Abstract
Sarcoidosis, lymphoma and tuberculosis can often present with similar clinical features – for example, lymphadenopathy, fever, malaise, weight loss, respiratory symptoms, hypercalcaemia – making the establishment of the diagnosis difficult. The authors present a case of a 62-year-old woman with an established diagnosis of sarcoidosis affecting the lymph nodes, who subsequently developed high-grade T cell non-Hodgkin’s lymphoma; the patient was also treated for active tuberculosis at the same time. This case highlights that these conditions can co-exist and that the occurrence of new and rapidly progressive symptoms in patients with an established diagnosis should alert clinicians to vigilantly search for another possible diagnosis.
Background
The differential diagnosis of generalised lymphadenopathy includes – among other conditions – sarcoidosis, tuberculosis and lymphoma. These three conditions often present with similar clinical features – lymphadenopathy, fever, malaise, weight loss, respiratory symptoms, hypercalcaemia – making the establishment of the diagnosis challenging. Furthermore, the presence of one condition does not preclude the development of another.
We present the case of a 62-year-old woman with an established diagnosis of sarcoidosis affecting the lymph nodes, who subsequently developed high-grade peripheral T cell non-Hodgkin’s lymphoma and who was treated for presumed tuberculosis at the same time. To our knowledge, there has been only one other report thus far where there was concurrent presentation of sarcoidosis, tuberculosis and lymphoma, with our case being the first reported in the UK.
With this report and by reviewing the relevant literature we highlight the importance of being aware that these conditions can co-exist and that the occurrence of new and rapidly progressive symptoms in patients with an established diagnosis should alert clinicians to vigilantly search for another possible diagnosis.
Case presentation
A 61-year-old woman was referred to our respiratory clinic in September 2007 from the haematology department where she presented with cervical and axillary lymphadenopathy; an excision lymph node biopsy showed ‘non-caseating granulomatous lymphadenitis’ consistent with the diagnosis of sarcoidosis. She appeared systemically well. She had no respiratory symptoms at the time of presentation but complained of dry eyes and mouth and of a scaly itchy rash on her upper body, arms and legs; this was originally thought to represent cutaneous sarcoidosis but was later found to be ‘granuloma annulare’ on skin biopsy. Her respiratory examination was normal. A CT of neck/thorax/abdomen/pelvis showed enlarged cervical and axillary lymph nodes and normal lung parenchyma. Her spirometry, full blood count, renal, bone, liver function tests, autoimmune screen and immunoglobulin profile were also normal. As she was symptomatic and uncomfortable due to the enlarged lymph nodes, she was started on steroid treatment with prednisolone 40 mg daily and on an oral bisphosphonate for bone prophylaxis.
Of note, a year earlier in May 2006, she had presented to the ENT (ear nose throat) department with an isolated right-sided cervical lymph node, excision biopsy of which concluded an ‘atypical reactive process’. Her medical history included depression, cervical spondylosis, treated cervical intraepithelial neoplasia grade 2 (CIN 2), total abdominal hysterectomy and bilateral breast augmentation.
She responded well to the steroid treatment. By January 2008 she was taking prednisolone 20 mg daily, her lymph nodes had significantly reduced in size and her cutaneous lesions had also significantly improved. Her steroid treatment was slowly being weaned down and on regular reviews in the chest clinic she remained clinically stable and asymptomatic.
In September 2008, a year after her initial presentation to our department, she complained of a 2-month history of being unwell with general malaise, loss of appetite, weight loss of 5 kg, musculoskeletal pains, gastro-oesophageal reflux, a dry cough, shortness of breath on exertion and headaches. On examination she had extensive cervical and axillary lymphadenopathy and a palpable spleen. She was at that point admitted for further investigation.
Investigations
On admission, her full blood count showed: normocytic anaemia with haemoglobin of 8 g/dl, elevated white cell count of 36.8×109/l – with a differential of 30.2×109/l neutrophils, 2.2×109/l lymphocytes, 3.5×109/l monocytes, 0.52×109/l eosinophils and 0.44×109/l basophils, thrombocytopenia with a platelet count of 52×109/l and a normal clotting profile. Her blood biochemistry showed: urea 11 mmol/l, creatinine 130 mmol/l, corrected calcium 2.81 mmol/l, albumin 30 g/l, C-reactive protein 87 mg/l, normal liver enzyme profile, normal immunoglobulin electrophoresis, normal parathyroid hormone level and normal ACE level.
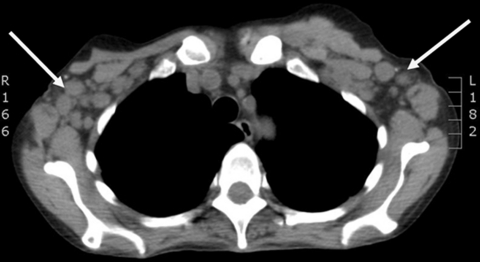
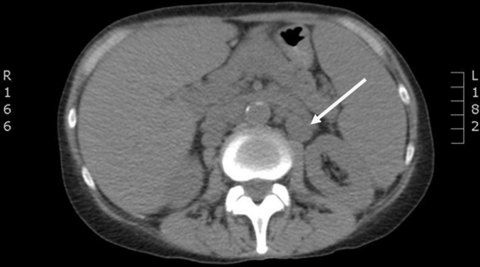
A chest x-radiograph was unremarkable but a non-contrast CT of thorax/abdomen/pelvis demonstrated widespread significant lymphadenopathy above and below the diaphragm – cervical, axillary, para-aortic, mesenteric and inguinal lymph nodes – splenomegaly, two liver lesions of unknown significance and a few pulmonary nodules (figures 1 and 2). Stage 4 lymphoma was suspected.
Figure 1.
CT thorax showing bilateral axillary lymphadenopathy (arrows).
Figure 2.
CT abdomen showing retroperitoneal lymphadenopathy (arrow).
Over the next 10 days as an inpatient, she deteriorated dramatically. She experienced night sweats and spiking fevers in the early hours of the morning. These episodes were associated with intermittent confusion and lactic acidosis, both of which resolved in the daytime. Her white cell count progressively increased and reached 60×109/l (mainly neutrophils and lymphocytes). Repeated septic screening (blood, urine, sputum) was negative; she, nevertheless, received intravenous antibiotics (meropenem, vancomycin and metronidazole) to cover possible sepsis.
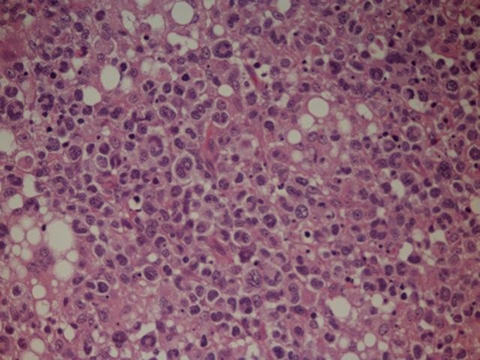
She underwent an axillary lymph node surgical excision, histological examination of which showed: a diffuse infiltrate of large atypical lymphoid cells which showed considerable nuclear pleiomorphism; immunohistochemistry showed that these T-cells expressed CD45, CD2 and CD3 and were negative for CD4, CD8, CD30 and ALK; in addition there were areas of necrosis and occasional multi-nucleated giant cells. These appearances were consistent with a high-grade peripheral T cell non-Hodgkin’s lymphoma (figure 3). Microbiological examination of the lymph node biopsy also showed the presence of acid fast bacilli on Zielh-Nielsen stain suggesting active tuberculosis. This was, nevertheless, not further confirmed or proven on TB culture.
Figure 3.
H&E slide showing a diffuse infiltrate of large atypical lymphoid cells consistent with peripheral T cell non-Hodgkin’s lymphoma (photomicrograph x40).
Differential diagnosis
Stage 4 high-grade peripheral T cell non-Hodgkin’s lymphoma
Possible active tuberculosis
On the background of known sarcoidosis.
Treatment
At this point, she was commenced on quadruple antituberculous treatment with isoniazid, rifampicin, pyrizinamide and ethambutol at the appropriate doses, pyridoxine to prevent peripheral neuropathy and also high dose of steroids with dexamethasone 8 mg twice daily (recommended by the oncologist for the lymphoma). She also received supportive treatment with intravenous fluids and nasogastric tube feeding.
Outcome and follow-up
Her condition rapidly deteriorated in the course of a few days; she became very weak, confused and metabolically unstable. She was considered not fit enough to withstand chemotherapy for her stage 4 high-grade lymphoma by the oncologists and she died 12 days after the day of admission to hospital.
Discussion
Sarcoidosis is a multi-system granulomatous disease of unknown aetiology with pulmonary and extrapulmonary manifestations. Extrapulmonary sarcoidosis can affect the lymph nodes giving rise to lymphadenopathy and systemic features can include fever, malaise and hypercalcaemia.1 Tuberculosis and lymphoma can also lead to similar clinical presentations. Thus, it is often difficult to establish the diagnosis. Furthermore, the presence of one condition does not preclude the development of another and this is discussed below.
Sarcoidosis and lymphoma
The association between sarcoidosis and lymphoma has been thoroughly reviewed.2
The sarcoidosis-lymphoma syndrome was first described by Brincker in 1986.3 He observed that malignant lymphoproliferative disease developed at least 5.5 times more often than expected in middle-aged patients with chronic active sarcoidosis. The syndrome has three characteristics: (1) the onset of sarcoidosis precedes the diagnosis of the associated lymphoma; (2) the median age of onset of sarcoidosis in patients who develop lymphoma is greater than 40 years and (3) Hodgkin’s lymphoma is the most commonly associated lymphoma followed by non-Hodgkin’s and then other lymphoproliferative disorders.
As an explanation for the syndrome, it was speculated that in sarcoidosis, the increased mitotic activity of lymphocytes due to the immune inflammatory response in the involved tissues may increase the risk of lymphocytes undergoing mutation and subsequent malignant transformation.4
Since then, a number of cases with co-existing sarcoidosis and lymphoproliferative disorders have been reported in the literature.5 One such report describes the case of a 71-year-old woman presenting with fever of unknown origin due to sarcoidosis-lymphoma syndrome, as active sarcoidosis and B cell lymphoma were found to be concurrently present.6
There is conflicting evidence as to whether patients with sarcoidosis are more likely to develop a cancer. Some studies suggest that sarcoidosis may be a predisposing factor for the development of a lymphoid malignancy in affected organs7 while others dispute such associative risk.8 9
In addition to the sarcoidosis-lymphoma syndrome, whereby sarcoidosis precedes the development of the malignancy by a number of years, there are also patients with cancer – and particularly haematological malignancies – in whom sarcoidosis subsequently appears.10 Sometimes, but not always, this is seen after antineoplastic treatment administration.
Sarcoidosis and tuberculosis
Both sarcoidosis and tuberculosis are granulomatous diseases. The association of sarcoidosis with tuberculosis is complex.11 Mycobacterium tuberculosis has been implicated in the pathogenesis of sarcoidosis.12 Tuberculosis has been described to precede but also to occur concurrently with sarcoidosis, usually as an opportunistic infection in sarcoidosis patients who are on immunosuppressant treatment.13 14
Sarcoidosis, lymphoma and tuberculosis
We found only one other case report whereby concurrent presentation of sarcoidosis, tuberculosis and lymphoma was reported in a single patient who presented with dyspnoea, fever and cough.15 This case bears similarities as well as differences to our case, as highlighted below.
This patient15 developed anaplastic T cell lymphoma 7 years after her initial diagnosis with sarcoidosis. In fact, she was completely asymptomatic without any treatment for 7 years before re-presenting with symptoms of exertional dyspnoea and cough. At this point, she was treated with systemic steroids for about 9 months but progressively deteriorated which led to her admission and establishment of the concurrent diagnoses.
In our patient, the time interval between the initial diagnosis of sarcoidosis and the development of lymphoma was much shorter at 1 year. Our patient had been stable for one year on systemic steroids before developing new and rapidly progressive clinical features – weight loss, fever of unexplained origin, cough and shortness of breath, new lymphadenopathy and new-onset hypercalcaemia – which prompted the pursue of an alternative diagnosis.
In the other case,15 acid fast bacilli were detected on Zielh–Nielsen stain but TB-PCR and subsequent culture were negative. Nevertheless, she was treated as a case of tuberculosis based on clinical suspicion, in view of the fact that tuberculosis has relatively high prevalence in that region of Turkey.
In our patient, an initial ZN stain on the lymph node biopsy sample showed the presence of acid fast bacilli. In our region in the UK tuberculosis is not very prevalent; however, at the time, the patient showed clinical signs of sepsis (with elevated neutrophils and repeated sterile blood cultures) and was on immunosuppressive medication (steroids), and was thus treated with anti-TB medications. Retrospectively, especially in view of the fact that the patient died within a few days, we cannot be sure of the significance of the acid fast bacilli detected, as tuberculosis was not subsequently confirmed on TB culture.
To summarise, we report a rare case – the first in the UK – of a 62-year old woman with an established diagnosis of sarcoidosis affecting the lymph nodes, who subsequently developed high grade T cell non-Hodgkin’s lymphoma and tuberculosis, at the same time. This case and the extensive review of the literature highlights three important leaning points, shown below.
Learning points.
-
▶
Lymphoma, sarcoidosis and tuberculosis can present with similar clinical features.
-
▶
The three conditions can co-exist.
-
▶
Clinicians should be alerted by the presentation of new-onset and rapidly progressive symptoms in patients with an established diagnosis – especially if they have been stable or on treatment – and to vigilantly search for another possible diagnosis.
Acknowledgments
The authors wish to thank Dr Margaret Wilkins (Department of Histopathology, Bedford Hospital) and Dr John Grant (Addenbrooke’s Hospital) for the pathological examinations.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.American Thoracic Society Statement on sarcoidosis. Am J Respir Crit Care Med 1999;160:736–55 [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 2007;25:326–33 [DOI] [PubMed] [Google Scholar]
- 3.Brincker H. The sarcoidosis-lymphoma syndrome. Br J Cancer 1986;54:467–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brincker H. Sarcoidosis and malignancy. Chest 1995;108:1472–4 [DOI] [PubMed] [Google Scholar]
- 5.Suvajdzic N, Milenkovic B, Perunicic M, et al. Two cases of sarcoidosis-lymphoma syndrome. Med Oncol 2007;24:469–71 [DOI] [PubMed] [Google Scholar]
- 6.de León DG, Shifteh S, Cunha BA. FUO due to sarcoidosis-lymphoma syndrome. Heart Lung 2004;33:124–9 [DOI] [PubMed] [Google Scholar]
- 7.Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest 1995;107:605–13 [DOI] [PubMed] [Google Scholar]
- 8.Rømer FK, Hommelgaard P, Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J 1998;12:906–12 [DOI] [PubMed] [Google Scholar]
- 9.Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suen JS, Forse MS, Hyland RH, et al. The malignancy-sarcoidosis syndrome. Chest 1990;98:1300–2 [DOI] [PubMed] [Google Scholar]
- 11.Baughman RP. Can tuberculosis cause sarcoidosis? New techniques try to answer an old question. Chest 1998;114:363–4 [DOI] [PubMed] [Google Scholar]
- 12.Drake WP, Pei Z, Pride DT, et al. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerging Infect Dis 2002;8:1334–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litinsky I, Elkayam O, Flusser G, et al. Sarcoidosis: TB or not TB? Ann Rheum Dis 2002;61:385–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papaetis GS, Pefanis A, Solomon S, et al. Asymptomatic stage I sarcoidosis complicated by pulmonary tuberculosis: a case report. J Med Case Reports 2008;2:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tufan A, Ranci O, Sungur A, et al. Concurrent presentations of the sarcoidosis, tuberculosis and lymphoma in a single patient. Respir Med 2006;100:951–3 [DOI] [PubMed] [Google Scholar]