Abstract
Perturbations in the normal functions of the endoplasmic reticulum (ER) trigger a signaling network that coordinates adaptive and apoptotic responses. There is accumulating evidence implicating prolonged ER stress in the development and progression of many diseases, including neurodegeneration, atherosclerosis, type 2 diabetes, liver disease, and cancer. With the improved understanding of the underlying molecular mechanisms, therapeutic interventions that target the ER stress response would be potential strategies to treat various diseases driven by prolonged ER stress.
Keywords: ER stress, unfolded protein response (UPR), neurodegenerative disease, liver disease, atherosclerosis, diabetes, cancer
ENDOPLASMIC RETICULUM STRESS
The endoplasmic reticulum (ER) is a specialized organelle that has crucial roles in cell homeostasis and survival, which include protein folding, lipid biosynthesis, and calcium and redox homeostasis (1). The lumen of the ER is the major site for proper protein folding and contains molecular chaperones and folding enzymes including Grp78 (BiP), Grp94, protein disulfide isomerase (PDI), calnexin, and calreticulin. Only properly folded proteins are exported to the Golgi organelle, while incompletely folded proteins are retained in the ER to complete the folding process or are delivered to the cytosol to undergo endoplasmic reticulum–associated degradation. Under physiologic conditions, there is an equilibrium between ER protein load and folding capacity. Alterations in ER homeostasis due to increased protein synthesis, accumulation of misfolded proteins, or alterations in the calcium or redox balance of the ER lead to a condition called ER stress (2).
To cope with this stress, cells have developed an adaptive signaling pathway called the unfolded protein response (UPR) or ER stress response. The initial objective of the UPR is to re-establish homeostasis and alleviate ER stress through two mechanisms: (a) increasing folding capacity via expression of protein-folding chaperones and (b) downregulation of ER protein client load via inhibiting general protein translation and promoting the degradation of misfolded proteins. However, if the stress is prolonged or severe, the UPR initiates programmed cell death (3). UPR-mediated cell death may contribute to the pathogenesis of many diseases (Table 1), including cancer, type 2 diabetes, neurodegeneration, and atherosclerosis (3, 4).
Table 1.
Diseases related to endoplasmic reticulum (ER) stress
| Disease | Role of ER stress | References |
|---|---|---|
| Alzheimer’s disease | Mutant presenilin 1 induces CHOP | 12, 13 |
|
| ||
| Parkinson’s disease | Accumulation of a substrate of Parkin in the ER activates ER stress | 18 |
|
| ||
| Amyotrophic lateral sclerosis | Mutant SOD1 aggregates and activates ER stress | 20 |
|
| ||
| Type 2 diabetes | Obesity induces ER stress | 22–24 |
| ATF6 interferes with gluconeogenesis | 25 | |
| Free fatty acids and hyperglycemia induce beta cell death through CHOP | 27–29 | |
|
| ||
| Atherosclerosis | Atherosclerosis-relevant stimuli induce macrophage death via CHOP | 36, 37 |
| Oxidized phospholipids, hyperhomocysteinemia, and cholesterol loading induce endothelial and smooth muscle cell death via CHOP | 34, 40, 43 | |
|
| ||
| Nonalcoholic fatty liver disease | ER stress induces SREBP-1c | 46 |
|
| ||
| HCV and HBV infection | HCV suppresses IRE1-XBP1 pathway | 49 |
| HBV induces Grp78 and Grp94 | 50 | |
|
| ||
| Alcoholic liver disease | Alcohol induces Grp78 and CHOP | 52, 53 |
|
| ||
| Cancer | Many cancers induce Grp78 and XBP1 | 55–58 |
Abbreviations: CHOP, C/EBPα-homologous protein; HBV, hepatitis B virus; HCV, hepatitis C virus
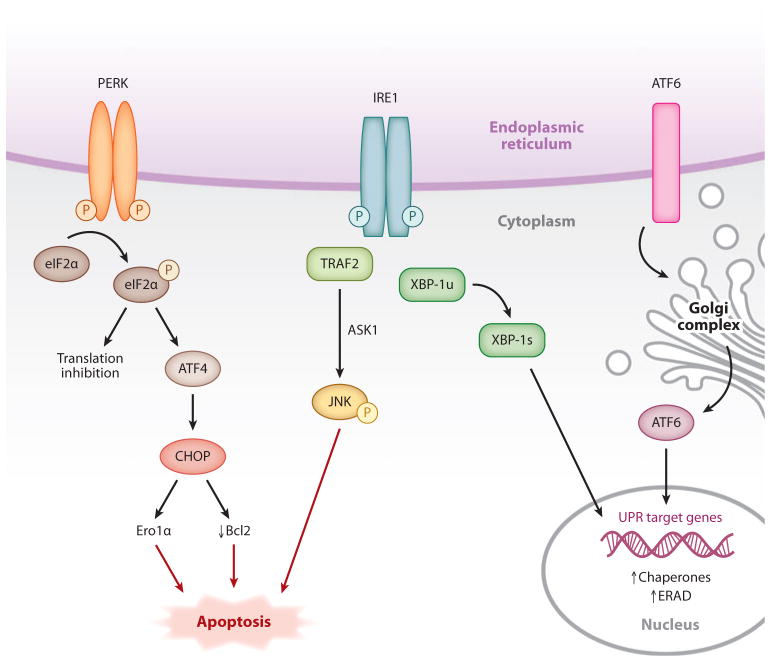
There are three major branches of UPR, which are each activated by a dedicated ER-localized transmembrane molecule: PERK (protein kinase RNA-like ER kinase); IRE1 (inositol requiring protein–1); and ATF6 (activating transcription factor–6) (Figure 1) (5). IRE1 is the most evolutionarily conserved ER stress sensor, and under physiologic conditions it is thought to be held in its inactive form through an interaction with immunoglobulin heavy chain–binding protein (BiP) (2). Upon accumulation of unfolded proteins in the ER lumen, BiP dissociates from IRE1, leading to its activation by transautophosphorylation. However, other mechanisms of activation, such as direct binding of IRE1 to unfolded proteins, are likely to be involved (6). IRE1 has a cytoplasmic endoribonuclease domain, which, upon activation, splices and enables the translation of the mRNA encoding X-box binding protein–1 (XBP1). Spliced XBP1 (XBP1s) is a transcription factor that induces many essential UPR genes that increase ER folding capacity and expand ER membrane surface area (7). Recently IRE1 has been shown to be required for cleavage and post-transcriptional degradation of mRNAs other than Xbp1, which may function as another mechanism to reduce client load on the ER (8). IRE1 also has an intrinsic kinase activity that appears to be involved in the regulation of its nuclease function, although the precise mechanisms have not yet been elucidated. Other functions of IRE1 may be related to the triggering of apoptosis (see below). For example, upon activation, IRE1 binds the adaptor protein, TNF receptor–associated factor–2 (TRAF2), which then promotes activation of c-Jun N-terminal kinase (JNK) through apoptosis signal–regulating kinase–1 (ASK1) (9). In addition to its endoribonuclease and kinase activities, in some cell types IRE1 activation has been reported to trigger the recruitment of a proapoptotic ER-resident cysteine protease, caspase 12 (10).
Figure 1.
Endoplasmic reticulum (ER) stress response. Upon accumulation of unfolded proteins in the ER lumen, three stress sensors—IRE1, PERK, and ATF6—are activated and initiate signal transduction events that control cell survival or death. PERK, protein kinase RNA-like ER kinase; IRE1, inositol requiring protein 1; ATF6, activating transcription factor 6; eIF2α, eukaryotic translation initiation factor 2 alpha; CHOP, C/EBPα-homologous protein; Bcl-2, B cell lymphoma 2; Ero1α, ER oxidase 1 alpha; TRAF2, TNF receptor–associated factor 2; JNK, c-Jun N-terminal kinase; ASK1, apoptosis signal-regulating kinase 1; XBP1, X-box binding protein 1; ERAD, endoplasmic reticulum–associated degradation; UPR, unfolded protein response.
PERK is a serine threonine kinase and very similar to IRE1. It has a luminal ER-stress-sensing domain and is activated through transautophosphorylation. Activated PERK phosphorylates eukaryotic translation initiation factor 2 alpha (eIF2α), which results in global translational attenuation and reduced ER protein load (2). However, phosphorylated eIF2α promotes the translation of ATF4 (activating transcription factor–4), which induces the UPR effector CHOP (C/EBPα-homologous protein, also known as GADD153). In pathologic settings, prolonged CHOP expression triggers apoptosis through a number of mechanisms, including downregulation of the antiapoptotic factor B cell lymphoma-2 (Bcl-2) and induction of a calcium-mediated apoptosis pathway triggered by the CHOP transcriptional target, ER oxidase–1α (3).
The third UPR pathway is initiated by a basic leucine zipper transcription factor, ATF6. When ER stress occurs, ATF6 translocates to the Golgi complex, where it gets cleaved by site 1 and site 2 proteases. The resultant transcription factor then migrates to the nucleus to increase the expression of ER chaperones such as Grp78 (1, 2).
ENDOPLASMIC RETICULUM STRESS AND DISEASE PROGRESSION
The ER has essential roles in physiologic regulation of many processes. Accumulating evidence indicates that pathologic conditions that interfere with ER homeostasis give rise to chronic activation of the UPR, which contributes to the pathogenesis of many diseases. These include neurodegenerative disorders, type 2 diabetes, atherosclerosis, liver disease, and cancer.
Neurodegenerative Diseases
Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive neurologic disorder characterized by a decline in cognitive processes, eventually leading to dementia. The hallmarks of this disease are accumulation of extracellular amyloid-β peptides and intracellular aggregates of phosphorylated tau proteins, along with the perturbation of calcium homeostasis and neuronal death (11). Genetic studies have revealed that mutations in the amyloid-β precursor protein (APP) and the presenilins PS1 and PS2 are associated with the familial form of the disease. Mutations in these proteins lead to alterations in the processing of amyloid-β peptides from APP, resulting in more toxic forms of amyloid-β peptides in the plaques. In vitro studies have shown that mutations in the PS1 gene interfere with the physiologic functions of the UPR and thus render cells more susceptible to ER-stress-induced death (12). In mice with mutant PS1, there is a prolonged ER stress response and elevated level of CHOP, which, as mentioned above, can trigger pathologic cell death (13).
Recent reports have indicated that the UPR is activated in the AD brain. Increased expression of the ER chaperone Grp78, which is indicative of UPR activation, is found in AD cases compared with controls (14). Autopsy studies have shown increased immunohistochemical staining of phosphorylated (activated) PERK, eIF2α, and IRE1 in the brains of patients with AD compared with specimens from subjects without the disease. UPR-positive staining was localized to neurons, not glial cells, which is consistent with a role for ER stress in AD pathogenesis (15). However, the exact role of the UPR in the pathogenesis of AD must await molecular-genetic causation studies in suitable animal models.
Parkinson’s disease
Parkinson’s disease (PD) is a multifactorial neurodegenerative disorder characterized by the death of dopaminergic neurons and accumulation of protein aggregates (Lewy bodies, LBs) in a distinct brain region (16). Although several hypotheses have been proposed in the pathogenesis of PD, recent evidence indicates that, like other neurodegenerative disorders, PD is a “protein misfolding” disorder. Current studies have shown that impaired protein quality control and dysfunction of the UPR have essential roles in the pathogenesis of this disease. Studies from familial forms of PD have revealed mutations in the Parkin gene, which encodes an enzyme involved in the degradation of unfolded proteins (17). The loss of activity of this protein results in the accumulation of a substrate of Parkin in the ER, leading to ER stress and apoptosis. Moreover, cell culture experiments have shown that Parkin expression is induced by ER stress, and overexpression of Parkin protects cells from ER-stress-induced death (18). Finally, studies from post mortem PD cases have shown that phosphorylated PERK (p-PERK) and phosphorylated eIF2α (p-eIF2α) are increased in the neurons of PD subjects. As with AD, the precise role of the UPR in PD pathophysiology will require mechanistically based molecular-genetic causation studies.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Charcot’s or Lou Gehrig’s disease, is characterized by muscle weakness, atrophy, and paralysis. The pathologic feature of ALS is the selective degeneration of brain and spinal cord motoneurons. Markers of ER stress are upregulated in the spinal cords of patients with ALS (19). Moreover, mutations in the superoxide dismutase–1 (SOD1) gene have been linked to the familial form of the disease, and mouse models with this mutation show improper folding of SOD1 and SOD1-aggregate-induced UPR activation (20). However, further studies are needed to evaluate the exact mechanisms responsible for this effect and its pathophysiologic consequences in ALS.
Type 2 Diabetes
Obesity induces type 2 diabetes (T2D), which is characterized by a combination of peripheral insulin resistance, dysregulated hepatic glucose production, and inadequate insulin secretion by pancreatic beta cells. At the molecular level, it entails defects in insulin signal transduction, such as reduced insulin receptor function and reduced post–insulin receptor phosphorylation steps (21). Recent studies have revealed that genetically obese (ob/ob) or diet-induced obese mice have signs of increased UPR parameters in liver and adipose tissues (22). Moreover, ER stress-mediated activation of JNK has been linked to insulin resistance through phosphorylating insulin receptor substrate–1 (IRS1) on Ser307, which results in reduced tyrosine phosphorylation and activation of IRS1. Most importantly, ER stress parameters, including Grp78, XBP1s, phospho-eIF2α, and phospho-JNK, are increased in the liver and adipose tissues of obese insulin-resistant nondiabetic humans (23), and these parameters are significantly reduced after gastric-bypass-induced weight loss (24). ER stress in obesity is thought to be induced by an augmented demand for protein synthesis under nutrient excess and by elevated levels of saturated free fatty acids, like palmitate, which have been shown to cause ER stress and to activate the UPR in vitro and in experimental animal models.
Increased hepatic glucose production due to enhanced gluconeogenesis is an important component of insulin resistance in T2D. Interestingly, recent work has shown an example in which one branch of the UPR may actually be beneficial in this area. ATF6-mediated interference of CREB–CRTC2 (CREB regulated transcription coactivator 2) interaction inhibits CRTC2-induced transcription of gluconeogenic genes, and ATF6 overexpression improves glucose balance by reversing the effects of CRTC2 on the gluconeogenic program and lowering blood glucose levels in ob/ob mice (25).
During the progression of T2D, there is an increased demand on the beta cells for insulin production in order to compensate for ongoing insulin resistance. Because maturation of proinsulin into insulin requires its processing in the ER, it is believed that this increased demand, together with increased circulating free fatty acids and hyperglycemia, triggers ER stress in islet beta cells (26). Chronic ER stress eventually leads to beta cell death, which further exacerbates hyperglycemia. In this context, recent studies have documented increased levels of eIF2α phosphorylation, increased splicing of XBP1 mRNA, and increased CHOP and Grp78 protein levels in the islets of mice with models of insulin resistance and beta cell failure (27). Moreover, islets from patients with T2D have elevated Grp78 and CHOP protein levels. Proof-of-concept experiments in multiple mouse models of T2D have shown that CHOP gene ablation results in improved glycemic control and expanded beta cell mass by protecting against oxidative stress in response to ER stress (28, 29). Moreover, consistent with the usual protective role of XBP1, mice lacking XBP1 selectively in beta cells of the pancreas have hyperglycemia and beta cell loss, possibly through impaired postnatal insulin secretory function of beta cells (30). Thus, mechanistic and causation data in animal models and correlative studies in humans suggest that the UPR can serve physiologic roles in normal glucose homeostasis but, when prolonged in the setting of nutrient excess, can contribute to the pathophysiology of both insulin resistance and hyperglycemia in obesity and T2D.
Atherosclerosis
Atherosclerosis involves complex interactions between lipoproteins, arterial vascular cells, and inflammatory cells. The key initiating event in atherogenesis is the subendothelial retention of apolipoprotein B–containing lipoproteins, which is followed by recruitment of inflammatory monocytes and their differentiation into macrophages (31). Macrophages ingest lipoproteins to become lipid-loaded “foam cells” and, together with other immune cells and intimal smooth muscle cells, contribute to the progressively heightened inflammatory state and expansion of atherosclerotic lesions. As lesions progress, dead and dying macrophages, coupled with defective clearance of the dead cells, contribute to the development of the so-called necrotic core, which is a key component of complex, rupture-prone plaques that are associated with acute myocardial infarction and sudden death (32). Necrotic cores are associated with plaque instability, probably because they are a reservoir of matrix proteases, inflammatory mediators, and prothrombotic molecules. Thus, macrophage apoptosis may be a key factor in converting lesions from a benign to an unstable phenotype by promoting necrotic core formation. As reviewed below, one cause of macrophage apoptosis in advanced atherosclerosis is the chronic activation of ER stress pathways promoting cell death (33). The significance of ER stress in other lesional cell types is less well understood, but the current evidence supports roles for ER stress in the regulation of cell survival in smooth muscle cells and endothelial cells as well (34, 35).
Recent mechanistic data in vitro and molecular-genetic causation data in vivo have demonstrated a strong, causal relationship between atherosclerosis progression, particularly the formation of necrotic lesions, and ER stress in macrophages. For example, studies have shown that advanced lesional macrophage death and plaque necrosis are decreased in atherosclerotic apolipoprotein E–deficient (Apoe−/−) mice in the setting of CHOP deficiency (36, 37). Moreover, Myoishi et al. have documented a close correlation among CHOP expression, apoptosis, and plaque vulnerability in human coronary artery lesions (38). Consistent with the role of ER stress in atherosclerosis, Erbay et al. reported reduced ER stress parameters and lesion size in mice deficient for fatty acid–binding protein–4 (aP2), which is required for lipid-induced ER stress and macrophage apoptosis (39).
Endothelial cells, which express atherogenic monocyte adhesion molecules such as VCAM1, initiate an ER stress response after exposure to oxidized phospholipids (40). In human atherosclerotic lesions, markers of ER stress are increased in areas of endothelium containing oxidized phospholipids (41). In addition, it has been proposed that vascular smooth muscle cell (SMC) death compromises plaque integrity by weakening the protective fibrous cap in advanced plaque (42). In vitro evidence suggests that ER stressors such as cholesterol loading, hyperhomocysteinemia, and high glucose and glucosamine upregulate ER stress markers such as CHOP in SMCs, which may promote the death of these cells (34, 43). In addition, 7-ketocholesterol, an oxysterol that is elevated in the plasma and advanced lesions of patients with a high cardiovascular risk, has been shown to activate the UPR and induce apoptosis of cultured human SMCs (38, 44). However, unlike the case with macrophages, molecular-genetic causation evidence for a role of the UPR in SMCs in atherosclerosis is still lacking.
Liver Disease
Nonalcoholic fatty liver disease
The liver is one of the major secretory organs and has essential roles in carbohydrate and lipid metabolism. Hepatic lipogenesis is activated upon uptake of excess carbohydrates and is controlled at the transcriptional level by sterol regulatory element-binding protein-1c (SREBP-1c), which is aberrantly activated in obesity (45). Nonalcoholic fatty liver disease (NAFLD) is a progressive disorder characterized by aberrant lipid storage in hepatocytes. Studies in humans have pointed out the role of de novo lipogenesis in the excessive accumulation of lipids in the livers of patients with NAFLD.
SREBP-1c is an ER localized transcription factor which, like ATF6, requires processing in the Golgi organelle to become active. Recent work has shown that ER stress, through induction of SREBP-1c, activates lipogenesis (46). Furthermore, in mouse models of obesity, when ER stress is relieved through Grp78 overexpression, SREBP-1c induction is suppressed and hepatic steatosis is decreased. Additionally, the link between ER stress and lipogenesis has been shown in genetic ablation studies in which genes involved in the UPR were silenced. In one scenario, mice deficient in XBP1 specifically in the liver exhibited downregulation in key lipogenic enzymes (47). In addition, alterations in the PERK-eIF2α arm of the UPR has also been linked to lipogenesis and thus may contribute to NAFLD (48).
Viral hepatitis
Viral infections lead to UPR activation, which is likely triggered by the high level of synthesis of viral structural proteins and the assembly of viral particles in the ER. In general, the UPR may have host-cell-protective roles, such as limiting viral protein production through translation attenuation or host cell apoptosis, or detrimental roles, such as enabling viral protein production through chaperone production. Accumulating evidence has shown that hepatitis C virus (HCV) and hepatitis B virus (HBV) infection lead to UPR activation in hepatocytes. Components of the HCV complex modulate the UPR, which in this case results in increased viral replication. For example, HCV can promote its replication through suppressing the IRE1–XBP1 pathway (49). Moreover, the large surface protein of HBV has been shown to induce Grp78 and Grp94 (50), which are chaperones that promote protein translation and folding. A recent study has demonstrated activation of three ER-resident stress sensors, ATF6, IRE1, and PERK, in livers from untreated patients with chronic HCV, although full induction of UPR-responsive genes was not found (51). Thus, regulation of the hepatocyte UPR by HCV and HBV may be an important pathophysiologic process utilized by the viruses.
Alcoholic liver disease
The major cause of liver disease in Western societies is alcoholic liver disease (ALD), which includes steatosis, fibrosis, and liver cirrhosis due to extensive hepatocyte damage. Recent studies with intragastric-alcohol-treated mice have implicated ER stress in the progression of this disease. ER-stress-related genes, such as those encoding Grp78, Grp94, and CHOP, were shown to be upregulated in mice fed with ethanol (52). Although mice deficient in CHOP displayed no change in markers of ER stress or fatty liver formation after ethanol feeding, CHOP knockout mice exhibited minimal hepatocyte apoptosis, further supporting the role of ER stress in ALD (53). One origin of ER stress in ALD may be elevated homocysteine, which has been shown to disrupt disulphide bond formation and thereby lead to accumulation of misfolded proteins in the ER. When mice were fed with betaine, which increases the conversion of homocysteine to methionine, ethanol-induced fatty liver formation was suppressed and CHOP and Grp78 mRNAs were decreased, further supporting the role of homocysteinemia in the ER stress–ALD link (54).
Cancer
Because of their high rates of growth and proliferation, cancer cells require an increased rate of protein folding and assembly in the ER. In addition, some cancer cells express mutant proteins that cannot be correctly folded and by this means activate the UPR. Following initiation of malignancy, poor vascularization of the tumor mass leads to nutrient starvation, hypoxia, and alterations in the redox environment, and these processes are also strong inducers of the activation of certain UPR pathways.
Accumulating evidence suggests that the UPR acts as an important cancer cell survival pathway. Increased expression of UPR markers has been reported in a number of cancers, and many ER chaperones show increased expression in human tumor specimens. For example, increased Grp78 expression has been shown in breast, colon, and adenocarcinoma cancer cell lines and in hepatocarcinomas (55). This high expression of Grp78 is thought to provide survival signals for cancer cells during oncogenesis and confer drug resistance. In addition, elevated Grp78 level has been reported to correlate well with higher pathologic grade, recurrence rate, and poor survival in patients with breast, liver, prostate, colon, and gastric cancers (56). Similarly, XBP1s overexpression has been demonstrated in a variety of human cancers including breast cancer and hepatocellular carcinomas. Moreover, a transgenic mouse model overexpressing XBP1s was shown to be capable of neoplastic transformation of plasma cells and development of multiple myeloma (57).
In contrast to the many studies showing that the UPR is activated in human tumors and animal tumor models, a recent report suggests that the UPR is downregulated in mouse prostate cancer models (58). This observation suggests that the role of ER stress in cancer may be more complex than initially anticipated.
THERAPEUTIC IMPLICATIONS
Enhancing ER Folding Capacity
In disease processes where ER function is perturbed, alleviating ER stress through enhancement of ER function could protect cells from irreversible damage and ameliorate disease. Chemical or pharmaceutical chaperones are a group of low-molecular-weight compounds that have been proposed to increase ER folding capacity by facilitating proper folding and decreasing the accumulation and aggregation of misfolded proteins in the ER lumen. In mouse models, 4-phenyl butyrate (4-PBA) and taurine-conjugated deoxycholic acid (TUDCA) have been shown to provide benefit for numerous ER-stress-related diseases including T2D, atherosclerosis, and leptin resistance (59, 60). However, their precise ER-stress-relieving properties remain unknown, and additional studies are needed to determine the mechanisms responsible for this improvement. Similarly, valproate, a drug used in epilepsy treatment, increases the expression of ER chaperones such as Grp78 and Grp94 and has beneficial effects in in vitro models of neurodegenerative diseases (61). In addition, a recent report showed that a specific inducer of Grp78/BiP called BiP inducer X (BIX) has beneficial effects in focal ischemia–stroke mouse models, further providing evidence that approaches aimed at enhancing the protective arms of the UPR may be beneficial in ER-stress-related disorders (62).
Targeting Individual UPR Pathways
Recent work has shown that a selective inhibitor of the dephosphorylation of eIF2α, salubrinal, provides protection against ER-stress-induced cell death in a PD cell culture model and in ischemia-induced ER stress in mice (63, 64). Because p-eIF2α suppresses global protein translation, the mechanism of action is thought to be protection from excess client load, which is one of the ways pathologic, prolonged UPR activation is maintained in disease states. However, not all preparations of commercially available salubrinal possess this beneficial activity, which raises uncertainty related to its usefulness as a drug and mechanism of action. Other studies have shown that Chop−/− mice are protected from ER-stress-related diseases where cell death is the main mechanism of the disease pathogenesis. For example, a protective effect of CHOP deficiency has been shown in atherosclerosis, T2D, neurodegenerative diseases, and ALD (28, 29, 36, 37, 53). Therefore, small molecules specifically targeting CHOP hold potential as a therapeutic tool.
Therapeutic Induction of the UPR
Therapeutic induction of ER-stress-induced apoptosis may be beneficial in killing cancer cells. As an example, inhibition of proteasome activity, which degrades misfolded proteins, induces ER stress in cancer cells. In this context, a selective proteasome inhibitor, bortezomib, has been reported to be beneficial in killing multiple myeloma cells and provides antitumor activity in the treatment of pancreatic cancer (65, 66). However, extreme caution should be used in ER-stress-targeted anticancer drug development because of the potentially toxic effects of inhibitors of this process on normal cells.
CONCLUSIONS
During the past decade, research on how cells can sense and cope with ER stress has rapidly progressed. There is also compelling ongoing research that suggests one or more branches of the UPR are important in the pathogenesis of several diseases. However, many unanswered questions remain. A detailed understanding of the mechanisms and consequences involved in these processes will be important for translating our knowledge into novel therapeutic approaches. Moreover, although relieving ER stress may be beneficial in certain diseases, such as T2D, atherosclerosis, and neurodegeneration, increasing ER stress may be helpful in other disease processes, such as cancer and possibly certain types of viral infections. Therefore, new therapeutic agents must be carefully tested in appropriate mouse models to avoid possible adverse effects.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–84. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 6.Oikawa D, Kimata Y, Kohno K. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J Cell Sci. 2007;120:1681–88. doi: 10.1242/jcs.002808. [DOI] [PubMed] [Google Scholar]
- 7.Glimcher LH. XBP1: the last two decades. Ann Rheum Dis. 2010;69(Suppl 1):i67–i71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- 8.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–7. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 9.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–66. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 11.Ittner LM, Gotz J. Amyloid-beta and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 12.Katayama T, Imaizumi K, Sato N, et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol. 1999;1:479–85. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 13.Milhavet O, Martindale JL, Camandola S, et al. Involvement of Gadd153 in the pathogenic action of presenilin-1 mutations. J Neurochem. 2002;83:673–81. doi: 10.1046/j.1471-4159.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoozemans JJ, Veerhuis R, van Haastert ES, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110:165–72. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 15.Unterberger U, Hoftberger R, Gelpi E, et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol. 2006;65:348–57. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 16.Healy DG, bou-Sleiman PM, Wood NW. PINK, PANK, or PARK? A clinicians’ guide to familial parkinsonism. Lancet Neurol. 2004;3:652–62. doi: 10.1016/S1474-4422(04)00905-6. [DOI] [PubMed] [Google Scholar]
- 17.Shimura H, Hattori N, Kubo S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 18.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–64. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 19.Atkin JD, Farg MA, Walker AK, et al. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400–7. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–36. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, White MF. Insulin receptor substrate proteins and diabetes. Arch Pharm Res. 2004;27:361–70. doi: 10.1007/BF02980074. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 23.Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–44. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Vera L, Fischer WH, et al. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–37. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 27.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–63. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 28.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song B, Scheuner D, Ron D, et al. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AH, Heidtman K, Hotamisligil GS, et al. Dual and opposing roles of the unfolded protein response regulated by IRE1α and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci USA. 2011;108:8885–90. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol. 1998;9:471–74. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedi X, Ming Y, Yongping W, et al. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. 2009;207:123–30. doi: 10.1016/j.atherosclerosis.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Nakano T, Watanabe H, Ozeki M, et al. Endoplasmic reticulum Ca2+ depletion induces endothelial cell apoptosis independently of caspase-12. Cardiovasc Res. 2006;69:908–15. doi: 10.1016/j.cardiores.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Tsukano H, Gotoh T, Endo M, et al. The endoplasmic reticulum stress-CHOP pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–32. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 37.Thorp E, Li G, Seimon TA, et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–33. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 39.Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanson M, Auge N, Vindis C, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–36. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- 41.Civelek M, Manduchi E, Riley RJ, et al. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–61. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–80. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 43.Werstuck GH, Khan MI, Femia G, et al. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006;55:93–101. [PubMed] [Google Scholar]
- 44.Pedruzzi E, Guichard C, Ollivier V, et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–17. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diraison F, Dusserre E, Vidal H, et al. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 46.Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee AH, Scapa EF, Cohen DE, et al. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–96. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oyadomari S, Harding HP, Zhang Y, et al. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tardif KD, Mori K, Kaufman RJ, et al. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem. 2004;279:17158–64. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 50.Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol. 1997;71:7387–92. doi: 10.1128/jvi.71.10.7387-7392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asselah T, Bieche I, Mansouri A, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264–74. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- 52.Ji C, Kaplowitz N. ER stress: Can the liver cope? J Hepatol. 2006;45:321–33. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Ji C, Mehrian-Shai R, Chan C, et al. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez PM, Tabbara SO, Jacobs LK, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 56.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–99. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 57.Carrasco DR, Sukhdeo K, Protopopova M, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–60. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.So AY, de la FE, Walter P, et al. The unfolded protein response during prostate cancer development. Cancer Metastasis Rev. 2009;28:219–23. doi: 10.1007/s10555-008-9180-5. [DOI] [PubMed] [Google Scholar]
- 59.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Bown CD, Wang JF, Chen B, et al. Regulation of ER stress proteins by valproate: therapeutic implications. Bipolar Disord. 2002;4:145–51. doi: 10.1034/j.1399-5618.2002.t01-1-40201.x. [DOI] [PubMed] [Google Scholar]
- 62.Kudo T, Kanemoto S, Hara H, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–75. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 63.Smith WW, Jiang H, Pei Z, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005;14:3801–11. doi: 10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- 64.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 65.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 66.Nawrocki ST, Carew JS, Pino MS, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–66. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]