This infection has a high death rate and is predominantly associated with healthcare.
Keywords: Population-based, candidemia, surveillance, epidemiology, Australia, healthcare-associated, Candida, research
Abstract
Population-based surveillance for candidemia in Australia from 2001 to 2004 identified 1,095 cases. Annual overall and hospital-specific incidences were 1.81/100,000 and 0.21/1,000 separations (completed admissions), respectively. Predisposing factors included malignancy (32.1%), indwelling vascular catheters (72.6%), use of antimicrobial agents (77%), and surgery (37.1%). Of 919 episodes, 81.5% were inpatient healthcare associated (IHCA), 11.6% were outpatient healthcare associated (OHCA), and 6.9% were community acquired (CA). Concomitant illnesses and risk factors were similar in IHCA and OHCA candidemia. IHCA candidemia was associated with sepsis at diagnosis (p<0.001), death <30 days after infection (p<0.001), and prolonged hospital admission (p<0.001). Non–Candida albicans species (52.7%) caused 60.5% of cases acquired outside hospitals and 49.9% of IHCA candidemia (p = 0.02). The 30-day death rate was 27.7% in those >65 years of age. Adult critical care stay, sepsis syndrome, and corticosteroid therapy were associated with the greatest risk for death. Systematic epidemiologic studies that use standardized definitions for IHCA, OHCA, and CA candidemia are indicated.
Bloodstream infections with Candida (candidemia) account for 8% to 15% of hospital-acquired sepsis in the United States (1,2). Death rates associated with candidemia are high (40%–70%) (3), with an estimated attributable death rate of 25% to 49% (4,5). Candidemia also results in prolonged hospital stay and substantial healthcare costs (4,6).
Studies have shown that incidence and etiology of hospital-acquired candidemia varied with geography, type of hospital, population studied, and clinical practice (3,7–9). Secular trends in the incidence of candidemia and etiologic species of Candida differ between Europe and the United States (10). Although candidemia is perceived as a hospital-acquired infection, changes in medical practice to more frequent use of home healthcare for many illnesses and long-term indwelling vascular devices have increased the number of susceptible patients in the community, potentially increasing incidence and changing epidemiology.
Two population-based surveys in the United States showed that 20% and 28% of cases were acquired outside hospitals (7,11), compared with only 1.2% in Finland (12). In a more recent study in Spain, 11% of episodes occurred in outpatients (13). These studies are not directly comparable because definitions of outpatient-acquired episodes were different. In addition, within this group, little distinction was made between cases of healthcare-associated candidemia and community-acquired (CA) candidemia.
A retrospective survey of candidemia in Australian hospitals from 1995 to 1998 showed an increase in the annual incidence of infection and a decrease in the proportion caused by Candida albicans (14). The Australian Candidemia Study Group was formed in 2001 to conduct the first countrywide, population-based, active laboratory surveillance for candidemia.
Methods
Study Design and Data Collection
Cases of candidemia were prospectively identified by blood culture surveillance at 50 of 52 public and private microbiology laboratories in Australia (population ≈20.1 million) from August 2001 to July 2004. One nonparticipating laboratory provided services to a university pediatric hospital where candidemia rarely occurred (A. Daley, pers. comm.) and the other provided services to an adult university hospital (36 cases identified; H. Sheorey, pers. comm.). Approval for the study was obtained from the human ethics review committees of institutions providing clinical data.
Adults, children, and neonates with <1 blood culture yielding Candida species were eligible for enrollment. State or territory coordinators were responsible for case identification. Information was collected on a standard form regarding age, sex, patient location at time of candidemia diagnosis, healthcare setting, risk factors within the preceding 30 days (including surgery, vascular access devices [VADs], hyperalimentation, and use of antimicrobial and systemic antifungal agents), major concomitant conditions (International Statistical Classification of Diseases and Related Health Problems, 10th revision, Australian modification) (15), portal of entry, clinical signs of sepsis (16), complications of candidemia, results of diagnostic studies, antifungal therapy, and clinical outcome <30 days after diagnosis. All data were collected prospectively, and forms were completed on days 5 and 30 after the date of the initial positive blood culture or at death if it occurred earlier. Data were collected and analyzed at a central site. Periodic audits of laboratory records ensured that all cases of candidemia were reported. For the study period, number of hospital beds and annual different day patient separations (defined as completed admissions) were obtained from 40 hospitals.
Definitions
A case was defined as incident isolation of Candida species from blood during the study period. For patients with >1 episode of candidemia, the second episode was defined as a new case if it occurred >30 days after the previous episode. A total of >2 episodes fulfilling the case definition and occurring in different patients who were epidemiologically linked (e.g., with regard to risk factors, location, and time) constituted a case cluster.
Episodes were classified as inpatient healthcare-associated (IHCA) if they occurred >48 hours after hospital admission and had not clinically manifested on admission (17). Cases occurring <48 hours after hospital admission were considered outpatient-acquired. Among outpatient-acquired candidemia, episodes associated with an indwelling medical device, surgical procedure, or chemotherapy-related neutropenia (<1 × 109 cells/L, adjusted for children) were classified as outpatient healthcare associated (OHCA), and CA infections were classified as those occurring in patients with no healthcare-related risk factors (17). Adult intensive care unit (ICU) acquisition was when candidemia developed >48 hours after ICU admission for nonneutropenic patients. Source of candidemia was considered VAD related if culture of the device tip grew the same species isolated from blood. Endocarditis was classified by modified Duke criteria (18).
Microbiologic Methods
All laboratories cultured blood specimens in BACTEC (Becton Dickinson, Sparks, MD, USA) or BacT/Alert 3D (bioMérieux, Marcy l'Etoile, France) automated systems. Candida organisms were speciated by using standard phenotypic methods (19). Isolates were forwarded to a reference laboratory (Women's and Children's Hospital, Adelaide) for susceptibility testing and species confirmation by using conventional methods (20). Candida dubliniensis was distinguished from C. albicans by PCR fingerprinting (21). Where identification was discordant, species determination at the reference laboratory was used.
Statistical Analysis
Population and age-specific incidences were calculated by using denominator data from the 2004 Australian census (22) and hospital-specific incidences by using day of separation denominator data from individual hospitals for the study period. Overall incidences were expressed as pooled mean rates calculated by aggregating numerators and dividing by the sum of denominators from all sites. Data were analyzed with SPSS version 10.0.7 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared with Student t test, and categorical variables were compared with χ2 or Fisher exact tests. Incidence data from university hospitals were pooled and compared with pooled data from other hospital types with χ2 test. A p value <0.05 was considered significant. Univariate analyses were performed to identify risk factors associated with overall illness. Candidate variables with a univariate p<0.15, or previously published risk factors, were analyzed by multiple logistic regression. The same independent predictors of death were identified with forward, backward, and stepwise variable selection methods.
Results
Incidence and Patient Demographics
Permission was denied to include 36 candidemia episodes identified at the nonparticipating adult hospital. Thus, the number of incident episodes was 1,095 (in 1,095 patients), with 337, 352, and 406 during the first, second, and third years, respectively, of the study. Data on hospital characteristics were available for 1,095 patients, demographic and clinical data for 1,005 (91.7%) episodes, and outcome data for 857 (78.3%). Species were identified for 1,068 (97.5%) isolates.
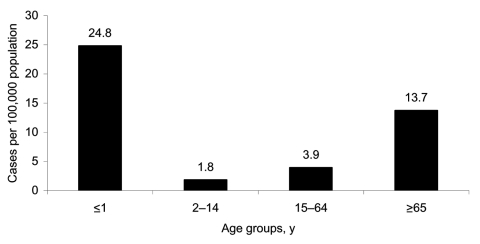
The average population-based incidence of candidemia was 1.81/100,000 (1.87/100,000 if inclusive of cases at the nonparticipant hospital) per year (Table 1) and was highest in infants (24.8/100,000) and persons >65 years of age (13.7/100,000; Figure 1). Most cases (36.4%) occurred in the most populous state, New South Wales, but incidence was highest in Queensland. Age composition of the population was similar by jurisdiction and similar to the national average (data not shown) except for the 2 least populated areas, Northern Territory and Australian Capital Territory, where the percentage of persons >65 years of age was 4.4% and 9.3%, respectively, compared with the national average of 13%.
Table 1. Number and incidence of candidemia cases reported in all jurisdictions, Australia, 2001–2004*.
| Parameter | State or territory |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NSW | VIC | QLD | SA | WA | TAS | NT | ACT | Total | |
| No. cases (%) | 399 (36.4) | 300 (27.4) | 273 (24.9) | 45 (4.1) | 40 (3.7) | 19 (1.7) | 5 (0.5) | 14 (1.3) | 1,095 (100.0) |
| Mean incidence per 100,000 population | 1.98 | 2.01† | 2.34 | 0.99 | 0.67 | 1.31 | 0.83 | 1.44 | 1.81† |
| Mean incidence per 1,000 separations‡ | 0.19 | 0.36 | 0.18 | 0.25 | 0.16 | 0.09 | 0.05 | 0.09 | 0.21 |
| No. institutions | 35 | 15 | 30 | 3 | 3 | 2 | 1 | 1 | 90 |
| Acquisition (% of episodes)§ | |||||||||
| IHCA | 79.1 | 83.4 | 79.3 | 90.2 | 87.5 | 89.5 | 50 | 85.7 | 81.5 |
| OHCA | 10.9 | 14.2 | 10.7 | 9.8 | 2.5 | 10.5 | 0 | 14.3 | 11.6 |
| CA | 10.0 | 2.4 | 10.0 | 0 | 10.0 | 0 | 50.0 | 0 | 6.9 |
*NSW, New South Wales; VIC, Victoria; QLD, Queensland; SA, South Australia; WA, Western Australia; TAS, Tasmania, NT, Northern Territory; ACT, Australian Capital Territory; IHCA, inpatient healthcare-associated; OHCA, outpatient healthcare-associated; CA, community-acquired. †Mean incidences per 100,000 in VIC and in Australia are 2.25 and 1.87, respectively, with inclusion of 36 cases identified at the nonparticipating adult hospital. ‡Data available for 17 hospitals in NSW, 8 in VIC, 8 in QLD, 2 in SA, 1 in WA, 2 in TAS, 1 in NT, and 1 in ACT. §Data for 919 candidemia episodes.
Figure 1.
Annual age-specific incidence of candidemia, Australia, 2001–2004.
The pooled mean annual hospital-specific incidence of candidemia (data from 40 institutions) was 0.21/1,000 separations (range by jurisdiction 0.05–0.36) (Table 1). No case clusters occurred. The estimated mean incidence in university (n = 28) and university-affiliated hospitals (n = 9) was similar (0.22/1,000 separations, range by jurisdiction 0.05–0.90) and greater than in private hospitals (n = 3, 0.1/1,000 separations, range by jurisdiction 0.04–0.16).
Neonates <1 month of age accounted for 33 (3.3%) of 1,005 cases, children 2 months to 14 years of age for 95 (9.5%) cases, adults 15–64 years of age for 527 (52.4%) cases, and patients >65 years of age for 350 (34.8%) cases. The median age was 56 years (range 0–98); 537 (53.4%) episodes affected male patients.
Most cases occurred in medical wards (including cancer and hemopoietic stem cell transplant [HSCT] units; 390 [35.6%] of 1,095 cases), critical care units (273 [24.9%] cases), and surgical wards (193 [17.3%] cases). Emergency (4.3%) and obstetrics and gynecology (0.5%) services reported small percentages of episodes. Pediatric cases occurred in pediatric critical care units (16 cases, 1.5%) and pediatric medical or surgical wards (24 cases, 2.2%). Neonatal infections occurred in premature or low birthweight infants in neonatal critical care units (35 cases, 3.2%).
Concomitant Conditions, Risk Factors, and Healthcare Settings for Candidemia
Concomitant conditions and risk factor data were available for 1,005 episodes. Cancer was the most common underlying condition (323 cases, 32.1%), with 164 episodes in solid tumor (50.8% cancers) cancer patients. Of 159 cases diagnosed in patients with hematologic malignancy, 91 occurred in patients with leukemia and 58 in those with lymphoma. Only 52 episodes occurred in transplant recipients (17 allogeneic and HSCTs, 11 autologous HSCTs, and 24 solid organ transplants). Gastrointestinal disorders (19%), chronic cardiovascular disease (13.8%), and diabetes (13.6%) were common, but pancreatitis was infrequent (2.5%), and coincident HIV infection was rare (0.6%). Common iatrogenic risk factors included indwelling VADs (72.6%), antimicrobial agents (77%), major surgery (37.1%), and hyperalimentation (33%, Table 2).
Table 2. Selected concomitant conditions, risk factors, and outcomes for 919 episodes of candidemia by healthcare setting, Australia, 2001–2004*.
| Characteristic | Healthcare-associated, outpatient acquired (n = 107) | Healthcare-associated, inpatient acquired (n = 749) | p value† | Community acquired (n = 63) | p value† | |
|---|---|---|---|---|---|---|
| Concomitant condition | ||||||
| Hematologic malignancy | 27 (25.2) | 131 (17.5) | 0.06 | 1 (1.6) | <0.001 | |
| Solid organ malignancy | 21 (19.6) | 135 (18.1) | 0.69 | 8 (12.7) | 0.30 | |
| Solid organ transplantation | 2 (1.9) | 22 (2.9) | 0.76 | – | – | |
| HSCT | 3 (2.8) | 25 (3.4) | 1.0 | – | – | |
| Prematurity | – | 35 (4.7) | – | 1 (1.6) | 0.37 | |
| Renal disease‡ | 10 (9.3) | 56 (7.5) | 0.44 | 2 (3.2) | 0.24 | |
| GI and liver disease§ | 17 (15.9) | 162 (21.6) | 0.20 | 12 (19.0) | 0.67 | |
| Pancreatitis | 1 (0.9) | 24 (3.2) | 0.35 | – | – | |
| Cardiovascular disease¶ | 17 (15.9) | 118 (15.8) | 1.0 | 4 (6.3) | 0.09 | |
| Diabetes mellitus | 14 (13.1) | 111 (14.8) | 0.77 | 12 (19.0) | 0.37 | |
| Risk factor | ||||||
| Surgery in past 30 d | 16 (15.7) | 353 (49.0) | <0.001 | 4 (6.9) | 0.08 | |
| Burns/trauma | – | 40 (5.5) | – | 1 (1.8) | – | |
| VAD | 72 (69.2) | 653 (90.8) | <0.001 | 5 (9.1) | <0.001 | |
| Hyperalimentation | 13 (12.6) | 318 (44.1) | <0.001 | 1 (1.8) | 0.04 | |
| Neutropenia | 19 (18.1) | 144 (19.6) | 0.79 | 1 (1.6) | 0.002 | |
| Antimicrobial agents | 69 (65.7) | 686 (95.5) | <0.001 | 19 (34.5) | <0.001 | |
| Corticosteroids | 29 (28.4) | 236 (33.0) | 0.43 | 1 (1.6) | <0.001 | |
| Chemotherapy | 27 (25.2) | 106 (14.2) | 0.01 | – | – | |
| Systemic antifungal use | 15 (14.0) | 106 (14.2) | 1.0 | – | – | |
| Intravenous drug use | 2 (1.9) | 13 (1.7) | 1.0 | 15 (23.3) | <0.001 | |
| Other BSI | 15 (14) | 256 (34.2) | <0.001 | 5 (8) | 0.33 | |
| Sepsis syndrome | 74 (69.2) | 594 (79.3) | 0.01 | 37 (58.7) | 0.71 | |
| Candida endocarditis | 7 (6.5) | 22 (2.9) | 0.08 | 5 (7.9) | 0.55 | |
| Mean time in hospital, d | 18.1 | 56.7 | <0.001 | 16.1 | 0.33 | |
| Death within 30 d | 13 (12.4) | 218 (31.1) | <0.001 | 6 (9.4) | 1.0 | |
*Data are no. (%) of total cases in each category, except for mean time in hospital. Some patients had >1 concomitant condition or risk factor. HSCT, hemopoietic stem cell transplant; GI, gastrointestinal; VAD, vascular access device; BSI, bloodstream infection. †By χ2 test using outpatient healthcare-associated data as baseline. ‡Hemodialysis or peritoneal dialysis. §Biopsy-proven cirrhosis with portal hypertension, past upper GI bleeding caused by portal hypertension, or prior episodes of hepatic failure. ¶Severe congestive heart failure. Symptoms at rest/inability to carry out physical activity without discomfort.
Of 919 episodes for which information on the healthcare setting was available, 749 (81.5%) were IHCA, 107 (11.6%, or 62.9% of outpatient-acquired cases) were OHCA, and 63 (6.9%) were CA. The proportion of disease acquired outside a hospital by jurisdiction ranged from 9.8% (4 of 41 cases in South Australia) to 50% (but only 1 of 2 cases in Northern Territory). Of the major states, Western Australia reported the lowest percentage of OHCA cases (1 [2.5%] of 40 episodes, Table 1). Comparison of major concomitant conditions and risk factors according to healthcare setting is summarized in Table 2; >4 risk factors were evident in 195 (21.2%) episodes. A total of 183 (20%) episodes were associated with use of an adult ICU. Twenty-nine (93%) neonates received hyperalimentation compared with 35.4% of the adults. Systemic antifungal agents were administered as prophylaxis in 67 (55.4%) of 121 instances; 48 (68.6%) of these prescriptions were for hematology/HSCT patients.
Most (90%–100%) episodes associated with prematurity, organ transplantation, and burn or trauma were IHCA (Table 2). Compared with all cases acquired outside a hospital, patients with IHCA infection were significantly more likely to have had recent surgery (49% vs. 12.5%, p<0.001), neutropenia (19.7% vs. 12.6%, p = 0.04), indwelling VADs (90.1% vs. 48.4%, p<0.001), corticosteroid therapy (33% vs. 19%, p<0.001), or parenteral nutrition (44.1% vs. 8.9%, p<0.001) or to have died within 30 days of infection (31.1% vs. 1.8%, p<0.001). These risk factors, with the exception of surgery, were more common in cases with OHCA candidemia than in those with CA candidemia (Table 2).
Recent surgery and VADs, hyperalimentation, and antimicrobial agents were more common in IHCA patients than in OHCA patients (Table 2). Conversely, coincident malignancy (44% vs. 34% episodes, p = 0.05) and cancer chemotherapy (25.2% vs. 14.3%, p = 0.01) were associated with OHCA infection. OHCA patients also included hemodialysis recipients and patients with diabetes. In CA candidemia, common concomitant conditions included intravenous drug use (IVDU, 23.8%), gastrointestinal disorders (19%), diabetes (19%), behavioral disorders such as chronic alcohol abuse (31.8%), and infectious diseases (23.4%); 15 (50%) of 30 episodes in IVDU patients were CA (Table 2). Differences in clinical manifestations were identified in the 3 groups. Patients with IHCA candidemia were more likely to have sepsis, die within 30 days, remain in hospital longer, and have concomitant healthcare-associated bacteremia (Table 2). Of 34 patients with Candida endocarditis (13 definite and 21 possible), 7 were OHCA with an increasing trend when compared with those with IHCA candidemia (Table 2).
Candida Species
Species of Candida was correctly identified in 96.7% instances in which identity was established. C. albicans was the most common species (505 [47.3%] of 1,068 episodes) followed by C. parapsilosis (19.9%) and C. glabrata (15.4%). C. tropicalis, C. krusei, and C. dubliniensis were identified in 5.1%, 4.3%, and 1.9%, respectively, of the patients. The remaining 42 (4.0%) episodes were caused by C. guilliermondi (n = 11), C. lusitaniae (n = 7), C. kefyr (n = 5), C. famata (n = 3), C. rugosa (n = 3), and C. pelliculosa (n = 3). There were 24 (2.2%) episodes of polycandidal infection and an increase in the proportion of C. glabrata candidemia from 10.1% episodes the first year to 19.5% episodes in the third year of the study (p = 0.02, data not shown).
C. albicans was the predominant species in Tasmania (60%), Australian Capital Territory (77%), and Western Australia (54.1%). However, this species caused only 37.8% of episodes in South Australia, whereas 31.1% were caused by C. parapsilosis (7.7%–21.9% of cases elsewhere). C. krusei and C. dubliniensis candidemia were rare in the least populous jurisdictions where complex procedures such as HSCT are not performed. Age distribution, including proportion of neonates and children in the population, was comparable for all jurisdictions (data not shown).
Species other than C. albicans were isolated more often from patients with candidemia acquired outside a hospital (60.5% vs. 49.9% for IHCA candidemia, p = 0.02). C. parapsilosis was recovered from 30.9% of these episodes (compared with 16.7% of IHCA episodes, p<0.001), although C. krusei and C. dubliniensis candidemia was rare. Relative proportions of infections caused by C. albicans and non–C. albicans species in cases of OHCA candidemia and CA candidemia were similar.
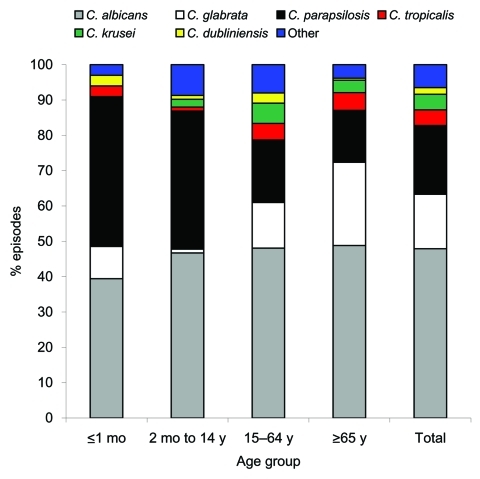
In neonates and children, C. parapsilosis accounted for a similar proportion of episodes as did C. albicans; other species were rare. C. albicans was the most common causative pathogen in adults (48.6% of cases) and C. glabrata, C. parapsilosis, and other Candida species were approximately equally distributed (Figure 2). Eighty (53.3%) of 150 episodes in patients >65 years of age were caused by C. glabrata.
Figure 2.
Distribution of causative pathogen according to patient age for 978 Candida species, Australia, 2001–2004.
C. albicans was the most common pathogen in ICUs (61.7% of episodes) and surgical (54.4% of episodes) patients but caused only 32% of episodes in hematology patients, who had the highest proportion (24 [66.7%] of 36 episodes) of infection with C. krusei. C. parapsilosis accounted for 42.9% of episodes in neonatal ICUs and for 42.4% of cases in patients without hematologic cancer. C. glabrata was present in all patient groups. Twelve (63.2%) of 19 episodes of C. dubliniensis candidemia were in surgical and nonhematology patients.
Outcome
The all-cause 30-day death rate was 27.7% (236 of 853 episodes) and similar in all age groups. Clinician-reported attributable deaths were lower (93 episodes, 10.9%). The death rate was higher in IHCA episodes than in either OHCA or CA episodes (Table 2). Univariate analysis showed that numerous variables were associated with increased death (Table 3). Multiple logistic regression analysis of 16 variables showed that an age >65 years, ICU stay, sepsis at diagnosis, and corticosteroid therapy (p<0.001, Table 4) were associated with the greatest risk for death. C. glabrata infection was also an independent predictor of death. Treatment with an antifungal agent (752 [85.8%] of 876 episodes) was independently associated with lower odds of death but removal of a VAD (76.4% of 878 instances where VADs were in situ at diagnosis) was not (Table 4). However, in the subset of VAD-related candidemia episodes (n = 409), removal of the VAD (n = 339) was associated with a better outcome (odds ratio 0.48, 95% confidence interval 0.26–0.88, p = 0.02).
Table 3. Univariate predictors of death by candidemia 30 days after diagnosis, Australia, 2001–2004*.
| Variable | Deaths, % (no./total) | Nondeaths, % (no./total) | p value |
|---|---|---|---|
| Age >65 y | 53.8 (127/236) | 28.4 (175/617) | <0.001 |
| Malignancy | 36 (85/236) | 33.2 (205/617) | 0.44 |
| Hematologic malignancy | 16.9 (40/236) | 16.5 (102/617) | 0.88 |
| Lymphoma | 8.1 (19/236) | 5.0 (31/617) | 0.09 |
| Surgery in past 30 days | 44.4 (104/234) | 41.2 (254/616) | 0.40 |
| VAD | 87.1 (203/233) | 82.2 (505/614) | 0.09 |
| Hyperalimentation | 44.2 (103/233) | 35.3 (217/615) | 0.02 |
| Hemodialysis | 16.7 (39/234) | 8.3 (51/615) | <0. 001 |
| Urinary catheter/drainage device | 69.2 (162/234) | 53 (325/613) | <0.001 |
| Trauma/burns | 1.7 (4/234) | 5.8 (36/616) | 0.01 |
| Corticosteroid therapy | 44.9 (105/234) | 25.6 (158/616) | <0.001 |
| Antimicrobial drug use | 96.1 (224/233) | 85.5 (526/615) | <0.001 |
| Neutropenia | 20.9 (49/234) | 16.4 (101/614) | 0.126 |
| ICU stay | 34.3 (81/236) | 15.9 (98/617) | <0.001 |
| Sepsis present (day 0) | 87.6 (205/234) | 78.8 (484/614) | 0.003 |
| Treatment with antifungal agent† | 70.5 (165/234) | 91.7 (564/615) | <0.001 |
| VAD removal | 61.6 (98/159) | 82.5 (406/492) | <0.001 |
| Candida glabrata infection | 22.5 (53/236) | 12.6 (78/617) | <0.001 |
| Polycandidal infection | 3.8 (9/236) | 1.8 (11/617) | 0.08 |
| Inpatient healthcare-associated | 91.9 (218/237) | 85.7 (531/620) | 0.02 |
*VAD, vascular access device; ICU, intensive care unit. †Includes all patients receiving >1 therapeutic dose of systemic antifungal agent(s) as advised by an infectious diseases physician.
Table 4. Multivariate predictors of death by candidemia 30 days after diagnosis, Australia, 2001–2004*.
| Characteristic | OR | 95% CI | p value |
|---|---|---|---|
| Age >65 y | 3.0 | 2.0–4.3 | <0.001 |
| ICU stay | 3.2 | 2.1–5.0 | <0.001 |
| Corticosteroid therapy | 2.8 | 1.9–4.1 | <0.001 |
| Hemodialysis | 2.4 | 1.4–4.1 | 0.002 |
| Hyperalimentation | 1.7 | 1.1–2.5 | 0.006 |
| Antimicrobial drug use | 2.1 | 0.9–4.4 | 0.07 |
| Neutropenia | 2.2 | 1.3–3.6 | 0.002 |
| Sepsis present | 3.1 | 1.7–5.4 | <0.001 |
| Treatment with antifungal agent | 0.01 | 0.06–0.2 | <0.001 |
| Candida glabrata infection | 1.8 | 1.1–2.9 | 0.01 |
*Sixteen candidate variables were included in the final model. OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
Discussion
This study provides the first contemporary, comprehensive, population-based description of candidemia across a continent. Overall disease incidence (1.81/100,000) was similar to that in Europe (1.4–4.9/100,000) (12,23) but different from that in the United States (6–10/100,000) (7,11,24). Most US and European surveys were not population-based and those that were included patients from specific areas (11,13,24). Age distribution of the population affected estimates, as shown by higher incidence among young and elderly people in Australia. Geographic variations in incidence were observed across Australia. However, this finding was unlikely to be caused by differences in age distribution because this distribution was similar in all states. Although the proportion of elderly persons was lower in Northern Territory and Australian Capital Territory, only 19 of 1,095 cases were in these jurisdictions.
As expected, mean incidence of candidemia was higher in university hospitals and university-affiliated hospitals, which have a higher proportion of patients at risk for candidemia (0.22/1,000 separations each) than private hospitals (0.1/1,000 separations). Since similar clinical management practices are used throughout Australia, jurisdictional differences are likely caused by different exposures to risk factors for infection. The highest incidences were observed in jurisdictions with organ transplantation, burn, and critical care centers.
Concomitant conditions and risk factors associated with candidemia were similar to those previously described (11,25), but pancreatitis (26) and HIV infection (8%–10% of cases in the United States) (7,11) were rare, and only 20% of episodes were associated with an ICU (33%–40% elsewhere) (7,13,25). We observed approximately equal numbers of cases in hematologic and solid tumor cancer patients. However, in previous studies, solid tumor patients were more common (6,11,25). Whether the prevalence of candidemia is increasing in the setting of chemotherapy for hematologic malignancy, despite use of antifungal prophylaxis in selected patients, is the subject of ongoing study.
Australian hospital statistics showed a 4% increase in community-based healthcare from 2000 through 2003 (27). Using population-based surveillance, we identified ≈20% of candidemia episodes that would not have been captured by nosocomial surveillance. This proportion is higher than in Europe (6%–10%) (12,13), but lower than in the United States (20%–28%) (7,11). However, these proportions are not directly comparable since previous studies have used different criteria to define outpatient-acquired episodes. Although most studies required blood cultures to be positive <48 hours of patient admission (1,13), others have used <72 hours (28) or <24 hours (7,11). If one considers the likely incubation period of candidemia, we suggest that 48 hours is appropriate. Furthermore, previous studies have not distinguished between CA and OHCA infections (7,11,13,28).
Using a national standard classification for bloodstream infections (17), we identified differences and similarities between IHCA candidemia and candidemia acquired outside a hospital and between OHCA and CA infections. Most (>60%) outpatient cases were healthcare associated. These cases resembled IHCA candidemia because cancer and other chronic diseases were common concomitant conditions and established risk factors were often present, although less frequently, than in IHCA infection; they differed from CA candidemia in instances in which IVDU was a risk factor. Thus, emergence of candidemia outside the hospital setting is likely due to the shift in persons with iatrogenic risk factors, such as chemotherapy and intravenous antimicrobial drug therapy, increasing management of more serious conditions outside the hospital, and implementation of early hospital discharge. Home-based therapies have been implicated in at least 1 outbreak of outpatient candidemia (29).
Compared with IHCA infection, the 30-day death rate and duration of hospital stay were lower in OHCA cases. Candidemia may have been more severe in the IHCA group because sepsis was initially identified in a higher proportion of these patients than in OHCA patients and was an independent predictor of death. Concomitant bacteremia was more common in IHCA patients. However, since no control group was studied, the difference may be explained by other characteristics of hospitalized patients compared with outpatients. Clinical outcomes of OHCA and CA candidemia were similar, although sepsis was present at diagnosis in a higher proportion of OHCA patients. Candidemia should be included in the differential diagnosis of patients with appropriate risk factors and sepsis who are admitted to emergency departments or in other healthcare settings.
Species distribution varied by jurisdiction, healthcare setting, age, and hospital service. In South Australia, C. albicans was less common and C. parapsilosis was more prevalent. This finding was not explained by differences in age distribution or proportions of IHCA and OHCA infections between jurisdictions. Overall, C. parapsilosis candidemia was more prevalent in outpatients, many of whom had VADs in situ, and in neonates, which is consistent with previous studies (7,11). Given the reduced susceptibility of C. glabrata to azole drugs (30), our observations that C. glabrata candidemia occurs more often in patients without hematologic malignancy and that these infections increased during the study provide useful data. In other studies, C. glabrata was more common in older persons (31) and patients with hematologic cancer (24).
The high overall death rate, albeit lower than reported elsewhere (35%–44%) (7,12,13), is another reminder of the role of candidemia in healthcare settings. The highest case-fatality rate was observed in the most vulnerable patients (elderly, ICU patients, and those who recently had medical interventions). Sepsis syndrome and failure to institute treatment with antifungal drugs (the latter occurred mainly in preterminal hematology patients) were independent predictors of death. Although removal of VADs did not independently protect against death, data from univariate analysis (Table 3) support current recommendations to remove VADs when candidemia is detected (32).
In conclusion, although candidemia is primarily a healthcare-associated entity in patients with established risk factors, many of these patients are observed in the outpatient setting. OHCA infections have characteristics intermediate between those of IHCA and CA infections. We propose that cases of candidemia be categorized as IHCA, OHCA, and CA, with the term CA reserved for those episodes occurring <48 hours of hospital admission and that do not meet criteria for healthcare-associated infections. Further study of secular trends and characteristics of candidemia acquired outside hospitals with standardized definitions is warranted. Surveillance is needed to track trends of this serious infection and provide guidelines for antifungal prophylaxis, treatment, and infection control strategies.
Acknowledgments
We thank Krystyna Maszewska and Wielund Meyer for confirming the identification of C. dubliniensis isolates by PCR fingerprinting, Karen Byth for advice with statistical analysis, Harsha Sheorey for providing cases at the nonparticipating adult hospital, and Catriona Halliday for critically reading the manuscript. The Australian Candidemia Study thanks all infectious diseases physicians, clinical microbiologists, and hospital scientists who contributed to the study.
The Australian Candidemia Study consists of the following members: Queensland: Cairns Base Hospital (J. McBride); Calboolture Hospital (C. Coulter); Mater Adult Hospital (J. McCormack, K. Walmsley); Princess Alexandra Hospital (D. Looke, B. Johnson, G. Nimmo, G. Playford); Queensland Medical Laboratories (D. Drummond, R. Forgan-Smith); Rockhampton Hospital (E. Preston); Royal Brisbane Hospital (A. Allworth, J. Faoagali, N. Gerns); Sullivan and Nicolaides Pathology (J. Botes, S. Cherian, J. Robson, R. Vohra); Townsville Hospital (R. Norton); The Prince Charles Hospital (C. Coulter, G. O'Kane); New South Wales: Albury Base Hospital (D. Robb); Concord Hospital (T. Gottlieb); Douglass Hanly Moir Pathology (I. Chambers); Gosford Hospital (D. DeWit); Griffith Hospital Base (M. Carroll); Hunter Area Pathology Service (P. Dobson, J. Ferguson, S. Graves, L. Tierney); Liverpool Hospital (F. Jozwiak, R. Munro, V. Tomasotos); Manning Base Hospital (R. Pickles); Mayne Health (J. Holland); Narrabri District Hospital (F. Groenwald); New Children's Hospital (K. Hale, M. Watson); Orange Base Hospital (R. Vaz); Prince of Wales Hospital (R. Hardiman, C. Baleriola, S. Ryan); Royal North Shore Hospital (R. Pritchard, K. Weeks); Royal Prince Alfred Hospital (R. Benn, N. Adams); St. George Hospital (R. Lawrence, P. Taylor); St. George Private Hospital (S. Lindstrom); St. Vincent's Private and St. Vincent's Public Hospital (J. Harkness, D. Marriott, Q. Nguyen); Sydney Children's Hospital (P. Palasanthrian); Sydney Adventist Hospital (R. Grant, R. McPetrie); Wagga Wagga Base Hospital (R. Johnson); Westmead Hospital (S. Chen, C. Halliday, K. Maszewska, O.C. Lee, W. Meyer, T. Sorrell); Wollongong Hospital (N. Dennis, P. Newton); Victoria: Alfred Hospital (C. Franklin, O. Morrisey, M. Slavin, D. Spelman, S. Wesselingh); Austin and Repatriation Hospital (B. Speed); Bendigo Health Care Group (J. Hellsten, B. Mayall, J. Russell); Frankston Hospital (S. Broughton, I. Woolley); Melbourne Pathology (S. Coloe); Melbourne Private Hospital (A. Sherman); Monash Medical Centre (T. Korman); PathCare Consulting Pathologists (S. Graves); Peter MacCallum Cancer Institute (M. Slavin, M. Huysmans); Royal Melbourne Hospital (M. Slavin, A. Sherman); South Australia: Flinders Medical Centre (D. Gordon); Royal Adelaide Hospital (K. Rowlands, D. Shaw, W. Ferguson); Women's and Children's Hospital (D. Ellis, B. Ritchie, R. Handke); Western Australia: Fremantle Hospital (M. Beaman, A. Chiam, J. McCarthy); Royal Perth Hospital (C. Heath); Sir Charles Gairdner Hospital (S. Altmann, I. Arthur, D. Speers); Tasmania: Launceston General (E. Cox); Royal Hobart Hospital (L. Cooley, A. McGregor); Northern Territory: Royal Darwin Hospital (B. Currie, G. Lum, D. Fisher); Australian Capital Territory: The Canberra Hospital (P. Collignon, J. Roberts, A. Watson).
The study was supported by a grant from Pfizer Inc. Tania Sorrell was supported by grant no. 264625 from the National Health Medical Research Council of Australia.
Biographies
Monica Slavin, Deborah Marriott, David Ellis, and Tania Sorrell are or have been on antifungal advisory boards of Gilead Sciences, Pfizer Australia, and Merck Sharp and Dohme, Australia; Monica Slavin, David Ellis, and Tania Sorrell are on the antifungal advisory board of Schering Plough; Monica Slavin is on the antifungal advisory board of Bristol-Myers Squibb; Tania Sorrell is on the advisory board of Pfizer Global; and Sharon Chen is a member of the antifungal advisory board of Gilead Sciences and Pfizer.
Dr Chen is a senior staff specialist in infectious diseases and microbiology at the Centre of Clinical Research Excellence in Infections and Bioethics in Haematological Malignancies, Centre for Infectious Diseases and Microbiology, Westmead Hospital. Her major research interests are clinical and molecular epidemiology of invasive fungal infections, pathogenesis of fungal infections, and development of new diagnostic approaches in medical mycology.
Footnotes
Suggested citation for this article: Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, Ellis D, et al. Active surveillance of candidemia, Australia. Emerg Infect Dis [serial on the Internet]. 2006 Oct [date cited]. http://dx.doi.org/10.3201/eid1210.060389
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial blood stream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–44. 10.1086/520192 [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent S, Seifert H, Wenzel R, Edmond M. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 3.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. 10.1016/S1473-3099(03)00801-6 [DOI] [PubMed] [Google Scholar]
- 4.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–5. 10.1001/archinte.148.12.2642 [DOI] [PubMed] [Google Scholar]
- 5.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–7. 10.1086/378745 [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–9. 10.1086/496922 [DOI] [PubMed] [Google Scholar]
- 7.Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, et al. Incidence of blood stream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27. 10.1128/JCM.42.4.1519-1527.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchetti O, Bille J, Fluckiger U, Eggiman P, Ruef C, Garbino J, et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis. 2004;38:311–20. 10.1086/380637 [DOI] [PubMed] [Google Scholar]
- 9.Sandven P. Epidemiology of candidemia. Rev Iberoam Micol. 2000;17:73–81. [PubMed] [Google Scholar]
- 10.Bille J, Marchetti O, Calandra T. Changing face of health-care associated fungal infections. Curr Opin Infect Dis. 2005;18:314–9. 10.1097/01.qco.0000171924.39828.fb [DOI] [PubMed] [Google Scholar]
- 11.Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, et al. The epidemiology of candidemia in two United States cities: results of population-based active surveillance. Clin Infect Dis. 1999;29:1164–70. 10.1086/313450 [DOI] [PubMed] [Google Scholar]
- 12.Poikonen E, Lyytokainen O, Anttila VJ, Ruutu P. Candidemia in Finland, 1995–1999. Emerg Infect Dis. 2003;9:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43:1829–35. 10.1128/JCM.43.4.1829-1835.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slavin MA; Australian Mycology Interest Group. The epidemiology of candidemia and mould infections in Australia. J Antimicrob Chemother. 2002;49(Suppl 1):3–6. [DOI] [PubMed] [Google Scholar]
- 15.National Centre for Classification in Health. International statistical classification of disease and related health problems. 10th revision. Australian modification (ICD-10-AM). Sydney: The Centre, University of Sydney; 1998. [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 17.Australian Government Department of Health and Aging. Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting. 2004. [cited 2006 Jul 10]. Available from http://www.icg.health.gov.au
- 18.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 19.Warren N, Hazen K. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray RP, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. Washington: American Society for Microbiology Press; 1999. p. 1184–99. [Google Scholar]
- 20.Kurtzman CP, Fell JW. The yeasts, a taxonomic study. 4th ed. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- 21.Meyer W, Maszewska K, Sorrell T. PCR fingerprinting: a convenient molecular tool to distinguish between Candida dubliniensis and Candida albicans. Med Mycol. 2001;39:185–93. [DOI] [PubMed] [Google Scholar]
- 22.Australian Institute of Health and Welfare. Australian hospital statistics 2003–2004. AIHW catalog no. HSE 37. Canberra: AIHW (Health Services Series no.23). 2005. [cited 2006 Jul 10]. Available from http://www.aihw.gov.au
- 23.Australian Bureau of Statistics. Population by age and sex, Australian states and territories. Canberra: The Bureau; 2004. [Google Scholar]
- 24.Asmundsdottir LR, Erlendsdottir H, Gottfredson M. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J Clin Microbiol. 2002;40:3489–92. 10.1128/JCM.40.9.3489-3492.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diekema DJ, Messer SA, Brueggemnaa AB, Coffman SL, Doern GV, Herwaldt LA, et al. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002;40:1298–302. 10.1128/JCM.40.4.1298-1302.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, et al. Epidemiology of candidemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital–based surveillance study. Eur J Clin Microbiol Infect Dis. 2004;23:317–22. 10.1007/s10096-004-1103-y [DOI] [PubMed] [Google Scholar]
- 27.Ostrosky-Zeichner L. New approaches to the risk of Candida in the intensive care unit. Curr Opin Infect Dis. 2003;16:533–7. 10.1097/00001432-200312000-00004 [DOI] [PubMed] [Google Scholar]
- 28.Pasqualotto AC, Nedel WL, Machado TS, Severo LC. A comparative study of risk factors and outcome among outpatient-acquired and nosocomial candidemia. J Hosp Infect. 2005;60:129–34. 10.1016/j.jhin.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 29.Cano MV, Perz JF, Craig AS, Liu M, Lyon GM, Brandt ME, et al. Candidemia in pediatric outpatients receiving home total parenteral nutrition. Med Mycol. 2005;43:219–25. 10.1080/13693780410001731592 [DOI] [PubMed] [Google Scholar]
- 30.Rex JH, Pfaller MA, Barry AL, Nelson PW, Webb CD. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as standard treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodey GP, Mardini M, Hanna HA, Boktur M, Abbas J, Girgaway E, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med. 2002;112:380–5. 10.1016/S0002-9343(01)01130-5 [DOI] [PubMed] [Google Scholar]
- 32.Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–89. 10.1086/380796 [DOI] [PubMed] [Google Scholar]