Abstract
Purpose
To report the clinical outcome and late side effect profile of proton radiotherapy in the treatment of children with parameningeal rhabdomyosarcoma (PM-RMS).
Methods and Materials
Seventeen consecutive children with PM-RMS were treated with proton radiotherapy at Massachusetts General Hospital between 1996 and 2005. We reviewed the medical records of all patients and asked referring physicians to report specific side effects of interest.
Results
Median patient age at diagnosis was 3.4 years (range, 0.4–17.6). Embryonal (n = 11), alveolar (n = 4), and undifferentiated (n = 2) histologies were represented. Ten patients (59%) had intracranial extension. Median prescribed dose was 50.4 cobalt gray equivalents (GyRBE) (range, 50.4–56.0 GyRBE) delivered in 1.8–2.0-GyRBE daily fractions. Median follow-up was 5.0 years for survivors. The 5-year failure-free survival estimate was 59% (95% confidence interval, 33–79%), and overall survival estimate was 64% (95% confidence interval, 37–82%). Among the 7 patients who failed, sites of first recurrence were local only (n = 2), regional only (n = 2), distant only (n = 2), and local and distant (n = 1). Late effects related to proton radiotherapy in the 10 recurrence-free patients (median follow-up, 5 years) include failure to maintain height velocity (n = 3), endocrinopathies (n = 2), mild facial hypoplasia (n = 7), failure of permanent tooth eruption (n = 3), dental caries (n = 5), and chronic nasal/sinus congestion (n = 2).
Conclusions
Proton radiotherapy for patients with PM-RMS yields tumor control and survival comparable to that in historical controls with similar poor prognostic factors. Furthermore, rates of late effects from proton radiotherapy compare favorably to published reports of photon-treated cohorts.
Keywords: Parameningeal rhabdomyosarcoma, Pediatric, Proton radiotherapy, Late effects, Conformal radiotherapy
INTRODUCTION
Rhabdomyosarcoma is the most common soft-tissue sarcoma of childhood and is a highly malignant, locally invasive neoplasm that accounts for 3.8% of solid tumors in children (1, 2). Approximately one quarter of rhabdomyosarcomas are found in parameningeal locations. Because of anatomic constraints, parameningeal rhabdomyosarcomas (PM-RMS) are rarely amenable to surgical resection. Consequently, radiotherapy (RT) and chemotherapy are the mainstays of treatment.
Five-year failure-free survival (FFS) for children with PM-RMS is approximately 67% but can drop as low as 52% if a patient has an unfavorable parameningeal site and meningeal impingement (3–5). Durable local control is critical to a favorable outcome. Isolated local recurrences account for more than one third of treatment failures, and local recurrence is a component of more than half of all failures (3). Additionally, salvage therapy after recurrence yields a median survival of only 15 months, highlighting the importance of local control for long-term survival, as well as quality of life (6).
Although survival for children with childhood rhabdomyosarcoma has dramatically increased over the last four decades with combined-modality treatment, RT can result in damage to normal surrounding tissues, leaving children with significant sequelae from treatment. Reported late effects of RT for patients with PM-RMS include endocrine deficits, facial hypoplasia, visual or orbital complications, hearing loss, neurocognitive deficits, and radiation-induced malignancies (3, 7). Because the impairment of tissue growth and organ function increases with higher radiation doses, greater volume of irradiated tissue, and younger age, the better the radiation dose localization to the tumor, the fewer and less severe the late effects will be.
We have previously shown that proton RT offers a dosimetric advantage over conventional photon RT for children with PM-RMS through enhanced normal tissue sparing (8). Proton RT is currently only available at a limited number of centers around the world, so very few children have been treated with this technology. Therefore, there are very few clinical data of the late effects of proton treatment in the pediatric literature, although late effects have been reported in the orbital RMS population (9). The goal of the present study was to report our institution’s experience with proton RT for children with PM-RMS, with particular attention paid to the late effects of treatment.
METHODS AND MATERIALS
Patient population
Seventeen consecutive patients with a diagnosis of PM-RMS were treated with proton RT at the Harvard Cyclotron Laboratory (HCL) and the Francis H. Burr Proton Therapy Center at Massachusetts General Hospital between 1996 and 2005 and constitute the study cohort on this institutional review board–approved study of late effects and clinical outcomes. Metastatic workup included CT and MRI scans of the head and neck, chest CT, cerebrospinal fluid sampling, bone marrow biopsies, and bone scan. All patients had histologically confirmed rhabdomyosarcoma by review at Massachusetts General Hospital or the Children’s Oncology Group (COG) Soft Tissue Sarcoma Central Pathology Review.
Simulation, treatment planning, and treatment
Patients underwent contrast-enhanced CT simulation of radiation fields with images obtained at 2.5-mm intervals from the vertex to the base of the neck. Patients were positioned supine, with head immobilization provided by an Aquaplast mask. The target volume was defined as the radiographically evident, prechemotherapy tumor volume. Pre- and postchemotherapy CT and MRI scans were used to define the clinical tumor volume, with image registration to the planning CT when electronically available. The gross target volume and clinical target volumes were manually drawn, and an additional margin of 7.5–10 mm was added to the field edge to account for 2 to 3 mm of setup uncertainty and penumbra lateral to the beam direction. Additional smearing of the compensator, range, and modulation were added for setup uncertainty at depth. Nine patients were treated at the Francis H. Burr Proton Therapy Center (230-MeV proton beam), and 8 patients were treated at the HCL (160-MeV proton beam). Brass apertures and Lucite compensators were fashioned for each treatment portal. Orthogonal diagnostic-quality port films were obtained daily before treatment, and isocenter shifts of 1 mm or more were made to ensure precise positioning. General anesthesia was administered to younger children when necessary to ensure proper patient immobilization during setup and treatment.
Follow-up
Patients able to maintain local follow-up (n = 6) were examined within 6 weeks of therapy completion and then at 6-month intervals for 2 years and at least annually thereafter. Eleven patients were unable to return to our institution for follow-up due to travel constraints (most were out of state or country). We contacted their referring physicians and asked a series of questions regarding treatment toxicity and requested follow-up clinic notes, laboratory results, and imaging studies.
Late effects
Late effects of treatment were derived from medical record review and physician answers to our questionnaire. We paid particular attention to the following categories derived from late effects previously noted in the literature: (1) endocrine deficits, including requirement for hormone replacement (thyroid hormone, cortisol, growth hormone, estrogen/testosterone), pubertal development, and growth velocity; (2) facial hypoplasia and the need for reconstructive or cosmetic plastic surgery; (3) vision and orbital complications, including cataracts, retinopathy, retinal hemorrhage, chronic conjunctivitis, and corneal erosion; (4) failure of permanent tooth eruption, dental caries, temporal mandibular joint dysfunction, and surgical dental procedures; (5) cranial neuropathies; (6) chronic nasal or sinus congestion; (7) auditory deficits; (8) central nervous system injury, including cognitive impairment, learning disabilities, impaired social functioning, poor balance/coordination, and seizures; and (9) second malignancies.
Growth
We assessed statural growth using height measurements before the initiation of RT and serially until the date of last follow-up according to the practices of the Intergroup Rhabdomyosarcoma Study Group (IRS) protocols (10). We plotted height measurements on standard National Center for Health Statistics growth charts. We organized patients into six height categories within their expected value for age and gender: >95th percentile, 75th–95th percentile, 50th–74th percentile, 25th–49th percentile, 5th–24th percentile, and <5th percentile. Decreased growth velocity was defined as a decrease of two or more height categories.
Statistical analysis
Treatment failures were characterized as local, regional, distant, or a combination thereof, and were histologically confirmed when appropriate. A local failure was defined as recurrence of disease in the radiation portal, a regional failure as disease in regional lymph nodes, and a distant failure as hematogenous metastases. Failure-free survival and overall survival (OS) were estimated using the Kaplan-Meier (FFS) method and were measured from the date of biopsy that resulted in a histologic diagnosis. The survival time was censored at the date of the last follow-up if a patient had not recurred or was still alive. A pointwise confidence interval (CI) for the survivor function is computed using the log–log transformation. The log–rank test was used to compare the survival difference between patients with and without intracranial extension at diagnosis. Survival estimates and 95% CIs were obtained using SAS 9.2 (SAS Institute, Cary, NC), and the two-sided p values for the log–rank test were computed by StatXact 6.0 (Cytel Software, Cambridge, MA).
RESULTS
Patient and treatment characteristics
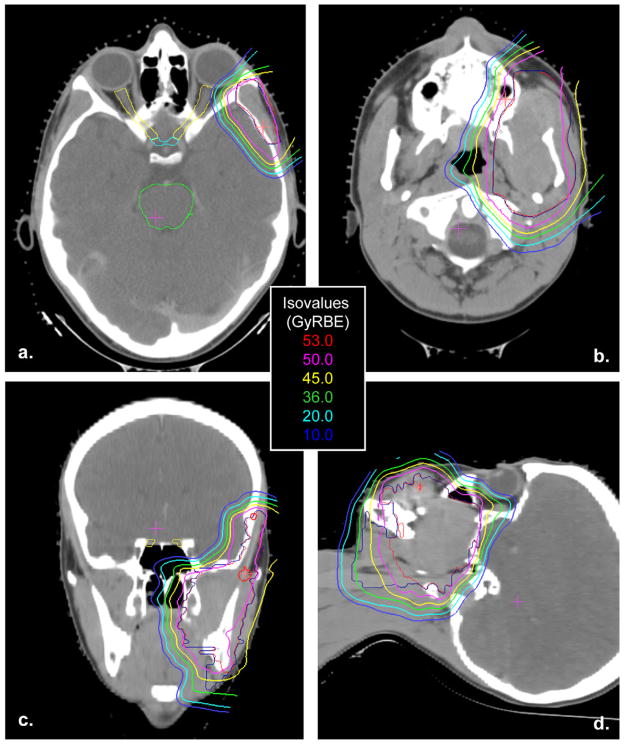
Patient clinical characteristics and treatment parameters are presented in Table 1. Median follow-up for all surviving patients was 5.0 years (range, 2–10.8 years). The median prescribed dose to the gross tumor volume was 50.4 cobalt gray equivalents (GyRBE) (range, 50.4–56 GyRBE), with a median fraction size of 1.8 GyRBE (range, 1.8–2 GyRBE). The 10 patients with intracranial extension, which includes cranial base bone erosion and cranial nerve palsies, began proton RTa median of 4 weeks (range, 1–27 weeks) after diagnosis. The median time from diagnosis to initiation of proton RT for patients without intracranial extension (n = 7) was 14 weeks (range, 11–58 weeks). All patients completed the planned course of RT. The HCL had the capacity to treat patients only 4 days per week. Seven patients treated at the HCL had a mixed photon/proton plan; a median of 9 Gy (range, 9–21.6 Gy) was delivered with photons. One patient treated at the HCL received proton RT in 2-GyRBE fractions four times per week for a total dose of 56 GyRBE with no photon component. A representative proton RT plan is shown in Fig. 1.
Table 1.
Clinical characteristics and radiation treatment parameters of 17 patients with parameningeal rhabdomyosarcoma
| Age at diagnosis (y) | |
| Median (range) | 3.4 (0.4–17.7) |
| 1–10 | 12 (71) |
| <1 or >10 | 5 (29) |
| Gender | |
| Female | 6 (35) |
| Male | 11 (65) |
| IRS Group III | 15 (88) |
| IRS Group IV | 2 (12) |
| Histology | |
| Embryonal | 11 (65) |
| Alveolar or undifferentiated | 6 (35) |
| ICE | 10 (59) |
| Primary site | 2 (12) |
| Middle ear/mastoid | 8 (47) |
| Infratemporal fossa | 3 (18) |
| Paranasal sinus | 4 (23) |
| Nasal cavity/nasopharynx | |
| Dose (GyRBE), median (range) | 50.4 (50.4–56) |
| Time from diagnosis to | |
| RT start (wk), median (range) | |
| Patients with ICE (n = 10) | 4 (1–27) |
| Patients without ICE (n = 7) | 14 (11–58) |
Abbreviations: ICE = intracranial extension; GyRBE = cobalt gray equivalents.
Values are number (percentage) unless otherwise noted.
Fig. 1.
Representative proton radiotherapy plan in the axial (a, b), coronal (c), and sagittal (d) planes for a tumor in a parameningeal location.
All patients received multiagent chemotherapy. Eleven patients were treated with vincristine, actinomycin, and cyclophosphamide (VAC) for 40 weeks according to the standard arm (Arm A) of the COG trial for children with intermediate-risk rhabdomyosarcoma, COG D9803 (4). Two patients received VAC alternating with vincristine, topotecan, and cyclophosphamide for 40 weeks on the experimental arm (Arm B) of COG D9803 (4). Three patients were treated according to a European protocol, Malignant Mesenchymal Tumors Study 98, with a complex multiagent chemotherapy regimen including the agents ifosfamide, vincristine, actinomycin, carboplatin, epirubicin, and etoposide for a total duration of 27 weeks (11). One patient who was 18 months old at diagnosis was treated with chemotherapy for 58 weeks before initiation of RT, which was delayed because of her young age. She received a combination of VAC, topotecan, etoposide, ifosfamide, carboplatin, and adriamycin.
According to the IRS clinical grouping and staging guidelines, 15 patients were categorized as Clinical Group III, and were either Stage II or III, and 2 patients were categorized as Clinical Group IV, Stage IV with subcentimeter lung lesions at diagnosis. One patient had a debulking of his nasopharyngeal mass, but gross residual disease remained. All other patients had an incisional or fine-needle biopsy to obtain a histologic diagnosis with no further attempts at resection.
Survival and patterns of failure
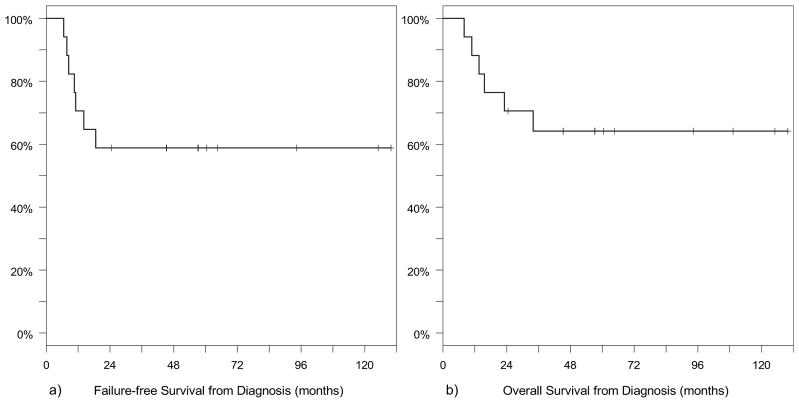
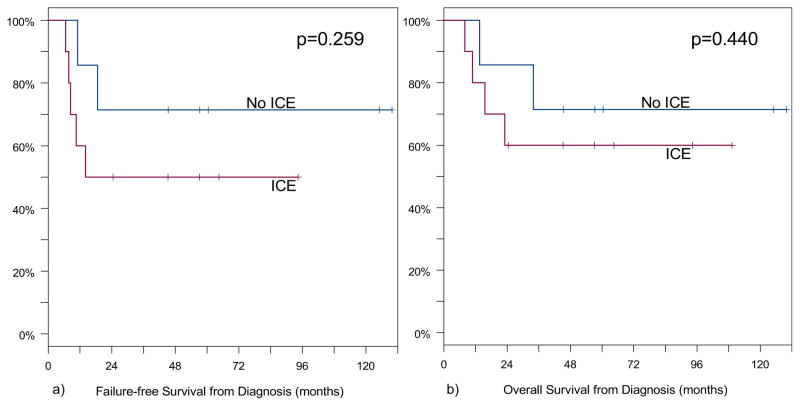
For all patients, 5-year FFS was 59% (95% CI, 33–79%) and 5-year OS was 64% (95% CI, 37–82%). Patients who had intracranial extension at diagnosis (n = 10) had a 5-year FFS of 50% (95% CI, 18–75%) and a 5-year OS of 60% (95% CI, 25–83%). Patients without intracranial extension (n = 7) had a 5-year FFS of 71% (95% CI, 26–92%) and a 5-year OS of 71% (95% CI, 26–92%). Seven of the 17 patients (41%) had a recurrence at a median time of 10.5 months (range, 7–18.5 months) after diagnosis. Sites of first recurrence were local only (n = 2), regional only (n = 2), distant only (n = 2), and local and distant (n = 1). Six of these patients died at a median time of 14.6 months (range, 8–34 months) after diagnosis. One patient had a recurrence in a regional lymph node 14 months after diagnosis, which was treated with radical neck dissection, matched-field RT, and additional chemotherapy. This was followed by a second, distant recurrence in the breast and treated with excision and additional chemotherapy. She is currently free of disease 9.2 years after initial diagnosis. Median survival after initial local–regional recurrence was 10 months (range, 2–95 months), and median survival after distant recurrence was 4 months (range, 1 week to 15 months). Kaplan-Meier survival curves for OS and FFS for all patients are shown in Fig. 2. Figure 3 shows the Kaplan-Meier survival estimates for OS and FFS according to the presence or absence of intracranial extension.
Fig. 2.
Failure-free survival (a) and overall survival (b) from diagnosis for 17 patients with parameningeal rhabdomyosarcoma treated with proton radiotherapy.
Fig. 3.
Failure-free survival (a) and overall survival (b) from diagnosis according to presence of intracranial extension (ICE).
Late effects
The late effects of treatment in the 10 patients without recurrence are compared with previously published reports, shown in Table 2. Median follow-up for these 10 patients was 5 years (range, 2–10.8 years). Late effects likely related to proton RT include failure to maintain height velocity (n = 3), endocrine deficits (n = 2; 1 patient required growth hormone, cortisol, and thyroid hormone replacement; 1 patient required growth hormone replacement diagnosed before loss of height velocity), mild facial hypoplasia (n = 7), failure of permanent tooth eruption adjacent to the treatment field (n = 3), dental caries adjacent to the treatment field (n = 5), and chronic nasal/sinus congestion (n = 2). Deficits present before RT included ipsilateral blindness (n = 1), ipsilateral ptosis (n = 1), xerophthalmia requiring partial tarsorrhaphy (n = 1), ipsilateral hearing loss (n = 5), speech delay attributed to hearing loss (n = 2), and trismus (n = 2).
Table 2.
Incidence of recorded toxicities in patients with parameningeal rhabdomyosarcoma: Comparison of proton data with previously published studies
| Toxicity | Protons: MGH (n = 10) Median f/u: 5 y |
IRS II-III (n = 213) Median f/u: 7 y |
IMRT: MSKCC† (n = 21) Median f/u: 2 y |
University of Iowa‡ (n = 17) Median f/u: 20 y |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Decreased growth velocity | 3/10 | 30 | 92/190 | 48 | NR | 9/15 | 60 | |
| Growth hormone replacement | 2/10 | 20 | 36/190 | 19 | 1/21 | 5 | 6/15 | 40 |
| Other endocrinopathies | 1/10 | 10 | 17/213 | 8 | NR | 1/15 | 7 | |
| Facial hypoplasia | 7/10 | 70 | 74/76 | 97 | 1/21 | 5 | 11/15 | 73 |
| Visual complications | 0 | 45/213 | 21 | 2/21 | 10 | 9/11 | 82 | |
| Auditory complications | 0 | 36/213 | 17 | NR | 6/8 | 75 | ||
| Dentition | 3/10 | 30 | NR | NR | 7/7 | 100 | ||
| Chronic nasal and sinus congestion | 2/10 | 20 | 35/71 | 49 | 4/21 | 19 | NR | |
| Secondary malignancies | 0 | 4/213 | 2 | 2/21 | 10 | 1/17 | 6 | |
DISCUSSION
Balancing the potential for cure with the possibility of incurring harmful late effects is one of the major challenges in treating children with malignancies. As survival for children with rhabdomyosarcoma continues to improve with combined-modality treatment, functional abilities and quality of life after treatment are increasingly important. Our clinical data suggest that proton RT provides tumor control that is comparable to that with conventional photon RT. Moreover, these data demonstrate that patients benefit from the normal tissue-sparing properties of proton RT through a reduction in late effects.
Experience from the IRS and European studies shows that local failure is the most common form of relapse for patients with PM-RMS, and such failures are associated with a dismal salvage rate (3, 6, 11–13). In our series, the crude local failure rate was 18% (3 of 17). In comparison, IRS II–IV trials had a cumulative local failure rate of 17% (3) and 16.5–18.5% on COG D9803 (4). Though our numbers are small, our follow-up exceeds the typical time period for local recurrences, which is usually within the first 2 to 3 years after diagnosis. Thus, these data suggest that a similar local control rate is achieved using proton RT compared with standard photon external-beam RT.
Our cohort experienced slightly lower 5-year FFS (59%) and OS (64%) compared with the most recent COG trial (4-year FFS and OS at 68–73% and 79%, respectively, on COG D9083). Any difference in these rates is likely due to the greater percentage of patients in our cohort with poor prognostic features compared with the IRS trial population. Most notably, 59% of our patients had intracranial extension, compared with 38% in IRS II–IV (3, 5). Patients treated on IRS II–IV with meningeal involvement (which includes intracranial extension, cranial base bone erosion, and cranial nerve palsies) had a reduced rate of local failure if RT began within 2 weeks after diagnosis (3). Unfortunately, the median time from diagnosis to the start of proton RT for our patient cohort was 8 weeks (range, 1–58 weeks) owing to young age or late referral patterns to our institution. Of the 4 patients with a component of local failure in our cohort, 3 had intracranial extension at the time of diagnosis and received proton RT at 8, 8, and 12 weeks after diagnosis.
Additional poor prognostic features in our cohort include parameningeal site, histology, and 2 patients with metastatic disease. Raney et al. (5) found that tumors in the paranasal sinus and pterygoid/infratemporal fossa connote a poorer prognosis over tumors located in other parameningeal sites. Sixty-five percent of the tumors in our cohort were located in the paranasal sinuses or pterygoid/infratemporal fossa, compared with 39% in IRS II–IV (5). Alveolar or undifferentiated histology has also been shown to be an adverse prognostic factor for relapse; the percentage of patients with these adverse histologies in our patient cohort was 35%, compared with 19% in the large national protocols (3, 13, 14).
Taken together, these data indicate that our cohort was typified by poor prognostic features and underscores the importance of prompt referral, especially because of the scarce availability of proton RT. However, patients in our cohort had very similar outcomes to the patients with poor prognostic features in IRS II–V (3). Additionally, our results with proton RT are similar to those from single institutions whose cohorts with parameningeal rhabdomyosarcoma were treated with intensity-modulated radiotherapy (IMRT) and had an abundance of patients with adverse prognostic factors as well (15, 16).
The data from our cohort of surviving patients with a median follow-up of 5 years demonstrates the ability of proton RT to reduce the incidence of many clinically significant late effects as compared with the reported toxicities in selected previous studies in Table 2 (10, 16, 17). In Table 2, we show toxicities likely related to RT from our data and from the University of Iowa and Memorial Sloan-Kettering Cancer Center, and tumor or treatment-related toxicities from the IRS trials. There was no formal system of collecting late sequelae data for the IRS trials, and data regarding many toxicities were not available for all patients, so the true incidence of toxicities is difficult to fully discern. Although follow-up times also differ, the data suggest that the dosimetric advantages of proton RT result in a benefit of proton RT in terms of the risk of significant late effects compared with similar populations treated with photons (8). Although the data for the IMRT population look good to date (17), it is important to note a higher median age (8 vs. 3 years), a median follow-up of only 2 years, and inclusion of adults, all of which have a mitigating effect on the development of late effects in the cohort, including growth abnormalities. Deficits in growth velocity were noted in only 3 patients (30%) in our cohort, compared with half of those in the previous IRS studies. No visual deficits have developed to date after proton RT, compared with 45 of 213 patients (21%) with available data in IRS II–III and 9 of 11 patients (82%) in the Iowa series and 10% in the Memorial Sloan-Kettering Cancer Center series. Although longer follow-up may result in the appearance of late toxicities, the median time to development of visual impairments in the Iowa series was approximately 2 years. Proximity of the tumor to the cochlea explains the hearing loss in 4 patients in our cohort, which occurred before proton RT. Proton RT actually improved audition in 2 of these patients, whose tumors were located in the infratemporal fossa; this likely resulted from a reduction in conductive hearing loss without a decrease in sensorineural hearing loss from radiation. Seven of our surviving patients (70%) had minimal to mild facial asymmetry, which is similar to previous trials. Asymmetry may actually be of greater concern in our patients because the absence of exit dose with proton beam RT may increase the differential radiation doses to ipsilateral and contralateral bony structures, yielding greater facial asymmetry. However, to date we have not observed this in our patient population. Half of the survivors in our cohort received extra help with school work or therapy for anxiety and behavioral issues. This is in accord with the prevalence demonstrated in large studies of childhood cancer survivors and highlights the importance of education and social functioning for the general health status and quality of life for survivors of childhood cancers (18–20).
Although cross-trial comparisons of treatment efficacy and toxicity are fraught with difficulties, these data demonstrate that proton RT reduces the long-term toxicities of therapy for PM-RMS compared with historical controls. One important caveat is that conformality of dose to the tumor has improved in the era of good three-dimensional imaging and planning with the use of smaller margins and sophisticated planning techniques, such as IMRT. Thus, larger series and longer follow-up is necessary in cohorts treated with modern photon RT to compare the late-effect profile with those treated with proton RT. The COG will be well placed to discern the clinical differences in late-effects outcomes between proton- and photon-treated patients because both techniques are allowed on many of their protocols and have been for more than 5 years.
Although none of the patients treated with protons in our cohort had a second malignancy to date, further follow-up and greater patient numbers will be necessary to determine whether the second malignancy rate is actually decreased with proton RT. However, emerging evidence suggests a significant reduction in the second malignancy rate with proton RT compared with photon-treated patients matched for site, age, and histology (21). Furthermore, mathematical modeling studies predict that the lower integral dose of proton RT compared with IMRT or three-dimensional conformal photon RT will decrease the risk of second malignancies (22). We will continue to follow these patients and others treated at our institution to determine the incidence of second malignancies after proton RT.
Despite radiation doses in excess of 50 Gy, local failures still occurred in a significant percentage of patients. These failures result in profound morbidity and are rarely salvageable. The increased conformality of proton RT may make it possible to escalate doses safely to further improve on local control. Although IRS-IV did not show any improvement in local control or OS when a hyperfractionated schema was used to deliver a higher total dose (59.4 Gy) of radiation compared with the standard dose (50.4 Gy), this escalation represents a very modest increase in biologically equivalent dose due to a decreased dose per fraction (14, 23). Furthermore, retrospective analysis of IRS trials suggest that there may be a dose–response relationship; for tumors 5 cm or larger, radiation doses of <47.5 Gy are associated with approximately twice as many local failures as doses of >47.5 Gy (3). This observation suggests that dose escalation may be of interest in select PM-RMS patients, and proton RT may be particularly useful in sparing additional dose to normal structures in the setting of dose escalation.
CONCLUSIONS
The present study shows that pediatric PM-RMS patients benefit from the normal tissue-sparing properties of proton RT through a reduction in late effects compared with previously published series of patients treated with photon RT. Local control, FFS, and OS for patients in our cohort are comparable to those in similar cohorts of patients with poor prognostic features. Prompt initiation of therapy and access to facilities with proton RT continue to be a challenge, both of which are essential for maximal patient benefit for disease control and normal-tissue sparing.
Footnotes
Conflict of interest: none.
References
- 1.Halperin EC. Pediatric radiation oncology. 5. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 2.Ries Lag SM, Gurney JG, Linet M, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. [Google Scholar]
- 3.Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys. 2004;59:1027–1038. doi: 10.1016/j.ijrobp.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 4.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: Experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978–1997. Med Pediatr Oncol. 2002;38:22–32. doi: 10.1002/mpo.1259. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoleni S, Bisogno G, Garaventa A, et al. Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer. 2005;104:183–190. doi: 10.1002/cncr.21138. [DOI] [PubMed] [Google Scholar]
- 7.Heyn R, Haeberlen V, Newton WA, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol. 1993;11:262–270. doi: 10.1200/JCO.1993.11.2.262. [DOI] [PubMed] [Google Scholar]
- 8.Kozak KR, Adams J, Krejcarek SJ, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 9.Yock T, Schneider R, Friedmann A, et al. Proton radiotherapy for orbital rhabdomyosarcoma: Clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63:1161–1168. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 10.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and -III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.McDowell HP, Foot AB, Ellershaw C, et al. Outcomes in paediatric metastatic rhabdomyosarcoma: Results of The International Society of Paediatric Oncology (SIOP) study MMT-98. Eur J Cancer. 2010;46:1588–1595. doi: 10.1016/j.ejca.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Bisogno G, De Rossi C, Gamboa Y, et al. Improved survival for children with parameningeal rhabdomyosarcoma: Results from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008;50:1154–1158. doi: 10.1002/pbc.21527. [DOI] [PubMed] [Google Scholar]
- 13.Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 14.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 15.Curtis AE, Okcu MF, Chintagumpala M, et al. Local control after intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2009;73:173–177. doi: 10.1016/j.ijrobp.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Wolden SL, Wexler LH, Kraus DH, et al. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Paulino AC, Simon JH, Zhen W, et al. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2000;48:1489–1495. doi: 10.1016/s0360-3016(00)00799-9. [DOI] [PubMed] [Google Scholar]
- 18.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2007;25:3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 19.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 20.Maunsell E, Pogany L, Barrera M, et al. Quality of life among long-term adolescent and adult survivors of childhood cancer. J Clin Oncol. 2006;24:2527–2535. doi: 10.1200/JCO.2005.03.9297. [DOI] [PubMed] [Google Scholar]
- 21.Chung CS, Keating N, Yock TY, Tarbell NJ. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72:8. [Google Scholar]
- 22.Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]