Abstract
Cocaine is highly addictive and there are no pharmacotherapeutic drugs available to treat acute cocaine toxicity or chronic abuse. Antagonizing an inhibitor such as cocaine using a small molecule has proven difficult. The alternative approach is to modify cocaine’s pharmacokinetic properties by sequestering or hydrolyzing it in serum and limiting access to its sites of action. We took advantage of a bacterial esterase (CocE) that has evolved to hydrolyze cocaine and have developed it as a therapeutic that rapidly and specifically clears cocaine from the subject. Native enzyme was unstable at 37°C, thus limiting CocE’s potential. Innovative computational methods based on the protein’s structure helped elucidate its mechanism of destabilization. Novel protein engineering methodologies were applied to substantially improve its stability in vitro and in vivo. These improvements rendered CocE as a powerful and efficacious therapeutic to treat cocaine intoxication and lead the way towards developing a therapy for addiction.
Cocaine is a tropane alkaloid produced by the South American plant Erythroxylon coca. Cocaine elicits its effects by binding to a myriad of sites in the human body. Cocaine’s psychological effects are induced via binding mainly to monoamine neurotransmitter reuptake transporters in the presynaptic nerve termini and blocking them, leading to prolonged presence of neurotransmitters in the synapse. Prolonged presence of dopamine and serotonin in the synapse results in feelings of euphoria and wellbeing [1–3]. Chronic and prolonged blockade of dopamine transporters can lead to reinforcement of self-administration and, therefore, to various forms of addiction [1]. Binding to noradrenergic transporters results in elevated noradrenergic signaling, which accounts for increased heart rate (HR), blood pressure and vasoconstriction seen in cocaine users [1–4]. Cocaine also binds cardiac and neuronal sodium channels leading to altered cardiac conductivity and its anesthetic effects, respectively [5].
Cocaine abuse is a serious public health problem. Emergency department (ED) visits due to cocaine abuse lead the charts among all other drugs. In 2008, cocaine was involved in 482,188 in a total of 993,379 ED visits involving an illicit drug (48.5%) [6]. Cocaine remains the most co-abused drug along with alcohol, again leading all other illicit drugs. It is estimated that there are still approximately 2 million Americans that self-report being cocaine users, although the actual number is most likely higher according to recent studies [101]. A total of 15% of Americans have tried cocaine and 6% of high school seniors have used the drug [101].
Despite being a highly addictive and widely abused drug, there is no US FDA-approved medication for the treatment of cocaine abuse or toxicity. ED physicians treat patients presenting with cocaine toxicity symptoms with standard emergency room agents that control arrhythmias, convulsions and high blood pressure but not with drugs that directly address the toxic levels of cocaine present in the patient still. Symptoms presented due to cocaine intoxication are similar to amphetamine overdose symptoms and emergency room clinicians have to solve this conundrum before utilizing drugs that rapidly lower cocaine levels in these patients.
Small-molecule discoveries & approaches
The discovery of a small-molecule antidote for cocaine has been hampered by the fact that compounds that compete with an inhibitor such as cocaine are likely to be inhibitors themselves. Approaches for treating cocaine abuse were attempted with agonists to replace cocaine [7], antagonists to block cocaine at the site of action [8,9] and modulators of cocaine, aimed at altering the effects of cocaine by acting at sites other than monoamine transporters [10–13]. Drugs targeting GABAergic and dopaminergic pathways are being extensively tested for treatments for cocaine abuse. GABAergic drugs, such as baclofen, topiramate and vigabatrin, which increase GABAergic activity both directly or indirectly reduced cocaine intake and increased cocaine-negative urine screens [14–17]. GABA release inhibitor, tigabine, also surprisingly decreased cocaine use [18]. Dopamine uptake inhibitor buproprion showed mixed results in human clinical trials [19,20], while antagonists of dopamine D2 receptor and serotonin 2A receptor risperidone and olanzapine showed promise in schizophrenic cocaine users but not in normal cocaine users [21–24]. Aripiprazole, a dopamine D2 and serotonin 1a receptor partial agonist, blocked cocaine self administration in mice [25] and a Phase II clinical trial is underway. Disulfiram, an agent used for chronic alcohol abuse treatment has shown a lot of potential in reducing cocaine use when used in low doses [26]. In spite of these varied attempts, cocaine’s myriad sites of action has challenged development of safe and specific pharmacodynamic-based small-molecule agents as therapeutics against cocaine (Table 1).
Table 1.
Pros and cons of various therapeutic approaches to alleviate cocaine abuse.
| Therapeutic approach | Pros | Cons |
|---|---|---|
| Small molecules | ||
| ▪ GABAergic drugs | ||
| – Baclofen and toporimate | Reduced cocaine intake | Drowsiness and depression |
| ▪ Dopaminergic drugs | ||
| – Bupropion | Already in the market for other indications | Negative results in human trials |
| – Disulfiram | Reduced cocaine intake | Mixed results in human trials |
| – Risperidone | Promising results in schizophrenics and co-morbid users | Non-schizophrenics failed to respond |
| – Aripiprazole | Acute treatment in humans was promising | Chronic treatment increased self-administration |
| ▪ Sertonergic drugs | ||
| – Selective serotonin reuptake inhibitors | Failed in controlled trials | |
| – 5HT3 antagonist | 10-week trial showed reduced cocaine intake | |
| Catalytic antibodies (monoclonal antibody 1510) | Monoclonal antibody 1510 showed efficacy in rodents by blocking the toxic and reinforcing effects of cocaine | Could be easily overcome by increasing the cocaine dose and the antibody was found to non-effective in humans |
| Vaccines (TA-CD) | Safe and reduced cocaine intake. In clinical Phase IIb trials | Highly variable antibody response. Only 40% of the patients showing high levels of antibody |
| Enzyme therapy | ||
| – Recombinant BchE | ‘Self’ protein and, hence, safe | Difficult to express. Does not last long enough in vivo |
| – Bacterial CocE | Easy to express/purify. Highly active and found safe in preliminary studies | Foreign protein. Does not last long enough in vivo |
Protein-based therapeutic approaches (biologics)
Pharmacokinetics-based approach of targeting cocaine molecule peripherally and changing its distribution or speeding up clearance has been gaining some momentum in recent years. Moving away from small-molecule discovery, researchers have focused on protein therapeutics to achieve this goal. Protein-based therapy to treat diseases has gained traction in recent years as there are many advantages for protein-based therapeutics. Protein drugs are highly specific with limited side effects, thereby safer and can perform complex tasks with high efficiency. Approaches that use protein biologics to sequester cocaine in the serum (antibodies) or hydrolyze cocaine in the serum before it reaches its site of action (enzymes or catalytic antibodies) have shown enough initial promise to continue research in this exciting avenue. Protein biologic agents cannot pass through the blood–brain barrier to enter the CNS, thereby reducing off-target effects in the CNS, which is a major concern for small-molecule therapeutics.
Whether using small-molecule agents or protein biologics to rapidly clear the elevated cocaine levels seen in cocaine overdosed patients, ED physicians should first identify the exact cause of symptoms because both cocaine and other stimulants like amphetamines present similar set of symptoms. Time permitting, ED physicians can offer such patients with a lower diagnostic dose of the cocaine-lowering drug as a tool to assess the underlying cause. We hypothesize that a cocaine-lowering drug or antidote even at a lower dose can elicit a response in a patient overdosing on cocaine, thereby allowing the physician to understand the cause of the patient’s symptoms.
Therapeutic antibodies & vaccination strategies
Passive immunization with monoclonal antibodies (mAbs) developed against cocaine reduced cocaine self-administration in rats [27,28] and blocked cocaine toxicity in overdose models [29]. Active immunization with cocaine conjugates resulted in the development of cocaine antibodies capable of sequestering cocaine in the periphery. The antibody titers were sufficient to block reinstatement induced by a single dose of drug, but this protective effect was overcome with either repeated cocaine dosing or increasing the dose [30,31]. The most effective vaccine to date has been against BSA- or cholera toxin B-conjugated norcocaine (TA-CD) [27,28] and its success in eliciting good antibody titers has resulted in the initiation of clinical trials in humans [32,33]. Reports from the clinical trials indicate that antibody response from this vaccine was usually delayed by 6 weeks, the response highly variable and antibody levels dropped off by 12 weeks [34,35]. Catalytic mAb, raised in mice immunized with transition state analogs of cocaine hydrolysis, not only sequesters cocaine but can also hydrolyze cocaine. However, the mAbs were not therapeutically active for more than 72 h post administration [36–38]. Other than TA-CD, none of the other immunotherapeutic-based biologics have shown promise.
Cocaine-hydrolyzing enzymes
Extensive work has gone into developing faster acting enzymes that can hydrolyze cocaine more rapidly. Cocaine-hydrolyzing enzymes have a distinct advantage over passive immunization strategies or cocaine vaccines. Slow dissociation rates found in antigen–antibody complexes in the order of seconds to minutes [39], mean rapid depletion of free antibodies to sequester free cocaine remaining in the serum. Whereas, highly efficient enzymes can bind and degrade cocaine at a very high rate, thereby eliminating the problem of slow off rates seen with antibodies. Since an antibody, typically a Fab fragment, can only bind one molecule of cocaine stoichiometric amounts of antibodies are required to ensure complete drug sequestration. In overdose situations, the concentrations of antibodies can and will be easily overcome, thereby resulting in ineffective therapy [30,31].
Butyrylcholinesterase
Cocaine is hydrolyzed primarily, albeit very slowly, by butyrylcholinesterase (BchE) in humans and delivery of exogenous BchE is a good strategy to accelerate its metabolism systemically [40]. A highly active BchE variant, which has a 1730-fold improvement in catalytic efficiency over wild-type (wt)-BchE in hydrolyzing the active stereoisomer of cocaine ((−)-cocaine), was shown to effectively protect mice from lethal doses of cocaine [41–43]. Since the BchE gene and function is closely related to acetyl cholinesterase, off-target hydrolysis, especially of acetylcholine by the presence of excess exogenous highly active BchE variants, is a concern. To circumvent this issue, a computational modeling approach was employed by Xue and colleagues to address the specificity issue and they identified a pentamutant variant of BchE, which hydrolyzes acetylcholine less efficiently than wt-BchE [42]. Other issues with exogenous BchE-based therapy include possible neurodegenerative disorders, since elevated BchE expression and protein are seen in neurodegenerative disorders [44] and Alzheimer’s patients [45], and the presence of BchE in β-amyloid plaques, which has been shown to increase toxicity of β-amyloid [46]. In addition, the highly laborious process of producing BchE is a major hindrance in successful translation of this drug into clinics. Efforts such as expressing BchE in transgenic goat’s milk or plants have alleviated some of the production issues [47,48], however, cost–ineffectiveness of creating transgenic animals and plants are issues that need to be overcome before BchE becomes a viable option. Meanwhile, our group and collaborators have focused on a bacterial enzyme cocaine esterase (CocE) that can get the field past the known hurdles.
Bacterial CocE
CocE isolated from Rhodococcus strain of bacteria, which grows in the rhizosphere soil surrounding coca plants, remains the fastest natural enzyme that can hydrolyze cocaine [49,50]. Similar to BchE, CocE cleaves cocaine at the benzoyl ester bond on cocaine to release ecgonine methyl ester and benzoic acid. The CocE gene has been cloned and it can be expressed in Escherichia coli cells in large quantities. CocE is expressed in the cytosol and contains 574 residues. Its crystal structure has been solved; it is a globular protein containing three domains. It belongs to α/β hydrolase superfamily of proteins and it is one of the largest protein families containing many lipases, esterases, amidases, epoxide hydrolases, dehalogenases and hydroxynitrile lyases [50,51]. Domain I is the α/β hydrolase fold-containing domain, domain II is mainly α-helical containing seven helices with helix 2 and 3 packs antiparallel and forms a lidlike structure over the active site and domain III has a jelly roll-like topology [50].
Native CocE cleaves cocaine with 800-times more efficiency than wt-BchE. In a rodent model for acute cocaine toxicity, CocE was able to induce a ten-fold shift in cocaine dose–effect curve, unlike any of the other cocaine hydrolyzing enzymes tested [52]. Intravenous injection of wt-CocE (1 mg) protected 100% of rats receiving intraperitoneal cocaine (180 mg/kg) but 13-mg intravenous injection of wt BchE, a tenfold higher molar equivalent dose of CocE failed to protect rats from cocaine-induced lethality. But wt-CocE was shown to be rapidly cleared in vivo with a half-life of 12.2 min [52]. Rapid clearance was shown to be due to thermolability since wt-CocE had an in vitro half-life of only 11 ± 0.9 min at 37°C [52,53].
This lack of stability at physiological temperature is a roadblock to developing CocE into a therapy for cocaine toxicity and making further improvements to its stability that are essential for an addiction indication. Thermostabilization of proteins using protein engineering has been employed successfully to biocatalysts and enzymes [54] and techniques that use directed evolution, such as B-FIT [55] and rational computational design [56], have been successfully employed to thermostabilize proteins.
Rational protein engineering of CocE
CocE was subjected to molecular dynamic simulation [53], where the simulation was performed at 575 K and root-mean-square deviation values for the movement of all atoms were calculated. Movement as time increased was progressively larger for the whole protein but especially for domain II atoms, whose root-mean-square deviation values were 9 Å. Thus, computational modeling predicted that domain II is the least stable region in CocE and it was speculated that disruptions in this region at higher temperatures would lead to loss of structural integrity and activity. Domain II was, therefore, targeted for mutations that would stabilize the protein at higher temperatures. Two approaches were used to select mutations: one calculated the change in interaction energy between mutated residue and the rest of the protein and the second calculated the folding energy of the mutant based on the Rosetta Design program. Mutations were considered valid if they were predicted by both approaches [53].
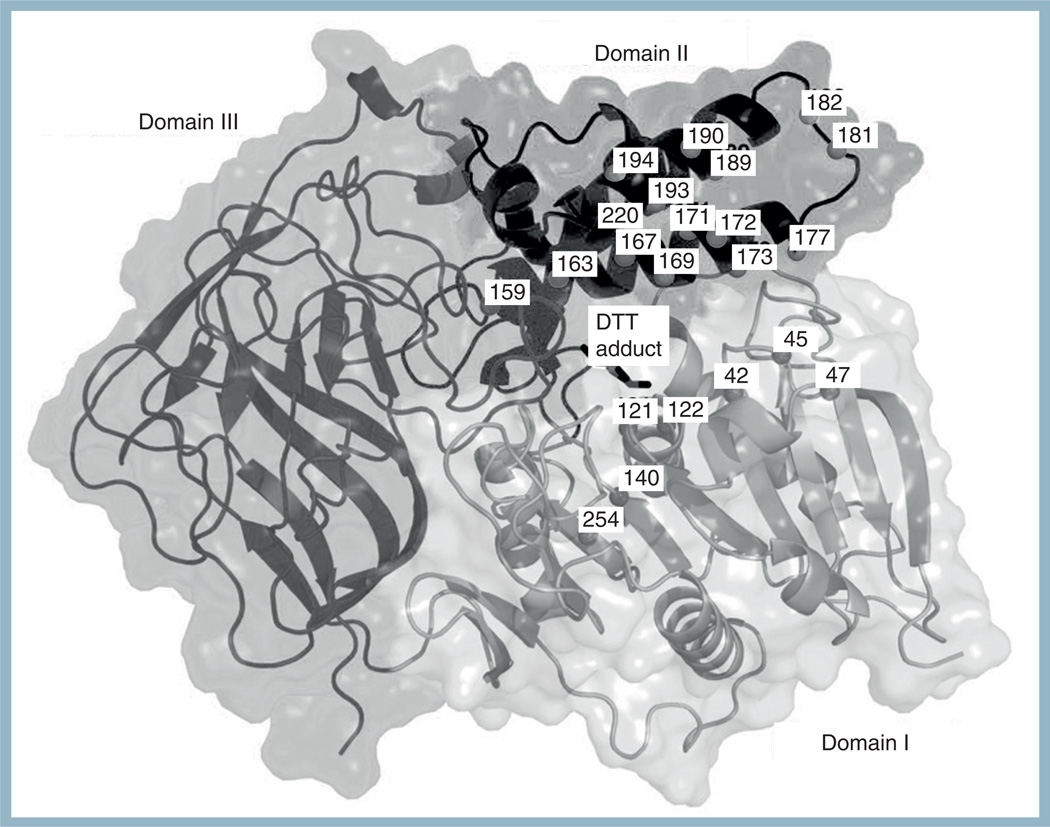
Based on a rational computational approach, 27 mutations were predicted and are listed as follows: N42V, D45R, F47K, F47R, W52L, V121D, T122A, Q123E, S159A, L163V, S167A, L169K, G171A, T172R, G173A, G173Q, L174R, S177Q, S179R, R182K, F189A, F189L, F189K, A193D, A194K, W220A and T254R [57]. These were predicted to either improve domain II stability by increasing either intra- or interdomain contacts with domain I. Locations of some these mutations are shown in Figure 1.
Figure 1. Structure of CocE.
CocE is composed of three distinct structural domains (domains I–III). Domain I contains the catalytic S117 and adopts a canonical α-/β-hydrolase fold. The locations of point mutations predicted by computational methods and tested for in vitro activity are shown as dark spheres. A DTT-carbonate adduct (DBC) is evident in all structures that contain DTT.
Study of thermostable CocE mutants
All these mutants and some combinations thereof were cloned, expressed and purified from E. coli. Of all the mutations tested, only T172R, G173Q and L169K showed significant in vitro stability at 37°C compared with wt-CocE. T172R had a 78-min half-life (τ1/2) at 37°C, whereas G173Q and L169K displayed half-lives of 75 and 403 min, respectively [53,57]. The G173Q mutant did not have any deleterious effects on the catalytic efficiency, T172R and the T172R-G173Q double mutant showed a threefold increase in Km compared with that of wt-CocE, whereas L169K exhibited an eightfold increase in Km [57].
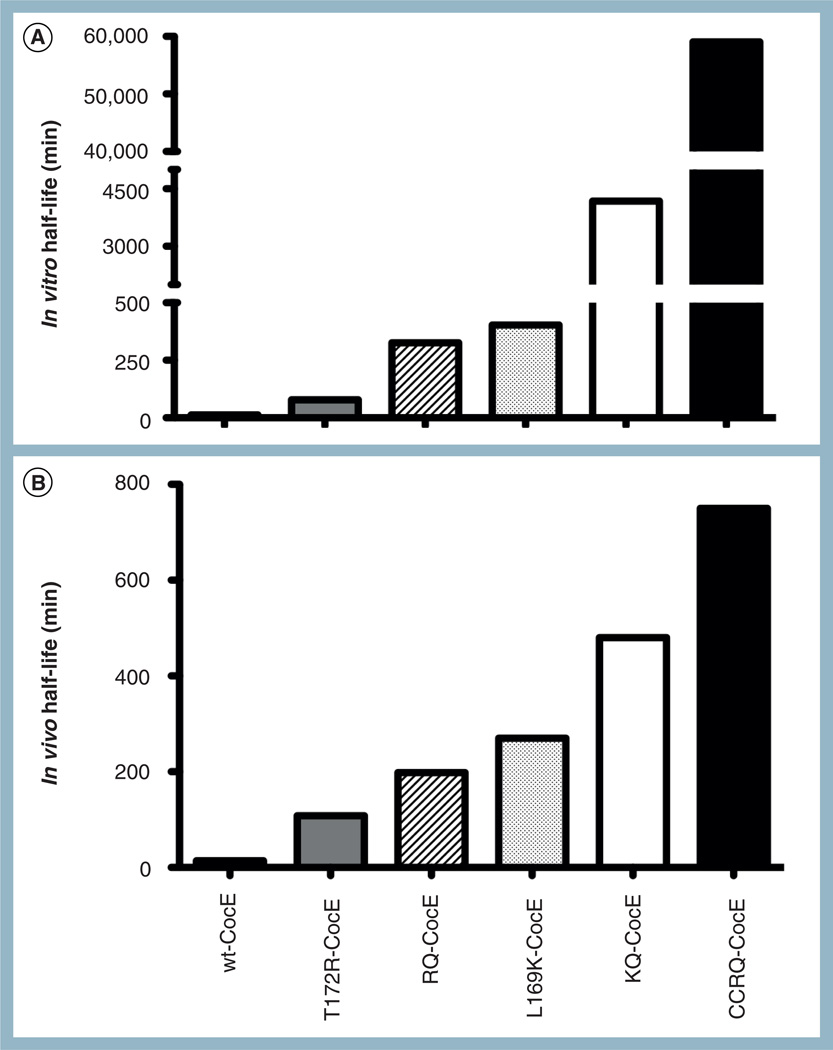
Combining the mutations in the form of double mutants T172R–G173Q (RQ-CocE) and L169K–G173Q (KQ-CocE) synergistically enhanced the stability of CocE with the observed τ1/2 of RQ-CocE being 326 min and for KQ-CocE being 2.9 days at 37°C (Figure 2A) [53,57,58]. Unfortunately, combining all three mutations resulted in an enzyme with no measurable activity and the alternate double-mutant combination T172R–L169K had a 5.6-fold loss in catalytic efficiency as compared with wt-CocE [57].
Figure 2. Comparison of in vitro and in vivo half-lives of wild-type CocE and stable mutants.
(A) For in vitro half-life calculation, decay in the capacity to convert cocaine to ecgonine methyl ester and benzoic acid was measured at 37°C. 50 ng/ml of wt-CocE, T172R-CocE, RQ-CocE, L169K CocE, KQ-CocE or CCRQ-CocE were incubated at 37°C and the activity calculated over time. τ1/2 values were measured from the resulting curves. (B) For in vivo half-life calculation, wt-CocE and the mutants were pre-injected into rodents and at several time points postinjection a lethal dose of cocaine (180 mg/kg) was given, and time taken to reach 50% lethality was calculated and plotted. wt: Wild-type.
CocE mutants displaying enhanced stability at 37°C also display enhanced thermostability as confirmed by a thermal inactivation assay and circular dichroism measurements and upon monitoring protein unfolding using Thermofluor. In a thermal inactivation assay, wt-CocE and its mutants were incubated at various temperatures prior to measurement of activity. wt-CocE exhibited a marked sensitivity to temperature, where its activity plummets precipitously following treatment for 30 min at 30–35°C; whereas, L169K and RQ-CocE are inactivated at higher temperatures (40–45°C) [53]. These data correlate well with circular dichroism measurements of thermal denaturation, which reveals a 3.1–3.5°C increase in denaturation temperature (Tm) for single mutants and a 7.1°C increase for the double mutant RQ-CocE [53]. The trend in temperature-dependent inactivation of wt-CocE and mutants was consistent with the temperature-dependent protein unfolding. Melting temperatures were also predicted by following ANS binding (fluorescence) using a Thermofluor reader. The T172R and G173Q each revealed increases in the melting temperature (Tm) by 3°C over wt-CocE, whereas RQ-CocE and L169K showed a Tm increase of 6°C [57]. Tm values for wt-CocE, RQ-CocE and KQ-CocE were measured by isothermal titration calorimetry and they were 37.62, 44.04 and 46.86°C, respectively [E Edwald, R Sunahara, Unpublished Data].
Structural analysis of thermostable CocE variants
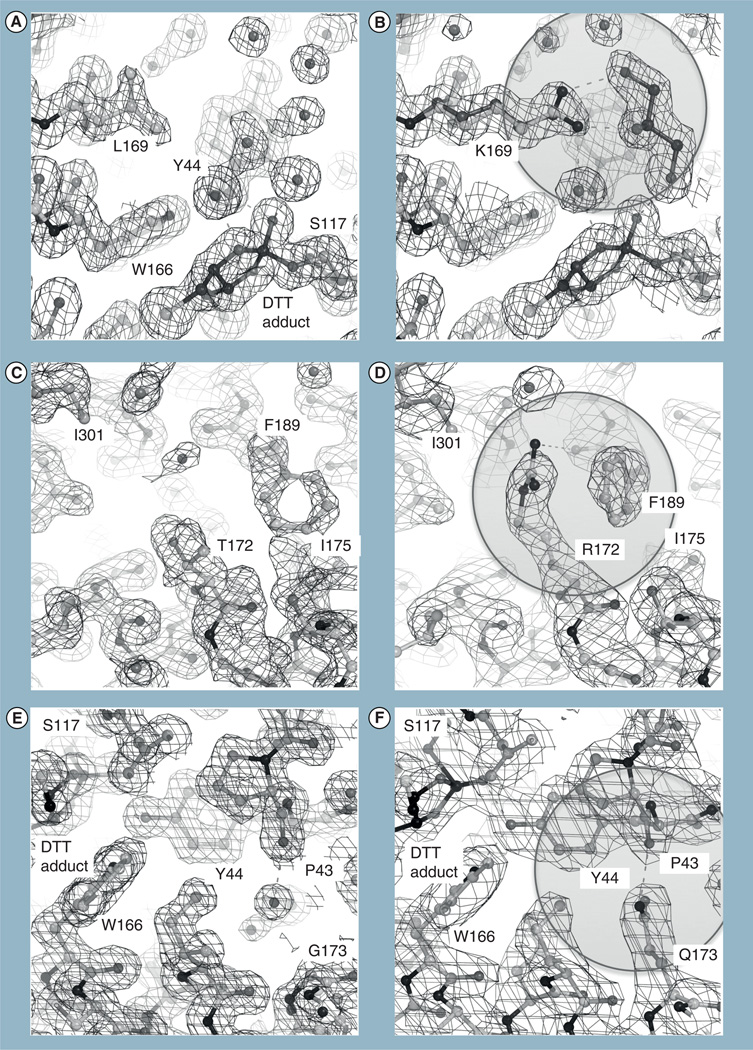
Structural analysis of wt-CocE and the thermostabilized variants mentioned above were performed to provide some insight into the mechanism by which these mutations enhance protein stability [57]. The primary differences in structures of the mutant forms were seen in interactions formed by the mutant side chains and in the conformation of the H2–H3 loop connecting the H2 (residues 159–175) and H3 (184–197) helices of domain II, which exists in two to three distinct alternative conformations. For the T172R mutation, a more extended alkyl chain at position 172 and the addition of a guanidinium moiety introduces van der Waals’ contacts with the aromatic ring of F189 in H3 and a hydrogen bond between the guanidinium moiety to the backbone oxygen of F189 (Figure 3C & D) [57].
Figure 3. Structural analysis of stabilizing CocE mutants.
The crystal structures of wild-type CocE (A, C & E) compared with T172R (B), G173Q (D) and L169K (F). The stabilizing effect of the mutants appears to result from enhanced interactions between the H2 and H3 of domain II or between subunits (T172R substitution), or from additional interdomain contacts (G173Q and L169K). The L169K side chain exhibits two conformations. Note that L169 is poorly ordered in the wild-type CocE structure and its side chain is solvent exposed, which is expected to be destabilizing. A DTT-carbonate adduct (DBC) is evident in all structures that contain DTT.
In the G173Q mutation, the longer side chain of G173Q occupies a small water-filled pocket between domains I and II in wt-CocE. The amide nitrogen of G173Q forms an interdomain hydrogen bond with the backbone carbonyl of P43 in domain I, in good agreement with predictions from molecular dynamic simulations. Interestingly, the analogous proline in Acetobacter turbidans alpha-amino acid ester hydrolase [59], the most similar structure in the PDB, also forms an interdomain hydrogen bond with a glutamine residue in domain II. Additional van der Waals’ contacts are formed by the glutamine side chain both with the main and side chain of Y44, and the side chains of L77, F78 and I170, and these interactions were not predicted by computational approaches (Figure 3E & F) [57].
In L169K, the Nζ atom of L169K is available to hydrogen bond with water molecules or the cryoprotectant, glycerol, in the active site. Modeling suggests that the longer side chain of lysine could sterically interfere with binding of the tropane ring of cocaine, which may account for the slightly higher Km value exhibited by this mutant (Figure 3A & B) [57].
CocE is a dimer
The guanidinium moiety of T172R packs against the side chain of I301 of a twofold crystallographic symmetry-related chain of CocE in the crystal lattice. Subsequent analysis of the lattice packing revealed that many residues from all three domains of CocE are involved in close contacts between these two protein chains, suggesting that CocE is in fact a homodimer. Size exclusion chromatography studies reveal that CocE resolves as a single peak with an apparent molecular weight of 140 kDa; most consistent with that of dimer. Upon heat treatment at 37°C, wt-CocE elutes as a large molecular weight entity in the void volume of the column, which we hypothesize to be an aggregated form of the protein. This aggregate lacks activity and wt-CocE fully aggregates within 1 h of incubation at 37°C, while the thermostable mutant forms take eightfold longer to aggregate [57]. We hypothesized that crosslinking CocE monomers through introducing disulfide linkage(s) at position 4 (G4C) and position 10 (S10C) would stabilize the enzyme and protect against aggregation [60]. G4C and S10C mutations in combination with RQ mutations (CCRQ-CocE) produced a synergistic stabilization effect and yielded an enzyme that retained almost all activity following treatment at 37°C for 41 days [60], which is a 4700-fold improvement over wt-CocE (Figure 2A).
CocE in rodent models of cocaine toxicity
Upon in vivo evaluation of single mutants T172R, G173Q and L169K, and double mutants RQ-CocE and KQ-CocE, it was observed that the improvement in their stability in vitro translated very well in animal models for cocaine toxicity. In this model, rodents were pre-administered with wt-CocE or mutants. At several time points post-enzyme injection, a lethal dose of cocaine was injected and the enzyme’s capacity to provide protection against lethality was evaluated. Pretreatment time that resulted in protection for 50% of animals was arbitrarily assigned to be in vivo half-life of the enzyme. Based on this it was found that wt-CocE, T172R, L169K, RQ-CocE and KQ-CocE had in vivo half-lives of 14 min, 1.8, 3.3, 4.5 and 8 h, respectively (Figure 2B) [57,58]. KQ-CocE showed an approximately 30-fold improvement over wt-CocE. Our longest acting mutant CCRQ-CocE at the highest dose tested (32 mg/kg) protected mice from lethal effects of cocaine no longer than 24 h [60].
The in vitro and in vivo half-lives of KQ-CocE (2.9 days vs 8 h, respectively) and CCRQ-CocE (41 days vs less than 24 h, respectively) appear to not correlate as well as for shorter acting CocE mutants. The observation that wt-CocE, KQ-CocE and RQ-CocE had similar serum half-lives (2.2 h for wt-CocE, 2.3 h for KQ-CocE and 2.4 h for RQ-CocE) by Western blot analysis demonstrating similar elimination rates, strongly suggesting that in vivo elimination may be due to factors beyond thermal instability [58,61].
Protease degradation and glomerular filtration are some of the processes that could shorten serum half-life of a protein biologic. The addition of stealth modification through conjugation with polyethylene glycol conjugation (PEGylation), albumin or other protein engineering methods are available to improve the in vivo half-life of CocE or variants thereof with improved in vitro half-lives [62]. In a preliminary report, Park et al. demonstrated that CocE can be PEGylated and still retain activity both in vitro (~60% of the unPEGylated enzyme) and in vivo. MALDI-TOF analysis of PEGylated CocE (by NHS coupling) revealed that one to four PEG chains were conjugated to each CocE monomer. The efficiency of cysteine-based maleimide-conjugated PEGylation of CocE was similar. PEG-CocE also provided protection against cocaine-induced lethality as efficiently as wt-CocE in mice [63]. Analyses of the in vivo time course were not performed on PEGylated forms of these versions. Expanding this initial report, PEGylation was performed on our longest lasting and the most stable form of CocE, CCRQ-CocE. When PEGylated with a 40-kDa branched PEG via maleimide coupling, PEG-CCRQ-CocE retained all activity both in vitro and in vivo [60]. We hypothesize that the improvement in the retention of full enzymatic activity of PEG-CCRQ-CocE was due to significant improvement of CCRQ-CocE’s stability over wt-CocE.
In the cocaine lethality studies, PEG-CCRQ-CocE at the highest dose tested (32 mg/kg) protected 100% of the animals for over 48 h, that is, pretreatment of animals with PEG-CCRQCocE at time 0 protected animals from lethal doses of cocaine administered at t = 0, 24 and 48 h post PEG-CCRQ-CocE injection [60]. Cocaine challenges with doses administered 72 h post PEG-CCRQ-CocE injection were lethal to approximately 75% of the animals. This dose of PEGylated protein corresponds to approximately 3.0 mg/kg for a typical human with blood volume of approximately 5 l.
CocE & its variants in cocaine self-administration models
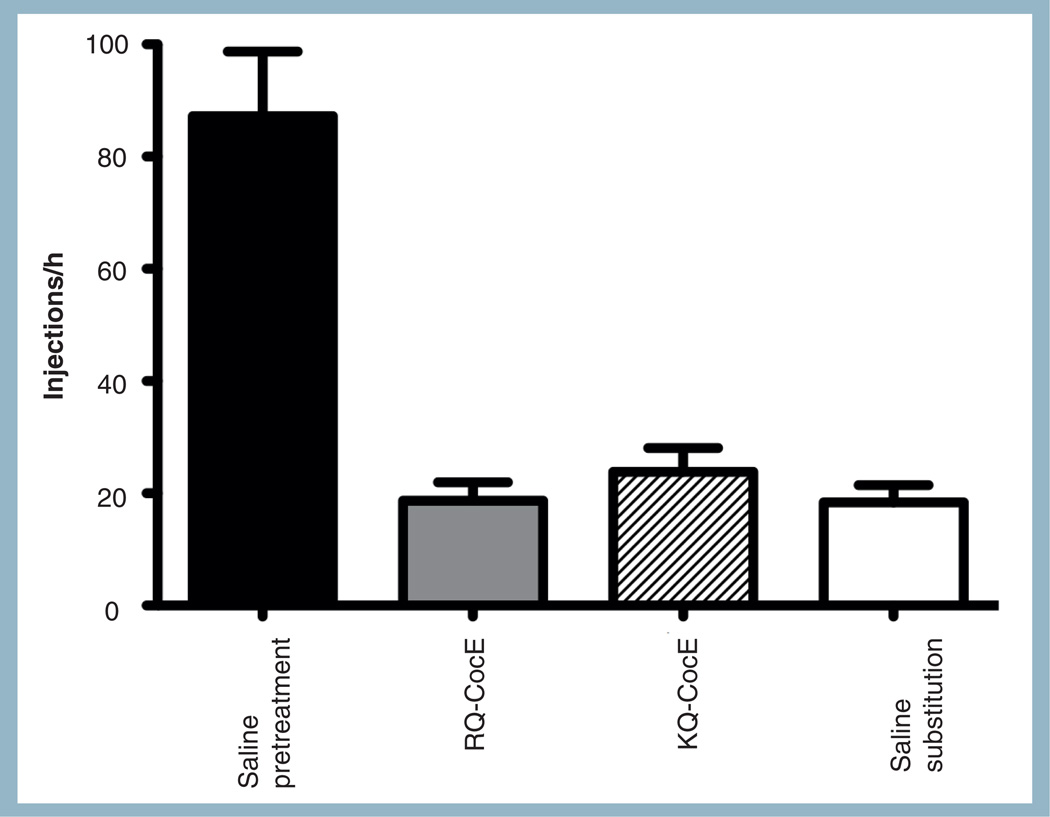
Assessment of drug self-administration in rodents has become an important tool to evaluate a drug candidate’s effect in reducing psychoactive drug use in humans [64]. Rats were trained to respond for 0.1 mg/kg/injection cocaine under a fixed ratio 5 schedule of reinforcement. RQ-CocE had a dose-dependent effect on reinforcing responding. As the dose was increased (0.03–1.0 mg/kg of RQ-CocE), cocaine-reinforced responding was completely eliminated for increasing periods of time [65]. In a similar analysis Brim et al. found that KQ-CocE reduced responding to levels seen when saline was substituted instead of cocaine if the enzyme was given immediately prior to the start of the session (Figure 4) [58]. If the enzyme was administered 1 h prior, the responding was half the level seen with saline. Animals pretreated 2 h with KQ-CocE prior no longer displayed reduced responding. These correlated well with the time course seen in cocaine toxicity assay and the Western blot analysis [58]. A similar effect on dose dependence is also seen in active vaccination trials in humans where significant increase in cocaine intake was seen in patients when circulating antibodies are low [34,66]. A significant improvement in the in vivo half-life of the best CocE candidate, along with increasing its catalytic efficiency, should circumvent this issue. To this effect, PEG-CCRQ-CocE completely inhibited responding that was maintained by doses of cocaine as large as 1.0 mg/kg/injection for greater than 48 h, with a significant antagonism of the reinforcing effects of cocaine apparent for more than 96 h after treatment [60,67].
Figure 4. Cocaine esterase protected against cocaine-reinforced operant responding in Sprague–Dawley rats.
Saline, 1-mg RQ-CocE or KQ-CocE was given as a pretreatment 1 min prior to cocaine self-administration sessions. Rats in the saline substitution condition (open bar) received no cocaine from nose pokes during the session.
Amelioration of cocaine-induced cardiovascular effects by CocE
Cocaine-induced chest pain accounts for approximately 40% of cocaine-related ED visits [68,69]. Therefore, any antidote to combat cocaine abuse has to be tested and evaluated as to how they alleviate cardiovascular related complications arising from cocaine use.
In rodents, acute cocaine toxicity is marked by increases in blood pressure, decreases in HR, respiratory suppression and tonic–clonic convulsions; the latter is associated with status epilepticus and thought to be the primary mechanism responsible for cocaine-induced lethality [70]. Rats administered cocaine (5.6 mg/kg intravenously) display hypertension and bradycardia. Administration of RQ-CocE 1 min prior to this sub-lethal dose of cocaine reversed these effects. Similarly, administrations of RQ-CocE following doses of cocaine that are sufficient to produce convulsions in rats (180 mg/kg intraperitoneally) can completely reverse the neurological and cardiovascular effects in rats [70].
In rhesus monkeys, intravenous administration of cocaine produces dose-dependent increases in mean arterial pressure and HR that eventually return to baseline levels following 0.1, 0.32 and 1 mg/kg cocaine doses. However. persistent increases are observed throughout a 2-h observation period following a dose of 3.2 mg/kg cocaine. Locomotor activity, body temperature and ECG parameters do not change in these monkeys for any of these cocaine doses. Administration of RQ-CocE produces a rapid and dose-dependent amelioration of the cardiovascular effects, with restoration to saline-like levels of mean arterial pressure within 5–10 min and HR within 20–40 min of RQ-CocE administration [71].
Immunogenicity of CocE & its variants
Development of antidrug antibodies due to immunosurveillance against protein biologics is an undesirable outcome that leads to reduced efficacy of the protein drug. This is attributable to altering of the drug’s pharmacokinetic properties due to the formation of immune complexes that accelerate the clearance rate of the protein and also formation of neutralizing antibodies that impact the pharmacological properties of the protein. Adverse effects due to immune reaction include hypersensitivity and immune cross-reactivity against endogenous homologs of administered protein biologic, if any, worsens the safety profile of the drug [72].
Analysis of the immunogenic potential of protein-based therapeutics, especially bacterial proteins such as CocE, is essential. CocE and its variants administered to both rodents and nonhuman primates elicited a weak immune reaction, suggesting that it is a poor immunogen. Repeated administration of RQ-CocE retained its effectiveness to prevent or block cocaine toxicity in mice [73]. Although antibodies were detected from multiple boosts (ten- to 100-fold over baseline) to levels that could neutralize the efficacy of RQ-CocE, these effects were easily surmountable with higher doses the RQ-CocE [73]. Early studies of the immune potential of CocE indicated significantly higher levels of anti-CocE antibodies (100- to 1000-fold increase) following a multidose regime [74]. However, it was later determined that these preparations contained high levels of endotoxin (lipopolysaccharides [LPS]), as a contamination carried over from the Gram-negative E. coli bacteria that CocE was expressed in. LPS itself can act as an immunogen or adjuvant to elicit immune response against the protein [73]. All subsequent studies were performed with enzyme preparations with significantly reduced LPS levels.
RQ-CocE was also analyzed in nonhuman primates for its capacity to elicit an immune response [71]. Repeated administration of RQ-CocE in rhesus monkeys elicited a similar ten- to 100-fold increase in anti-CocE. In contrast to the rodent studies, the increase in anti-CocE titers did not appear to significantly alter the capacity of RQ-CocE to reduce the cardiovascular effects of cocaine in these monkeys, again confirming that CocE is a weak immunogen. Importantly, these anti-CocE antibodies were easily surmountable with higher doses of RQ-CocE. The PEGylated form of CCRQ-CocE also showed negligible increase in anti-CocE antibodies after four doses in mice, whereas the unPEGylated CCRQ protein showed a 1000-fold increase in anti-CocE titer [60]. Further studies with longer trial periods are required to fully understand CocE’s immunogenic potential, if there are any.
Effect of co-administered drugs on CocE
Cocaine is often abused along with other psychoactive drugs introducing the potential of these co-abused drugs to have inhibitory or activity-altering capacity on CocE. In order for future successful development of CocE as a therapeutic for cocaine abuse treatment, it is essential that the enzyme’s effectiveness to break down cocaine in the presence of such drugs should be investigated. A representative panel of co-abused drugs, as well as drugs currently administered as emergency treatments for cocaine overdose, was assessed for their capacity to alter the hydrolytic activity of CocE [75]. The panel included alcohol, nicotine, morphine, phencyclidine, ketamine, methamphetamine, naltrexone, naloxone, midazolam and diazepam. None of the drugs other than diazepam significantly slowed cocaine hydrolysis by CocE (RQ-CocE), even at ten-times the concentrations seen in drug abusers [75].
Diazepam is usually given to sedate the cocaine-overdosing patient and can easily be replaced by any other benzodiazepine, especially midazolam. The fact that engineered human BchE can also hydrolyze succinylcholine, procaine and mivacurium highlights the therapeutic advantage CocE and its variants have over BchE and its variants with regards their intrinsic promiscuously on nonspecific substrates [75].
Future perspective
Protein-based therapeutics used to treat a variety of diseases is a rapidly expanding area of R&D. The relatively smaller off-target potential of the biologics coupled with the higher specificity offered by protein drugs present very attractive options. Protein-based therapies also provide an attractive alternative to gene therapy, which has been plagued by safety issues. Another advantage of protein biologic development is that FDA approval, when compared with small-molecule drugs, is slightly faster [76]. At present, more than 130 different proteins or peptides are approved for clinical use by FDA [76]. Currently, the use of protein therapeutics to combat substance abuse is restricted to vaccination approaches [77]. At the moment, only vaccines against nicotine and cocaine are in clinical trials. To our knowledge, other than the highly active BchE, bacterial CocE and vaccines, no other protein or peptide therapeutic drugs are in the development pipeline.
CocE variants RQ-CocE or KQ-CocE are good therapeutic options for cocaine-overdose indications. The development of dimer crosslinked CCRQ-CocE for the use in treatment of cocaine addiction, however, is significantly more challenging. A form of the enzyme whose bioavailability greatly exceeds the current level seen with PEG-CCRQ-CocE (72–96 h) is the need of the hour. Mass spectrometry analysis [60] indicates that PEG-CCRQ has only two 40-kDa PEG moieties attached to it and those may not fully shield the dimeric CocE from immunosurveillance and proteolysis. Of the three solvent exposed cysteines on the protein, only one cysteine, at position 551, is efficiently getting PEGylated (data not shown). Efforts are underway to engineer new cysteines located on the enzyme surface that is diametrically opposite to position 551 in order to offer more complete stealth-like behavior of PEGylation. The in vivo life of the protein drug can be increased by several other protein-engineering methods such as albumination, or through the application of sustained-release formulations.
Proteomic and bioinformatics approaches should help in identifying potential proteolysis sites and immunogenic epitopes on CocE, respectively. Bioinformatics approaches that identify T-cell epitopes [78], which facilitate CD4 (+) T-cell-dependent antibody response to exogenous proteins or identify potential antigenic surfaces (e.g., EMBOSS server and the antigenic prediction suite based on algorithms developed by Kolaskar and Tonganokar, and implemented by Peter Rice and Alan Bleasby at the European Bioinformatics Institute at Cambridge, UK) should also be implemented. Altering these sites may offer additional protection to PEGylation and combine to improve CocE’s in vivo lifespan.
Other avenues used to extend the residence time of biologics are extended-release formulations and more recently encapsulation in red blood cells or endothelial cells [79,80]. Encapsulation of CocE in red blood cells or endothelial cells may provide a depot of the protein that is stable for more than 40 days. Controlled-release formulations such as PLGA-based microspheres or millicylinders can be designed to release the protein slowly and steadily over a period of 3 months or longer.
Conclusion
In summary, we have utilized computational approaches to identify highly active and stable mutant forms of CocE. We have followed these initial findings with rigorous structure-based protein engineering to further enhance CocE stability in vitro. The improvements to enzyme stability have allowed us to identify elimination processes that limit the residence time of the therapeutic in live animals. We have identified proteolysis, excretion in urine and minor immunogenicity as the primary pathways that have limited CocE’s residence time in vivo. Through the application of more advanced pharmaceutical approaches we believe that CocE may now be developed into a legitimate, potential therapeutic that extends beyond treatment of cocaine intoxication and into the realm of addiction treatment.
Executive summary.
-
▪
Cocaine is highly addictive and lack of small-molecule therapeutics to treat its abuse makes the development of an alternative protein-based therapy a high priority.
-
▪
Almost two decades after the initial proposal to combat substance abuse, which addressed the feasibility of pharmacokinetic therapy to sequester cocaine or hydrolyze cocaine in the periphery, tremendous progress has been made in anticocaine vaccine development recently.
-
▪
Accelerated hydrolysis of cocaine by application of exogenous enzymes has several advantages over simple sequestration of the abused drug in the periphery. Stoichiometric binding of the antigen by antibodies limits the utility of anticocaine vaccines.
-
▪
Bacterial cocaine esterase is the fastest known natural enzyme that can hydrolyze cocaine and the ease with which it can be manufactured on a large scale as compared with human butyrylcholinesterase gives it a distinct advantage for development into a suitable therapy.
-
▪
Structure-based computational design has led to the discovery of highly thermostable mutants of bacterial cocaine esterase.
-
▪
In vivo testing in rodent models for cocaine toxicity and self-administration proved the effectiveness of using bacterial cocaine esterase as therapy. In addition, cocaine esterase very rapidly ameliorated cocaine-induced cardiovascular disturbances in both rodents and Rhesus monkeys.
-
▪
PEGylation and other protein-engineering methods should be applied to bacterial cocaine esterase to improve the in vivo lifetime by reducing the proteolysis, immunogenicity and elimination of the protein so that the protein can be used effectively in long-term therapy. Sustained-release formulation development of a long-lasting cocaine esterase will ease patient compliance.
-
▪
The knowledge gained in the process of developing this enzyme will stand well in identifying, stabilizing and applying new enzyme-based therapies for other substance-abuse drugs.
Acknowledgments
Funding was provided by NIDA grants DA021416 (JH Woods and RK Sunahara) and NIGMS GM07767 (University of Michigan, USA).
Key Terms
- PEGylation
Process of covalently attaching polyethylene glycol polymer to a drug molecule (small molecule, protein, virus or peptide) to alter the pharmacokinetic and pharmacodynamic properties of the drug molecule.
- Fixed ratio schedule of reinforcement
Rats or animals are trained to respond (by pressing the lever) to obtain a reward (reinforcer). Fixed ratio schedule means animals are rewarded with reinforcements after nth response, in this case after fifth response (FR5).
Footnotes
Financial & competing interests disclosure
The NIDA and the NIGMS had no further role in study design; the collection, analysis and interpretation of data; the writing of the report; or in the decision to submit the paper for publication. D Narasimhan, JH Woods and RK Sunahara are authors on patent PCT/US2008, ‘Thermostabilization of Proteins’. JH Woods and RK Sunahara have consulted for Reckitt Benckiser Corporation.
The authors have no other relevant affifiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Benowitz NL. Clinical pharmacology and toxicology of cocaine. Pharmacol. Toxicol. 1993;72(1):3–12. doi: 10.1111/j.1600-0773.1993.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 1989;41(1):3–52. [PubMed] [Google Scholar]
- 3.Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol. Psychiatry. 2002;7(1):21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- 4.Crumb WJ, Jr, Kadowitz PJ, Xu YQ, Clarkson CW. Electrocardiographic evidence for cocaine cardiotoxicity in cat. Can. J. Physiol. Pharmacol. 1990;68(5):622–625. doi: 10.1139/y90-090. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br. J. Clin. Pharmacol. 2010;69(5):427–442. doi: 10.1111/j.1365-2125.2010.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball JK, Albright V. National Estimates of Drug-Related Emergency Department Visits. Rockville, MD, USA: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008. [Google Scholar]
- 7.Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004;29(7):1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J. Med. Chem. 2005;48(11):3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 9.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem. Pharmacol. 2008;75(1):2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dackis C, O’Brien C. Glutamatergic agents for cocaine dependence. Ann. NY Acad. Sci. 2003;1003:328–345. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- 11.Mello NK. Preclinical evaluation of the effects of buprenorphine, naltrexone and desipramine on cocaine self-administration. NIDA Res. Monogr. 1990;105:189–195. [PubMed] [Google Scholar]
- 12.Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl.) 2002;163(3–4):265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- 13.Roberts DC, Brebner K. GABA modulation of cocaine self-administration. Ann. NY Acad. Sci. 2000;909:145–158. doi: 10.1111/j.1749-6632.2000.tb06680.x. [DOI] [PubMed] [Google Scholar]
- 14.Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse. 2003;50(3):261–265. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- 15.Kahn R, Biswas K, Childress AR, et al. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 2009;103(1–2):59–64. doi: 10.1016/j.drugalcdep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Shoptaw S, Yang X, Rotheram-Fuller EJ, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence. preliminary effects for individuals with chronic patterns of cocaine use. J. Clin. Psychiatry. 2003;64(12):1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87(1):1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Margolin A, Kosten TR, Avants SK, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 20.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch. Gen. Psychiatry. 2006;63(2):219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski J, Rhoades H, Stotts A, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29(5):969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- 22.Kampman KM, Pettinati H, Lynch KG, Sparkman T, O’Brien CP. A pilot trial of olanzapine for the treatment of cocaine dependence. Drug Alcohol Depend. 2003;70(3):265–273. doi: 10.1016/s0376-8716(03)00009-7. [DOI] [PubMed] [Google Scholar]
- 23.Reid MS, Casadonte P, Baker S, et al. A placebo-controlled screening trial of olanzapine, valproate, and coenzyme Q10/L-carnitine for the treatment of cocaine dependence. Addiction. 2005;100 Suppl. 1:43–57. doi: 10.1111/j.1360-0443.2005.00990.x. [DOI] [PubMed] [Google Scholar]
- 24.Smelson DA, Ziedonis D, Williams J, Losonczy MF, Steinberg ML, Kaune M. The efficacy of olanzapine for decreasing cue-elicited craving in individuals with schizophrenia and cocaine dependence: a preliminary report. J. Clin. Psychopharmacol. 2006;26(1):9–12. doi: 10.1097/01.jcp.0000194624.07611.5e. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen G, Sager TN, Petersen JH, et al. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology (Berl.) 2008;199(1):37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- 26.Oliveto A, Poling J, Mancino MJ, et al. Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend. 2011;113(2–3):184–191. doi: 10.1016/j.drugalcdep.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox BS, Kantak KM, Edwards Ma, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat. Med. 1996;2(10):1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 28.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl.) 2000;148(3):251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 29.Carrera MR, Trigo JM, Wirsching P, Roberts AJ, Janda KD. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol. Biochem. Behav. 2005;81(4):709–714. doi: 10.1016/j.pbb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc. Natl Acad. Sci. USA. 2001;98(4):1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc. Natl Acad. Sci. USA. 2000;97(11):6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Rev. Vaccines. 2010;9(9):1109–1114. doi: 10.1586/erv.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosten TR, Rosen M, Bond J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20(7–8):1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 34.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol. Psychiatry. 2005;58(2):158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry. 2009;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science. 1993;259(5103):1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita M, Hoffman TZ, Ashley JA, Zhou B, Wirsching P, Janda KD. Cocaine catalytic antibodies: the primary importance of linker effects. Bioorg. Med. Chem. Lett. 2001;11(2):87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang G, Chun J, Arakawa-Uramoto H, et al. Anti-cocaine catalytic antibodies: a synthetic approach to improved antibody diversity. J. Am. Chem. Soc. 1996;118(25):5881–5890. [Google Scholar]
- 39.Foote J, Eisen HN. Breaking the affinity ceiling for antibodies and T cell receptors. Proc. Natl Acad. Sci. USA. 2000;97(20):10679–10681. doi: 10.1073/pnas.97.20.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gorelick DA. Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend. 1997;48(3):159–165. doi: 10.1016/s0376-8716(97)00119-1. ▪▪ First study to suggest a pharmacokinetic method using exogenous enzyme to accelerate cocaine metabolism.
- 41.Brimijoin S, Gao Y, Anker JJ, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33(11):2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue L, Ko MC, Tong M, et al. Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Mol. Pharmacol. 2011;79(2):290–297. doi: 10.1124/mol.110.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng F, Yang W, Ko MC, et al. Most efficient cocaine hydrolase designed by virtual screening of transition states. J. Am. Chem. Soc. 2008;130(36):12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mack A, Robitzki A. The key role of butyrylcholinesterase during neurogenesis and neural disorders: an antisense-5´butyrylcholinesterase–DNA study. Prog. Neurobiol. 2000;60(6):607–628. doi: 10.1016/s0301-0082(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 45.Inestrosa NC, Dinamarca MC, Alvarez A. Amyloid-cholinesterase interactions. Implications for Alzheimer’s disease. FEBS J. 2008;275(4):625–632. doi: 10.1111/j.1742-4658.2007.06238.x. [DOI] [PubMed] [Google Scholar]
- 46.Ballard C. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur. Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- 47.Geyer BC, Kannan L, Cherni I, Woods RR, Soreq H, Mor TS. Transgenic plants as a source for the bioscavenging enzyme, human butyrylcholinesterase. Plant Biotechnol. J. 2010;8(8):873–886. doi: 10.1111/j.1467-7652.2010.00515.x. [DOI] [PubMed] [Google Scholar]
- 48.Huang YJ, Huang Y, Baldassarre H, et al. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc. Natl Acad. Sci. USA. 2007;104(34):13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bresler MM, Rosser SJ, Basran A, Bruce NC. Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl. Environ. Microbiol. 2000;66(3):904–908. doi: 10.1128/aem.66.3.904-908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen NA, Heine A, De Prada P, et al. Structure determination of a cocaine hydrolytic antibody from a pseudomerohedrally twinned crystal. Acta Crystallogr. D Biol. Crystallogr. 2002;58(Part 12):2055–2059. doi: 10.1107/s0907444902017420. [DOI] [PubMed] [Google Scholar]
- 51.Jochens H, Hesseler M, Stiba K, Padhi SK, Kazlauskas RJ, Bornscheuer UT. Protein engineering of alpha/beta-hydrolase fold enzymes. Chembiochem. 2011;12(10):1508–1517. doi: 10.1002/cbic.201000771. [DOI] [PubMed] [Google Scholar]
- 52. Cooper ZD, Narasimhan D, Sunahara RK, et al. Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol. Pharmacol. 2006;70(6):1885–1891. doi: 10.1124/mol.106.025999. ▪▪ First study to show the effectiveness of cocaine esterase in rodents.
- 53. Gao D, Narasimhan DL, Macdonald J, et al. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol. Pharmacol. 2009;75(2):318–323. doi: 10.1124/mol.108.049486. ▪▪ Molecular dynamics simulation at a high temperature was used to study the destabilization of cocaine esterase and to design thermostable mutants of the enzyme.
- 54.Kazlauskas RJ, Bornscheuer UT. Finding better protein engineering strategies. Nat. Chem. Biol. 2009;5(8):526–529. doi: 10.1038/nchembio0809-526. [DOI] [PubMed] [Google Scholar]
- 55.Reetz MT, Carballeira JD, Vogel A. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew. Chem. Int. Ed. Engl. 2006;45(46):7745–7751. doi: 10.1002/anie.200602795. [DOI] [PubMed] [Google Scholar]
- 56.Korkegian A, Black ME, Baker D, Stoddard BL. Computational thermostabilization of an enzyme. Science. 2005;308(5723):857–860. doi: 10.1126/science.1107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Narasimhan D, Nance MR, Gao D, et al. Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng. Des. Sel. 2010;23(7):537–547. doi: 10.1093/protein/gzq025. ▪▪ Structure analysis of stable cocaine esterase forms and first study to describe the dimer form of cocaine esterase.
- 58.Brim RL, Nance MR, Youngstrom DW, et al. A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol. Pharmacol. 2010;77(4):593–600. doi: 10.1124/mol.109.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barends TR, Polderman-Tijmes JJ, Jekel PA, et al. Acetobacter turbidans alpha-amino acid ester hydrolase: how a single mutation improves an antibiotic-producing enzyme. J. Biol. Chem. 2006;281(9):5804–5810. doi: 10.1074/jbc.M511187200. [DOI] [PubMed] [Google Scholar]
- 60. Narasimhan D, Collins GT, Nance MR, et al. Subunit stabilization and polyethylene glycolation of cocaine esterase improves in vivo residence time. Mol. Pharmacol. 2011;80(6):1056–1065. doi: 10.1124/mol.111.074997. ▪▪ Dimer stabilization by disulfide bridging and PEGylation are shown to improve in vivo half-life of cocaine esterase.
- 61.Brim RL, Noon KR, Collins GT, et al. The fate of bacterial cocaine esterase (CocE): an in vivo study of CocE-mediated cocaine hydrolysis, CocE pharmacokinetics, and CocE elimination. J. Pharmacol. Exp. Ther. 2012;340(1):83–95. doi: 10.1124/jpet.111.186049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009;23(2):93–109. doi: 10.2165/00063030-200923020-00003. [DOI] [PubMed] [Google Scholar]
- 63. Park JB, Kwon YM, Lee TY, et al. PEGylation of bacterial cocaine esterase for protection against protease digestion and immunogenicity. J. Control. Release. 2010;142(2):174–179. doi: 10.1016/j.jconrel.2009.10.015. ▪ Study that describes the PEGylation of cocaine esterase.
- 64.Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J. 2006;8(1):E196–E203. doi: 10.1208/aapsj080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collins Gt, Brim Rl, Narasimhan D, et al. Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J. Pharmacol. Exp. Ther. 2009;331(2):445–455. doi: 10.1124/jpet.108.150029. ▪▪ Describes the effects of cocaine esterase on reducing the self-administration of cocaine by rats.
- 66.Kosten TMB, Poling J, Gardner T. Cocaine vaccine: 6-month follow-up during drop in antibody levels (abstract 394); College on Problems of Drug Dependence Annual Meeting; 14–18 June 2008; San Juan, Puerto Rico. [Google Scholar]
- 67.Collins GT, Narasimhan D, Cunningham AR, et al. Long-lasting effects of a PEGylated mutant cocaine esterase (CocE) on the reinforcing and discriminative stimulus effects of cocaine in rats. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.226. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am. J. Med. 1990;88(4):325–331. doi: 10.1016/0002-9343(90)90484-u. [DOI] [PubMed] [Google Scholar]
- 69.Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. Triggering of myocardial infarction by cocaine. Circulation. 1999;99(21):2737–2741. doi: 10.1161/01.cir.99.21.2737. [DOI] [PubMed] [Google Scholar]
- 70.Collins GT, Zaks ME, Cunningham AR, et al. Effects of a long-acting mutant bacterial cocaine esterase on acute cocaine toxicity in rats. Drug Alcohol Depend. 2011;118(2–3):158–165. doi: 10.1016/j.drugalcdep.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Collins GT, Carey KA, Narasimhan D, et al. Amelioration of the cardiovascular effects of cocaine in rhesus monkeys by a long-acting mutant form of cocaine esterase. Neuropsychopharmacology. 2011;36(5):1047–1059. doi: 10.1038/npp.2010.242. ▪▪ Describes the application of cocaine esterase in nonhuman primates.
- 72.Gupta S, Devanarayan V, Finco D, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J. Pharm. Biomed. Anal. 2011;55(5):878–888. doi: 10.1016/j.jpba.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 73. Ko MC, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Woods JH. Effects of cocaine esterase following its repeated administration with cocaine in mice. Drug Alcohol Depend. 2009;101(3):202–209. doi: 10.1016/j.drugalcdep.2009.01.002. ▪ Covers the development of immunogenicity observed in rodents against cocaine esterase.
- 74.Ko MC, Bowen LD, Narasimhan D, et al. Cocaine esterase: interactions with cocaine and immune responses in mice. J. Pharmacol. Exp. Ther. 2007;320(2):926–933. doi: 10.1124/jpet.106.114223. [DOI] [PubMed] [Google Scholar]
- 75.Brim RL, Noon KR, Nichols J, Narasimhan D, Woods JH, Sunahara RK. Evaluation of the hydrolytic activity of a long-acting mutant bacterial cocaine in the presence of commonly co-administered drugs. Drug Alcohol Depend. 2011;119(3):224–228. doi: 10.1016/j.drugalcdep.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 2008;7(1):21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 77.Orson FM, Kinsey BM, Singh RAK, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Ann. NY Acad. Sci. 2008;1141(1):257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Groot AS, Moise L. Prediction of immunogenicity for therapeutic proteins: state of the art. Curr. Opin. Drug Discov. Devel. 2007;10(3):332–340. [PubMed] [Google Scholar]
- 79.Kwon YM, Chung HS, Moon C, et al. l-asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) J. Control. Release. 2009;139(3):182–189. doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2002;19(1):73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
Website
- 101.National Institute on Drug Abuse. www.drugabuse.gov/infofacts/understand.html.