Abstract
Purpose of the Review
To highlight recent research pertaining to the cellular mechanisms linking amino acid availability, mTORC1 signaling, and muscle protein metabolism.
Recent Findings
Activation of the mTORC1 pathway in response to amino acids may be dependent upon cellular re-localizaiton of mTORC1, a process that appears to involve the Rag GTPases. Recent studies have also identified other intracellular proteins, such as hVps34 and MAP4K3, and specific amino acid transporters as necessary links between amino acid availablity and mTORC1. In human skeletal muscle, it appears that mTORC1 activity increases the expression of several amino acid transporters, which may be an important adaptive response to sensitize muscle to a subsequent increase in amino acid availability.
Summary
The precise cellular mechansims linking amino acids to mTORC1 signaling and muscle protein metabolism are currently not well understood. More defined cellular mechansims are beginning to emerge suggesting a role for several intracellular proteins including hVps34, MAP4K3, and the Rag GTPases. Additionally, specific amino acid transporters may have a role both upstream and downstream of mTORC1. Continued investigation into the precise cellular mechanisms linking amino acid availablity and muscle protein metabolsim will help facilitate improvements in existing therapies for conditions of muscle wasting.
Keywords: mTORC1, amino acid transporters, protein synthesis
Introduction
The associated loss of skeletal muscle mass and strength represents an important hurdle in the treatment and/or rehabilitation for numerous clinical conditions such as cancer, sepsis, AIDS, and aging. In fact, decreases in skeletal muscle size and function not only negatively impact physical function, but muscle wasting also greatly impairs overall metabolic health since muscle serves as a primary substrate storage site and donor for metabolic and immune processes during injury, malnutrition, and disease [1]. Furthermore, the deleterious effects of muscle wasting are particularly evident in the growing elderly population, as the dramatic reduction in muscle size that accompanies advancing age, termed sarcopenia, increases injury risk, extends rehabilitation time, and decreases physical independence and overall quality of life [2–4]. Given these overwhelming side effects and the multitude of clinical conditions associated with muscle wasting, interventions that can be utilized in a variety of clinical settings to help maintain, or even increase muscle size are critical to reduce complications, increase function, and improve quality of life.
Nutritional interventions, in particular those involving an increase in amino acid availability, have routinely been shown to rapidly elevate the rate of muscle protein synthesis [5–13], and therefore represent a unique strategy that may help counteract muscle wasting and can easily be employed in most if not all clinical settings. While there is little question about the ability for increased intracellular amino acid availability to promote muscle anabolism, the particular cellular mechanisms responsible are only recently beginning to be unraveled. Understanding these precise cellular mechanisms will help facilitate the development of more effective therapeutic strategies for conditions of muscle wasting and decreased muscle function. Therefore, the focus of this review is to highlight recent advancements in the understanding of the cellular mechanisms that regulate human muscle protein metabolism during amino acid sufficiency.
Amino Acid Availability and Human Muscle Protein Synthesis
It is very well documented that increased amino acid availability stimulates human muscle protein synthesis [5–13]. Moreover, the ability for nutrients to increase protein synthesis is observed to a similar degree in multiple human muscles that display unique morphological and functional characteristics [5,14]. The elevation in muscle protein synthesis following an increase in amino acid availability is transient such that it peaks after approximately one to two hours which is subsequently followed by a rapid decline [7,15]. Although the time course for muscle protein synthesis is relatively short lived, the peak synthesis rate elicited by increased amino acids is greater than that elicited by acute resistance exercise performed in the fasted state [16,17]. Furthermore, in addition to stimulating muscle protein synthesis, ingestion of amino acids also rapidly alters gene expression, which may be regulated by various microRNAs [18].
It has previously been suggested that human muscle protein synthesis is regulated by extracellular amino acid availability during a continuous amino acid infusion [19]. In contrast, more recent work indicates that intracellular amino acid availability, particularly leucine, may be a principle regulator of human skeletal muscle protein synthesis following ingestion of amino acids [15]. The discrepancy between studies may be explained by differences in the method of amino acid delivery or by differences in the length of the protein synthesis measurement and relative timing of the determination of intracellular amino acid concentration. Nevertheless, it is likely that both intracellular and extracellular amino acid availability in some manner contribute to the regulation of muscle protein synthesis [20], however, future research is necessary to better define the specific role and mechanistic link of each amino acid pool to human muscle protein synthesis.
The stimulatory effect of amino acids on muscle protein synthesis appears to be driven primarily by increases in essential amino acid availability [11]. Of the essential amino acids, leucine appears to have a profound effect on stimulating muscle protein synthesis [21,22] and therefore recent investigations have examined the effect of leucine-enriched solutions on muscle protein metabolism [15,23–26]. These studies have shown that addition of extra leucine to a high-quality protein or essential amino acid solution does not further increase muscle protein synthesis at rest or following exercise in young individuals, however, additional leucine may promote a more anabolic environment through a slight decrease in the rate of muscle protein breakdown [15,23,25,26]. For example, Glynn et al. [15] recently examined muscle protein synthesis in two groups of individuals who ingested a solution of either 10 g of essential amino acids representative of a high-quality protein (leucine, 1.8 g) or 10 g of essential amino acids enriched in leucine (leucine, 3.5 g). Despite a higher arterial leucine concentration and a greater leucine delivery to the muscle in the leucine enriched group, intracellular leucine availability and muscle protein synthesis were elevated to a similar extent in both groups following ingestion. These results suggest, at least in young individuals, that a leucine concentration representative of a high-quality protein is sufficient to induce a maximal muscle protein synthesis rate and that muscle protein synthesis may be regulated by intracellular leucine availability. On the other hand, the extra leucine did appear to decrease markers of muscle protein breakdown, suggesting that additional leucine instead may promote a more anabolic response through a reduction in muscle protein breakdown [15]. Future research is necessary to more clearly define the role of leucine in human muscle protein breakdown.
In contrast to young individuals, older individuals appear to have an attenuated anabolic response to a lower dose of essential amino acids [27] and the inability to respond as robustly to nutrition has been suggested as a key factor responsible for the development of sarcopenia. Acutely, it appears the attenuated muscle protein anabolic response can be restored to that experienced by younger individuals by ingesting greater amounts (>15 g) of amino acids [12] or increasing the leucine content of a supplement [23,24]. On the other hand, Verhoeven et al. [28] recently examined changes in muscle mass and strength following three months of leucine supplementation (7.5 g/day) in 71 year old male subjects. These investigators did not observe any changes in muscle mass or strength following the supplementation period, which may have been due to the subjects already consuming a diet sufficiently high in protein.
mTORC1 Regulation of Muscle Protein Synthesis
The ability of essential amino acids, in particular leucine, to stimulate muscle protein synthesis relies upon key intracellular signaling pathways that transmit a cellular signal (i.e., elevation in essential amino acids) to initiate translation initiation. Specifically, the increase in leucine availability in the muscle cell, in conjunction with the transient elevation in insulin levels brought about by oral leucine ingestion [15,29], activate independent pathways that appear to converge at the mammalian target of rapamycin complex 1 (mTORC1) [21,30]. Upon activation, mTORC1 phosphorylates its down stream targets, ribosomal protein S6 kinase 1 (S6K1) and 4E binding protein 1 (4E-BP1), subsequently enhancing translation initiation. In particular, the hyperphosphorylation of 4E-BP1, via mTORC1 kinase activity, stimulates the release of eukaryotic initiation factor 4E (eIF4E) from 4E-BP1, therefore allowing eIF4E to bind with eIF4G and eIF4A to form the eIF4F translation initiation complex [31]. Increased leucine availability has been shown to stimulate the formation of the eIF4F complex, both dependent and independent of insulin [29,32], indicating leucine stimulates muscle protein synthesis primarily through an increased capacity to bind mRNA during translation initiation. Moreover, the ability for increased leucine availability to stimulate muscle protein synthesis and the formation of the eIF4F complex has been shown to be completely attenuated with administration of rapamycin, a potent mTORC1 inhibitor, indicating the ability for leucine to stimulate muscle protein synthesis is dependent on mTORC1 signaling [21].
Intracellular Proteins Linking Amino Acid Availability and mTORC1 Activation
While knowledge regarding the events downstream of mTORC1 in response to amino acid availability are relatively well understood, a looming question in the study of amino acids and their effect on protein metabolism is how do amino acids regulate mTORC1 activity (i.e., what are the events upstream of mTORC1)? Recent investigations using non-human models have begun to uncover several potential upstream effectors of mTORC1 activity that appear to be sensitive to amino acid availability. However, it should be noted that although these studies provide compelling evidence, a notable lack of human studies currently exist.
The class III PI3K hVps34 (human vacuolar protein sorting-34) has been shown to be inhibited under instances of amino acid starvation, whereas the activity of this protein is increased with amino acid re-addition [33,34]. In addition, the increased activity of this protein appears to be concomitant with increased S6K1 phosphorylation suggesting a role for hVps34 in amino acid sensing to mTORC1 [33]. However, it does not appear that amino acids themselves directly stimulate hVps34 activity, but rather increased amino acid availability stimulates the activity of hVps34 in a calcium dependent manner [35]. Specifically, the proposed model is that increased amino acid levels modulate an influx of calcium into the cell which facilitates an interaction between Ca2+/CaM and hVps34 leading to production of PI(3)P and subsequent mTORC1 activation [35]. However, recent in vivo research in Drosophila with null mutations in Vps34 have failed to observe a link between Vps34 activity and mTORC1 activation [36]. Such contrasting results between studies may be in part due to differences in cell type and/or experimental design (in vivo vs. in vitro). Further in vivo investigation is clearly warranted regarding the role of hVps34 in amino acid sensing to mTORC1.
Another protein that has been implicated as an amino acid sensor to mTORC1 is the Ste20 kinase family member MAP4K3 (mitogen activated protein kinase kinase kinase kinase-3), which has been shown to be both sensitive to amino acid availability and required for the phosphorylation of S6K1 induced by amino acid sufficiency [37]. Moreover, whereas treatment of cells with rapamycin eliminated the MAP4K3 induced phosphorylation of S6K1 and 4E-BP1, MAP4K3 activity itself was not inhibited by rapamycin treatment suggesting that MAP4K3 acts upstream of mTORC1 and induces phosphorylation of S6K1 and 4E-BP1 in an mTORC1 dependent manner [37]. Furthermore, in contrast to studies of hVps34 [33], over expression of MAP4K3 in amino acid starved cells profoundly delayed the dephosphorylation of S6K1 that accompanies amino acid withdrawal [37]. While these findings provide strong support for a role for MAP4K3 as an amino acid sensor to mTORC1, the role of MAP4K3 in vivo remains to be examined.
More recently, compelling data has surfaced providing evidence that the Rag (Ras-related GTPase) small GTPases play a role in mediating the amino acid induced increase in mTORC1 activity [38,39]. Rag proteins function as heterodimers, consisting of RagA or RagB and RagC or RagD (in mammalian cells) [38], and have been shown to interact with mTORC1 via association with Raptor. Stimulation of cells by amino acids enhances the affinity for the Rag heterodimer-raptor association by increasing the GTP loading of RagA and RagB [38]. Furthermore, in cells over expressing active forms of Rag GTPases, the mTORC1 pathway is not only activated (as measured by Thr389 phosphorylation of S6K1), but activity of the pathway becomes insensitive to amino acid withdrawal [38,39]. Similarly, knockdown of RagA or RagC in Drosophila dramatically reduces S6K1 phosphorylation in response to amino acid stimulation [39].
While these data provide a strong link between Rag proteins and mTORC1 activation, in vitro data show the ability for the Rag proteins to stimulate mTORC1 activity is not direct [38]. Rather, in the presence of elevated amino acids it appears that the increased GTP loading of the RagA/B proteins mediates the re-localization of mTORC1 to the peri-nuclear region and large vesicular structures of the cell [38]. Rheb, which is the direct upstream activator of mTORC1, also appears to localize to these regions of the cell [38] and thus the Rag proteins may increase mTORC1 activity indirectly by facilitating an interaction between mTORC1 and Rheb. Further, in addition to blocking the amino acid induced phosphorylation of S6K1, knockdown of RagB or RagC expression also suppresses the insulin-stimulated phosphorylation of S6K1 [38]. This finding suggests that the ability to transmit the anabolic signal from Rheb to mTORC1 is dependent upon the amino acid induced cellular re-localization of mTORC1. Consequently, the cellular translocation of mTORC1 may provide molecular insight into the observations that insulin signaling through mTORC1, and subsequent stimulation of protein synthesis, is reliant on amino acid availability, which has been documented in cell [40] and human [41] studies, and thus provides further support for the theory of mTORC1 re-localization. In addition, it has recently been observed that uncontrolled activity of Rab5 or Arf1, members of the Rab and Arf family GTPases, respectively, profoundly inhibit mTORC1 activity under amino acid stimulated conditions [42]. The inhibitory effect of the constitutively active form of Rab5 is also observed in the presence of an active form of RagA, suggesting that proper functioning of Rab5 is necessary for the activation of mTORC1 via the Rag GTPases. The Rab and Arf family of GTPases are key regulators of vesicle trafficking [42], and therefore these data suggest that proper intracellular trafficking is necessary for mTORC1 activation in response to amino acids. Taken together, it appears that the cellular localization of mTORC1, and potentially other proteins as well, play a pivotal role in activating mTORC1 in the presence of elevated amino acids. We have summarized the potential cellular mechanisms responsible for amino acid control of muscle protein synthesis in Figure 1.
Figure 1.
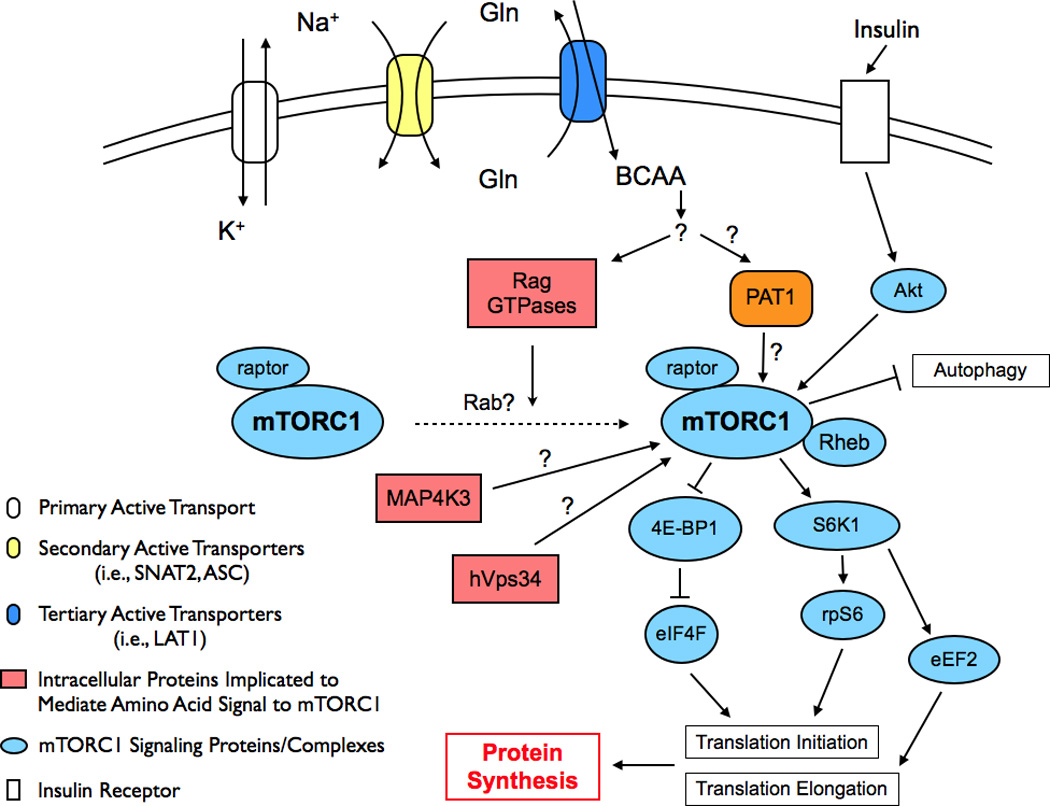
Schematic of potential cellular mechanisms responsible for amino acid control of muscle protein synthesis. Gln, glutamine; BCAA, branch chain amino acids; RAG, Ras-related GTPase; PAT1, proton-assisted amino acid transporter 1; Akt, protein kinase B; mTORC1, mammalian target of rapamycin complex 1; Rab, member of the Rab family of GTPases; Rheb, ras-homologue enriched in brain; MAP4K3, mitogen activated protein kinase kinase kinase kinase-3; 4E-BP1, 4E binding protein 1; S6K1, p70 ribosomal S6 kinase 1; hVps34, human vacuolar protein sorting-34; eIF4F, eukaryotic initiation factor 4F; rpS6, ribosomal protein S6; eEF2, eukaryotic elongation factor 2.
Amino Acid Transporters and mTORC1
The function of specific classes of amino acid transporters and the associated movement of amino acids across the membrane has received recent attention as a key cellular mechanism linking amino acid availability and protein metabolism [43–45]. Specifically, two amino acid transport systems that have been most closely related to mTORC1 signaling are the system L and system A amino acid transporters. System L transporters (i.e., LAT1/SLC7A5) form heterodimers with a glycoprotein (CD98/SLC3A2) and are primarily responsible for the influx of branch chain amino acids, such as leucine, in exchange for the efflux of accumulated cytoplasmic amino acids. Thus, system L transporters are able to produce net intracellular accumulation of a particular amino acid (i.e., leucine) without altering the overall intracellular amino acid concentration. In contrast, system A transporters (i.e., SNAT2) concentrate amino acids within the cell by coupling the influx of amino acids with that of Na+ through secondary active transport (for review of amino acid transporters, see [20,46]).
Recent data have demonstrated a unique coupling mechanism between system L and system A amino acid transporters that ultimately facilitates the influx of branch chain amino acids into the cell [47]. Specifically, system A transporter activity leads to an accumulation of glutamine within the cell, which in turn, provides the driving force for system L transporter exchange and subsequent influx of leucine in exchange for intracellular glutamine efflux. An identical link between system L and system ASC amino acid transporters has also been recently demonstrated, in which glutamine also serves as a substrate for both transporter systems. Moreover, it appears that the coupling mechanism between system L and system ASC amino acid transporters is required for activation of mTORC1 and cell growth as inhibition and knockdown of either of the two transporters diminishes mTORC1 activation and cell growth, respectively, in the presence of amino acids [44]. However, the precise mechanism(s) in which the activity of these transporters elicits an increase in mTORC1 activity remains to be elucidated.
Another class of amino acid transporters that has received attention for a role in mTORC1 signaling is the proton-assisted amino acid transporters (PATs) [45,48,49]. Heublein et al. [45] recently demonstrated that two ubiquitously expressed human PATs, PAT1 and PAT4, are required for the amino acid induced activation of mTORC1 signaling. However, these investigators showed that PAT1 is not present at the cell membrane, but rather is localized intracellularly. This finding suggests that PATs do not enhance mTORC1 signaling through transporting amino acids into the cell, but instead likely serve, in some fashion, to alter the sensitivity of mTORC1 to intracellular amino acid availability.
In addition to a potential role for amino acid transporters upstream of mTORC1, the activation of mTORC1 has also been shown to increase the expression level of several amino acid transporters [50]. In a recent human investigation, Drummond et al. [43] observed that one hour after ingesting 10 g of essential amino acids the expression of LAT1, CD98, SNAT2, and PAT1 mRNA was increased in skeletal muscle, which was subsequently followed by an increase in LAT1 and SNAT2 protein levels. These increased expression levels coincided with an increase in mTORC1 activity, indicating that the increased amino acid transporter expression likely occurred downstream of mTORC1 activation, which may represent a key adaptive response to increase the sensitivity of the cell to a subsequent increase in amino acid availability.
Conclusion
In summary, while the precise cellular mechanisms linking amino acid availability, mTORC1 signaling, and ultimately muscle protein synthesis are still yet to be defined, recent investigations have highlighted a potential role for several intracellular proteins, including hVps34, MAP4K3, and the Rag GTPases. Additionally, recent work also identifies a role for specific amino acid transporters that may not only be necessary for mTORC1 activation, but may also be up regulated as a consequence of mTORC1 activity. The latter likely serves as an adaptive response to sensitize muscle to subsequent amino acid stimulation. In addition, although the focus of this review was primarily on mechanisms linking amino acid availability to protein synthesis, elevated amino acid levels may also contribute to muscle anabolism through a decrease in muscle protein breakdown, which in contrast to protein synthesis, may be augmented by leucine enriched protein or amino acid supplements. Therefore, to improve existing strategies to counteract muscle wasting, future studies are not only necessary to further uncover the mechanisms linking amino acids and muscle protein synthesis, but a better understanding of the link between amino acid availability and muscle protein breakdown is also warranted.
Acknowledgements
Supported by the U.S. NIH/National Institute of Arthritis and Musculoskeletal and Skin Disease grant R01 AR049877, NIH/National Institute on Aging P30 AG024832, and 1UL1RR029876-01 from the NIH/National Center for Research Resources.
Funding Disclosure: Supported by the U.S. NIH/National Institute of Arthritis and Musculoskeletal and Skin Disease grant R01 AR049877, NIH/National Institute on Aging P30 AG024832, and 1UL1RR029876-01 from the NIH/National Center for Research Resources.
REFERENCES
- 1.Matthews DE, Battezzati A. Regulation of protein metabolism during stress. Curr Opin Gen Surg. 1993:72–77. [PubMed] [Google Scholar]
- 2.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90:2070–2074. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 3.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 4.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 5.Carroll CC, Fluckey JD, Williams RH, et al. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab. 2005;288:E479–E485. doi: 10.1152/ajpendo.00393.2004. [DOI] [PubMed] [Google Scholar]
- 6.Fujita S, Dreyer HC, Drummond MJ, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohe J, Low JF, Wolfe RR, et al. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- 9.Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 10.Volpi E, Ferrando AA, Yeckel CW, et al. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorfer B, Andersen JL, Plomgaard P, et al. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glynn EL, Fry CS, Drummond MJ, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010 doi: 10.3945/jn.110.127647. In Press. This paper demonstrates that a leucine content representative of a high-quality protein elicits a maximal muscle protein synthesis response. However, additional leucine instead may play a role in reducing muscle protein breakdown and thus promote a greater overall anabolic response.
- 16.Drummond MJ, Dreyer HC, Fry CS, et al. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72:551S–557S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 18. Drummond MJ, Glynn EL, Fry CS, et al. Essential amino acids increase microRNA-499, −208b, and −23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr. 2009;139:2279–2284. doi: 10.3945/jn.109.112797. This study shows that ingestion of essential amino acids rapidly alters the expression of several microRNA and growth related genes in human skeletal muscle.
- 19.Bohe J, Low A, Wolfe RR, et al. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 22.Smith K, Barua JM, Watt PW, et al. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol. 1992;262:E372–E376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 23.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopman R, Wagenmakers AJ, Manders RJ, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 26.Koopman R, Verdijk LB, Beelen M, et al. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- 27.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 28.Verhoeven S, Vanschoonbeek K, Verdijk LB, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 29.Anthony JC, Anthony TG, Kimball SR, et al. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 30.Atherton PJ, Smith K, Etheridge T, et al. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 33.Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 35.Gulati P, Gaspers LD, Dann SG, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juhasz G, Hill JH, Yan Y, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Findlay GM, Yan L, Procter J, et al. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. This study demonstrates that the Rag GTPases are necessary to activate mTORC1 in the presence of elevated amino acids. Rag GTPases may mediate this effect by re-localizing mTORC1 within the cell.
- 39. Kim E, Goraksha-Hicks P, Li L, et al. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. This paper demonstrates that the Rag GTPases are necessary for the activation of mTORC1 during amino acid sufficiency.
- 40.Nave BT, Ouwens M, Withers DJ, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344 Pt 2:427–431. [PMC free article] [PubMed] [Google Scholar]
- 41.Bell JA, Fujita S, Volpi E, et al. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289:E999–E1006. doi: 10.1152/ajpendo.00170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Kim E, Yuan H, et al. Regulation of mTORC1 by the Rab and Arf GTPases. J Biol Chem. 2010;285:19705–19709. doi: 10.1074/jbc.C110.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drummond MJ, Glynn EL, Fry CS, et al. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–E1018. doi: 10.1152/ajpendo.00690.2009. First study to characterize amino acid transporter expression in human skeletal muscle in response to essential amino acid ingestion. This paper demonstrates that mTORC1 activation likely triggers the expression of specific amino acid transporters which may serve to sensitize muscle to a subsequent increase in amino acid availability.
- 44. Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. Glutamine is necessary for mTORC1 stimulation in the presence of leucine, likely due to the role of glutamine as a substrate that couples system L and system ASC amino acid transporters and subsequent leucine influx into the cell.
- 45. Heublein S, Kazi S, Ogmundsdottir MH, et al. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. This paper demonstrates that PATs are significant regulators for intracellular amino acid sensing and are required for the activation of mTORC1
- 46.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baird FE, Bett KJ, MacLean C, et al. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–E829. doi: 10.1152/ajpendo.00330.2009. Describes that system A and system L amino acid transporters are coupled through tertiary active transport, which serves to increase intracellular leucine concentrations
- 48.Goberdhan DC, Meredith D, Boyd CA, et al. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development. 2005;132:2365–2375. doi: 10.1242/dev.01821. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds B, Laynes R, Ogmundsdottir MH, et al. Amino acid transporters and nutrient-sensing mechanisms: new targets for treating insulin-linked disorders? Biochem Soc Trans. 2007;35:1215–1217. doi: 10.1042/BST0351215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem. 2007;282:16744–16753. doi: 10.1074/jbc.M610510200. [DOI] [PubMed] [Google Scholar]


