and biofilm formation at some level. Instead, bacteria with resistance to β-defensins, such as T. denticola and P. gingivalis, survive and colonize on epithelial surfaces, and, eventually, invade gingival tissues (65). With the bacterial invasion, β-defensins stimulate the secretion of chemokines, such as IL-8 and MCP-1, from dendritic cells, and, in addition, act as chemoattractants, which bring phagocytes and lymphocytes to the site of infection (47). Taken together, the activated immune response limits innate response and, hence, secretion of β-defensins (Fig. 3).
Fig. 3.
A five-step hypothesis of the recruitment and dismissal of β-defensins during infection. (a) Within the healthy tissue, epithelial cells secrete only hBD-1, (b) the presence of commensal (C) and pathogenic (P) bacteria induce hBD-2 and hBD-3 secretions from the epithelium, (c) enhanced proportions of bacteria activate the variety of hBD responses, (d) the increasing β-defensin levels suppress the amount of bacteria, however, some defensin-tolerant pathogenic strains are able to pass through the hBD barrier, when enhanced β-defensin levels trigger the chemotactic activity through lymphocytes and monocytes, (e) at the final phase, hBD secretions return to the levels seen in a healthy condition, while the second phase of inflammation continues with the interplay between invaded bacteria/bacterial components and immune cells.
In the third hypothesis, genetic polymorphisms in β-defensin genes are emphasized (63, 66); β-defensin expression and secretion are not decreasing in periodontitis, but subjects with low β-defensin secretion due to some genetic variations are susceptible to develop periodontitis. Polymorphisms in hBD-encoding genes were previously linked with the bacterial carriage or disease activity (67–69). For example, in subjects with type 1 diabetes mellitus and single-nucleotide polymorphisms in their hBD-1-encoding genes high rates of C. albicans have been detected (67). Recently, functional polymorphisms of hBD genes were related to the presence of caries lesions (68). The link between functional polymorphisms of defensin genes and the susceptibility to periodontitis is likely, but needs to be proven.
Concluding remarks
β-defensins constitute major antimicrobial peptides in the initial response against bacteria in gingival tissues. In addition, they function as both proinflammatory and anti-inflammatory agents in the periodontal disease pathogenesis. It was previously considered that all hBDs play the same role, but in a coordinated manner. This coordination starts with hBD-1 secretion, which is performed constantly in the oral and sulcular epithelium. With the bacterial infection, the second response comes from hBD-2, which is strongly secreted in the superficial layers of the epithelium. With the progression of the infection, hBD-3 secretion, starts in the basal cell layers of the epithelium, then extending towards its superficial layers. Currently, it is considered that the re-localization of β-defensins in gingiva regulates the host reaction, first by initiating and, at some level, by limiting the immune response. Yet, the function of β-defensins in the periodontium is not limited to its role in defense, but these peptides may also have a role in wound healing by regenerating the damaged epithelium. Indeed, these antibacterial peptides can even take part in periodontal regeneration, by promoting the attachment and proliferation of fibroblasts on the diseased root surfaces (70). It is not an exaggeration to consider β-defensins, despite their small sizes, as major players in the epithelial functioning and homeostasis.
Conflict of interest and funding
The authors declare that they have no conflict of interest.
References
- 1.Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, O'Neal R, et al. Localized antimicrobial peptide expression in human gingiva. J Periodontal Res. 2001;36:285–94. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–6. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensch KW, Raida M, Mägert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 4.Ouhara K, Komatsuzawa H, Shiba H, Uchida Y, Kawai T, Sayama K, et al. Actinobacillus actinomycetemcomitans outer membrane protein 100 triggers innate immunity and production of beta-defensin and the 18-kilodalton cationic antimicrobial protein through the fibronectin-integrin pathway in human gingival epithelial cells. Infect Immun. 2006;74:5211–20. doi: 10.1128/IAI.00056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine DA. Antimicrobial peptides in defence of the oral and respiratory tracts. Mol Immunol. 2003;40:431–43. doi: 10.1016/s0161-5890(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 6.Marshall RI. Gingival defensins: linking the innate and adaptive immune responses to dental plaque. Periodontol 2000. 2004;35:14–20. doi: 10.1111/j.0906-6713.2004.003568.x. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer RI. Immunology: Peptide gets in shape for self-defence. Nature. 2011;469:309–10. doi: 10.1038/469309a. [DOI] [PubMed] [Google Scholar]
- 8.Vardar-Sengul S, Demirci T, Sen BH, Erkizan V, Kurulgan E, Baylas H. Human beta defensin-1 and -2 expression in the gingiva of patients with specific periodontal diseases. J Periodontal Res. 2007;42:429–37. doi: 10.1111/j.1600-0765.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomes Pde S, Fernandes MH. Defensins in the oral cavity: distribution and biological role. J Oral Pathol Med. 2010;39:1–9. doi: 10.1111/j.1600-0714.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 10.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cell Mol Life Sci. 2006;63:1294–313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pazgier M, Li X, Lu W, Lubkowski J. Human defensins: Synthesis and structural properties. Curr Pharm Des. 2007;13:3096–118. doi: 10.2174/138161207782110381. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Liu ZH, Tewary P, Chen Q, Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr Pharmaceutic Des. 2007;13:3131–9. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 13.Wimley WC, Selsted ME, White SH. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 1994;3:1362–73. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–23. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt NW, Mishra A, Lai GH, Davis M, Sanders LK, Tran D, et al. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J Am Chem Soc. 2011;133:6720–7. doi: 10.1021/ja200079a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowdish DM, Davidson DJ, Hancock RE. Immunomodulatory properties of defensins and cathelicidins. Curr Top Microbiol Immunol. 2006;306:27–66. doi: 10.1007/3-540-29916-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL-37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 18.Huang GT, Zhang HB, Kim D, Liu L, Ganz T. A model for antimicrobial gene therapy: demonstration of human beta-defensin 2 antimicrobial activities in vivo. Hum Gene Ther. 2002;13:2017–25. doi: 10.1089/10430340260395875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forssmann WG, Jübner M, et al. The novel β-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 2006;20:1701–2. doi: 10.1096/fj.05-4970fje. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Jun HK, Lee HR, Chung CP, Choi BK. Antibacterial and lipopolysaccharide (LPS)-neutralizing activity of human cationic antimicrobial peptides against periodontopathogens. Int J Antimicrob Agents. 2010;35:138–45. doi: 10.1016/j.ijantimicag.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible models of action. J Biol Chem. 2006;281:1636–43. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 22.Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun. 2004;72:7107–14. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q, Darveau RP, Samaranayake LP, Wang CY, Jin L. Differential modulation of human β-defensins expression in human gingival epithelia by Porphyromonas gingivalis lipopolysaccharide with tetra- and penta-acylated lipid A structures. Innate Immun. 2009;15:325–35. doi: 10.1177/1753425909104899. [DOI] [PubMed] [Google Scholar]
- 24.Otto M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol. 2006;306:251–8. doi: 10.1007/3-540-29916-5_10. [DOI] [PubMed] [Google Scholar]
- 25.Maisetta G, Brancatisano FL, Esin S, Campa M, Batoni G. Gingipains produced by Porphyromonas gingivalis ATCC 49417 degrade human-β-defensin 3 and affect peptide's antibacterial activity in vitro. Peptides. 2011;32:1073–7. doi: 10.1016/j.peptides.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Shin JE, Choi Y. Treponema denticola suppresses expression of human beta-defensin-2 in gingival epithelial cells through inhibition of TNF-α production and TLR2 activation. Mol Cells. 2010;29:407–12. doi: 10.1007/s10059-010-0048-5. [DOI] [PubMed] [Google Scholar]
- 27.Shin JE, Kim YS, Oh JE, Min BM, Choi Y. Treponema denticola suppresses expression of human β-defensin-3 in gingival epithelial cells through inhibition of the toll-like receptor 2 axis. Infect Immun. 2010;78:672–9. doi: 10.1128/IAI.00808-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyret-Lacombe A, Brunel G, Watts M, Charveron M, Duplan H. TLR2 sensing of F. nucleatum and S. sanguis distinctly triggered gingival innate response. Cytokine. 2009;46:201–10. doi: 10.1016/j.cyto.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Ji S, Shin E, Kim YS, Oh JE, Min BM, Choi Y. Toll-like receptor 2 and NALP2 mediate induction of human beta-defensins by Fusobacterium nucleatum in gingival epithelial cells. Infect Immun. 2009;77:1044–52. doi: 10.1128/IAI.00449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human β-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: Multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–15. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Ghosh SK, Scott ME, Bainbridge B, Jiang B, Lamont RJ, et al. Fusobacterium nucleatum-associated beta-defensin inducer (FAD-I): identification, isolation, and functional evaluation. J Biol Chem. 2010;285:36523–31. doi: 10.1074/jbc.M110.133140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vankeerberghen A, Nuytten H, Dierickx K, Quirynen M, Cassiman JJ, Cuppens H. Differential induction of human beta-defensin expression by periodontal commensals and pathogens in periodontal pocket epithelial cells. J Periodontol. 2005;76:1293–303. doi: 10.1902/jop.2005.76.8.1293. [DOI] [PubMed] [Google Scholar]
- 34.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–85. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis . Front Biosci. 2007;1:3965–74. doi: 10.2741/2363. [DOI] [PubMed] [Google Scholar]
- 36.Yang HW, Huang YF, Chan Y, Chou MY. Relationship of Actinobacillus actinomycetemcomitans serotypes to periodontal condition: prevalence and proportions in subgingival plaque. Eur J Oral Sci. 2005;113:28–33. doi: 10.1111/j.1600-0722.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 37.Ji S, Hyun J, Park E, Lee B-L, Kim K-K, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42:410–9. doi: 10.1111/j.1600-0765.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 38.Taguchi Y, Imai H. Expression of beta-defensin-2 in human gingival epithelial cells in response to challenge with Porphyromonas gingivalis in vitro . J Periodontal Res. 2006;41:334–9. doi: 10.1111/j.1600-0765.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 39.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–27. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–8. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder BO, Stange EF, Wehkamp J. Waking the wimp: Redox modification activates human beta-defensin 1. Gut Microbes. 2011;2:262–6. doi: 10.4161/gmic.2.4.17692. [DOI] [PubMed] [Google Scholar]
- 43.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steeves CH, Potrykus J, Barnett DA, Bearne SL. Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum. Proteomics. 2011;11:2027–37. doi: 10.1002/pmic.201000631. [DOI] [PubMed] [Google Scholar]
- 45.Kanda N, Kamata M, Tada Y, Ishikawa T, Sato S, Watanabe S. Human β-defensin-2 enhances IFN-γ and IL-10 production and suppresses IL-17 production in T cells. J Leukoc Biol. 2011;89:935–44. doi: 10.1189/jlb.0111004. [DOI] [PubMed] [Google Scholar]
- 46.Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol. 2007;37:2474–86. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- 47.Yin L, Chino T, Horst OV, Hacker BM, Clark EA, Dale BA, et al. Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria. BMC Immunol. 2010;11:37. doi: 10.1186/1471-2172-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura M, Abiko Y, Kurashige Y, Takeshima M, Yamazaki M, Kusano K, et al. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts, and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36:87–95. doi: 10.1016/j.jdermsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Chotjumlong P, Khongkhunthian S, Ongchai S, Reutrakul V, Krisanaprakornkit S. Human β-defensin-3 up-regulates cyclooxygenase-2 expression and prostaglandin E2 synthesis in human gingival fibroblasts. J Periodontal Res. 2010;45:464–70. doi: 10.1111/j.1600-0765.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 50.Semple F, Webb S, Li HN, Patel HB, Perretti M, Jackson IJ, et al. Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol. 2010;40:1073–8. doi: 10.1002/eji.200940041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol. 2000;24:127–52. [PubMed] [Google Scholar]
- 52.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation, and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 53.Otte JM, Werner I, Brand S, Chromik AM, Schmitz F, Kleine M, et al. Human beta defensin 2 promotes intestinal wound healing in vitro. J Cell Biochem. 2008;104:2286–97. doi: 10.1002/jcb.21787. [DOI] [PubMed] [Google Scholar]
- 54.Lu Q, Samaranayake L, Darveau R, Jin L. Expression of β-defensin-3 in gingival epithelia. J Periodontal Res. 2005;40:474–81. doi: 10.1111/j.1600-0765.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 55.Supp DM, Karpinski AC, Boyce ST. Expression of human β-defensin HBD-1, HBD-2, and HBD-3 in cultured keratinocytes and skin substitutes. Burns. 2004;30:643–8. doi: 10.1016/j.burns.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Gursoy UK, Pöllänen M, Könönen E, Uitto VJ. A novel organotypic dento-epithelial culture model: Effect of Fusobacterium nucleatum biofilm on β-defensin-2, -3, and LL-37 expression. J Periodontol. 2012;83:242–7. doi: 10.1902/jop.2011.110177. [DOI] [PubMed] [Google Scholar]
- 57.Lu Q, Jin L, Darveau RP, Samaranayake LP. Expression of human β-defensins-1 and -2 peptides in unresolved chronic periodontitis. J Periodontal Res. 2004;39:221–7. doi: 10.1111/j.1600-0765.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 58.Bissell J, Joly S, Johnson GK, Organ CC, Dawson D, McCray PB, Jr, et al. Expression of β-defensins in gingival health and in periodontal disease. J Oral Pathol Med. 2004;33:278–85. doi: 10.1111/j.0904-2512.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 59.Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, et al. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–25. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Offenbacher S, Barros SP, Paquette DW, Winston JL, Biesbrock AR, Thomason RG, et al. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J Periodontol. 2009;80:1963–82. doi: 10.1902/jop.2009.080645. [DOI] [PubMed] [Google Scholar]
- 61.Dunsche A, Açil Y, Dommisch H, Siebert R, Schröder JM, Jepsen S. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur J Oral Sci. 2002;110:121–4. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- 62.Kuula H, Salo T, Pirilä E, Hagström J, Luomanen M, Gutierrez-Fernandez A, et al. Human β-defensin-1 and -2 and matrix metalloproteinase-25 and -26 expression in chronic and aggressive periodontitis and in peri-implantitis. Arch Oral Biol. 2008;53:175–86. doi: 10.1016/j.archoralbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Brancatisano FL, Maisetta G, Barsotti F, Esin S, Miceli M, Gabriele M, et al. Reduced human beta defensin 3 in individuals with periodontal disease. J Dent Res. 2011;90:241–5. doi: 10.1177/0022034510385686. [DOI] [PubMed] [Google Scholar]
- 64.Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB, Jr, O'Neill S, et al. Inactivation of human β-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–7. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 65.Brissette CA, Simonson LG, Lukehart SA. Resistance to human β-defensins is common among oral treponemes. Oral Microbiol Immunol. 2004;19:403–7. doi: 10.1111/j.1399-302x.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 66.Rivas-Santiago B, Serrano CJ, Enciso-Moreno JA. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect Immun. 2009;77:4690–5. doi: 10.1128/IAI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human β-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–6. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozturk A, Famili P, Vieira AR. The antimicrobial peptide DEFB1 is associated with caries. J Dent Res. 2010;89:631–6. doi: 10.1177/0022034510364491. [DOI] [PubMed] [Google Scholar]
- 69.Tiszlavicz Z, Szabolcs A, Takács T, Farkas G, Kovács-Nagy R, Szántai E, et al. Polymorphisms of beta defensins are associated with the risk of severe acute pancreatitis. Pancreatology. 2010;10:483–90. doi: 10.1159/000276987. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Watanabe H, Ogita M, Ichinose S, Izumi Y. Effect of human β-defensin-3 on the proliferation of fibroblasts on periodontally involved root surfaces. Peptides. 2011;32:888–94. doi: 10.1016/j.peptides.2011.02.002. [DOI] [PubMed] [Google Scholar]