Abstract
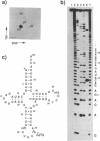
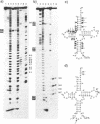
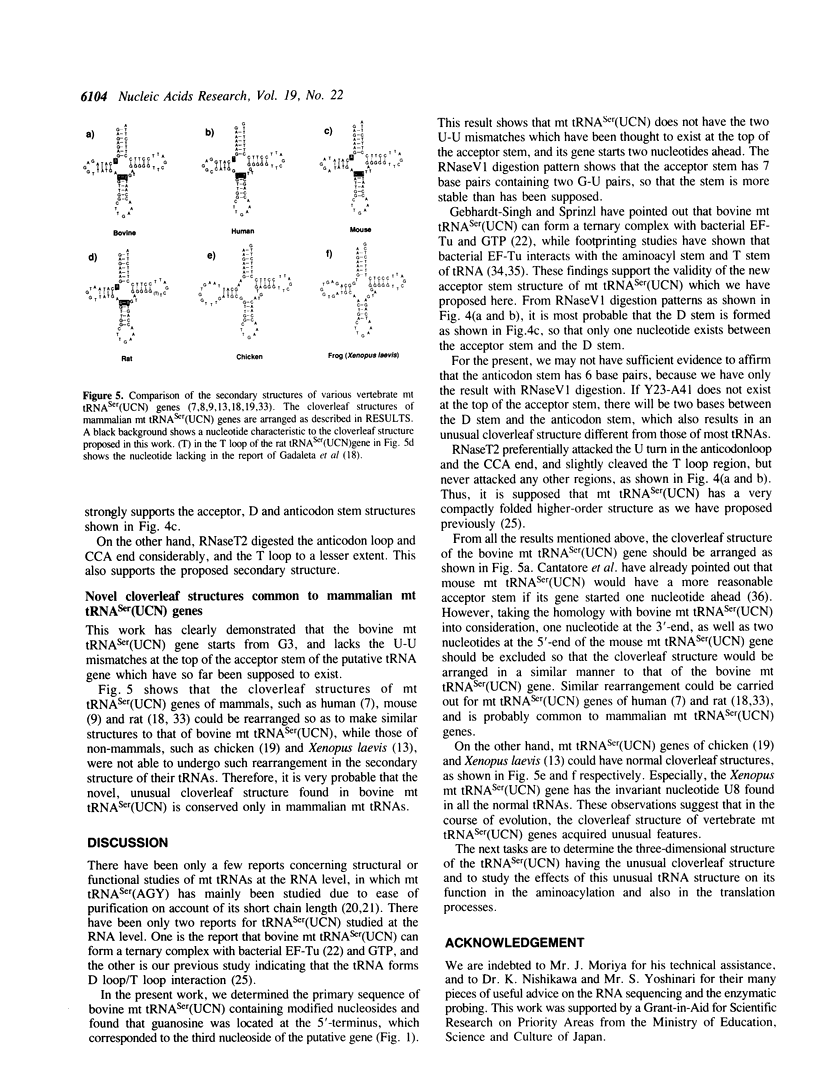
Bovine mitochondrial tRNA(Ser) (UCN) has been thought to have two U-U mismatches at the top of the acceptor stem, as inferred from its gene sequence. However, this unusual structure has not been confirmed at the RNA level. In the course of investigating the structure and function of mitochondrial tRNAs, we have isolated the bovine liver mitochondrial tRNA(Ser) (UCN) and determined its complete sequence including the modified nucleotides. Analysis of the 5'-terminal nucleotide and enzymatic determination of the whole sequence of tRNA(Ser) (UCN) revealed that the tRNA started from the third nucleotide of the putative tRNA(Ser) (UCN) gene, which had formerly been supposed. Enzymatic probing of tRNA(Ser) (UCN) suggests that the tRNA possesses an unusual cloverleaf structure with the following characteristics. (1) There exists only one nucleotide between the acceptor stem with 7 base pairs and the D stem with 4 base pairs. (2) The anticodon stem seems to consist of 6 base pairs. Since the same type of cloverleaf structure as above could be constructed only for mitochondrial tRNA(Ser) (UCN) genes of mammals such as human, rat and mouse, but not for those of non-mammals such as chicken and frog, this unusual secondary structure seems to be conserved only in mammalian mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Hieter P. A., Levy C. C. Identification of a cytidine-specific ribonuclease from chicken liver. J Biol Chem. 1980 Mar 10;255(5):2160–2163. [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Cantatore P., De Benedetto C., Gadaleta G., Gallerani R., Kroon A. M., Holtrop M., Lanave C., Pepe G., Quagliariello C., Saccone C. The nucleotide sequences of several tRNA genes from rat mitochondria: common features and relatedness to homologous species. Nucleic Acids Res. 1982 May 25;10(10):3279–3289. doi: 10.1093/nar/10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Saccone C., Gadaleta M. N. Clustering of tRNA genes in Paracentrotus lividus mitochondrial DNA. Curr Genet. 1988;13(1):91–96. doi: 10.1007/BF00365762. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. A cluster of six tRNA genes in Drosophila mitochondrial DNA that includes a gene for an unusual tRNAserAGY. Nucleic Acids Res. 1984 Mar 12;12(5):2367–2379. doi: 10.1093/nar/12.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin D. T., HsuChen C. C., Cleaves G. R., Timko K. D. Sequence and structure of a serine transfer RNA with GCU anticodon from mosquito mitochondria. J Mol Biol. 1984 Jun 25;176(2):251–260. doi: 10.1016/0022-2836(84)90423-6. [DOI] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Gebhardt-Singh E., Sprinzl M. Ser-tRNAs from bovine mitochondrion form ternary complexes with bacterial elongation factor Tu and GTP. Nucleic Acids Res. 1986 Sep 25;14(18):7175–7188. doi: 10.1093/nar/14.18.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno H., Masaki H., Kawai T., Ohta T., Kumagai I., Miura K., Watanabe K. Unusual genetic codes and a novel gene structure for tRNA(AGYSer) in starfish mitochondrial DNA. Gene. 1987;56(2-3):219–230. doi: 10.1016/0378-1119(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Nishimura S. Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol. 1987;155:379–396. doi: 10.1016/0076-6879(87)55026-1. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Yokogawa T., Hasegawa E., Miura K., Watanabe K. The aminoacylation of structurally variant phenylalanine tRNAs from mitochondria and various nonmitochondrial sources by bovine mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1989 Aug 5;264(22):13005–13011. [PubMed] [Google Scholar]
- Levy C. C., Karpetsky T. P. The purification and properties of chicken liver RNase: An enzyme which is useful in distinguishing between cytidylic and uridylic acid residues. J Biol Chem. 1980 Mar 10;255(5):2153–2159. [PubMed] [Google Scholar]
- Lockard R. E., Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981 Oct 10;9(19):5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Wolstenholme D. R. A set of tRNAs that lack either the T psi C arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990 Oct;9(10):3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Kim S. H. The three-dimensional structure of transfer RNA. Sci Am. 1978 Jan;238(1):52–62. doi: 10.1038/scientificamerican0178-52. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Ueda T., Ohta T., Watanabe K. Large scale isolation and some properties of AGY-specific serine tRNA from bovine heart mitochondria. J Biochem. 1985 Nov;98(5):1275–1284. doi: 10.1093/oxfordjournals.jbchem.a135394. [DOI] [PubMed] [Google Scholar]
- Vary C. P., Vournakis J. N. RNA structure analysis using T2 ribonuclease: detection of pH and metal ion induced conformational changes in yeast tRNAPhe. Nucleic Acids Res. 1984 Sep 11;12(17):6763–6778. doi: 10.1093/nar/12.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Clary D. O. Sequence evolution of Drosophila mitochondrial DNA. Genetics. 1985 Apr;109(4):725–744. doi: 10.1093/genetics/109.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T., Kumazawa Y., Miura K., Watanabe K. Purification and characterization of two serine isoacceptor tRNAs from bovine mitochondria by using a hybridization assay method. Nucleic Acids Res. 1989 Apr 11;17(7):2623–2638. doi: 10.1093/nar/17.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire 'dihydrouridine' loop and stem. EMBO J. 1983;2(8):1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]