Abstract
We diagnosed leptospirosis in 2 patients exposed to southern flying squirrels imported from the United States to Japan. Patients worked with exotic animals in their company. Leptospira isolates from 1 patient and 5 of 10 squirrels at the company were genetically and serologically identical and were identified as Leptospira kirschneri.
Keywords: Exotic animals, Leptospira, Leptospirosis, Southern flying squirrel
Leptospirosis is a worldwide zoonosis caused by infection with Leptospira interrogans sensu lato species. Leptospira is mostly transmitted to humans through contaminated water or soil and by direct contact with a variety of infected animals (1–3). To date, a variety of wild animals have been imported from foreign countries to Japan. In this study, 2 men working at an animal trading company were infected with Leptospira spp. To determine the source of infection, Leptospira spp. were isolated from animals in their company and sequenced.
The Cases
An animal trading company in Shizuoka, Japan, imported 106 southern flying squirrels from Miami, Florida, on March 27, 2005. Three workers handled these animals, which were housed 10 animals to a cage. Before patient 1 became ill, the workers dressed casually and touched the animals with bare hands in their routine work. Wild rats (such as Rattus norvegicus or R. rattus) had not invaded the animal house.
On April 22, 2005, patient 1, a 29-year-old man who handled a variety of exotic animals at the company, was hospitalized in Shizuoka Saisei-kai General Hospital with fever (temperature 40°C), headache, chills, nausea, vomiting, jaundice, and uremia, symptoms similar to those of locally acquired leptospirosis. Leptospirosis was diagnosed by polymerase chain reaction (PCR) targeted to the flagellin gene (flaB) and confirmed serologically with convalescent-phase serum by microscopic agglutination test. The patient was seronegative and PCR-negative for hantavirus, which causes symptoms similar to those observed in the patient. He was treated with an intramuscular injection of streptomycin (2 mg/day) for 7 days, which is the recommended treatment for leptospirosis in Japan (4); he consequently recovered.
On June 1, 2005, patient 2, a 28-year-old man who worked at the same company, was hospitalized in Shizuoka Saisei-kai General Hospital with fever (temperature 39°C), headache, chills, nausea, vomiting, jaundice, and uremia. The patient had been in contact with imported animals. He recovered with intramuscular injections of streptomycin (2 mg/day) for 3 days, followed by treatment with oral amoxicillin for 3 days.
Leptospira DNA was detected in serum samples from patient 1 and whole blood from patient 2 by flaB PCR (5). Sequences were determined by Prism 3130-avant DNA Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences of flaB detected from both patients were identical and showed a high degree of similarity to L. kirschneri.
Diagnosis was performed serologically by microscopic agglutination test with a panel of Leptospira reference strains (3). Convalescent-phase serum samples from both patients reacted to L. kirschneri strain Moskva V and strains isolated from southern flying squirrels, although serum collected on the day of hospitalization was negative in both patients (Table 1). To cultivate Leptospira, a few drops of blood from patient 2 were placed in several tubes of Ellinghausen-McCullough-Johnson-Harris medium supplemented with 2.5% rabbit serum. After 7 days of incubation at 30°C, Leptospira was detected from the culture (isolates P5.4, P10.1, P10.2).
Table 1. Microscopic agglutination titer of patients' sera collected while hospitalized and during the convalescent phase.
| Patient | Leptospira strain used as antigen* | Patient serum |
|
|---|---|---|---|
| Hospitalized | Convalescent-phase | ||
| 1 | Serovar Grippotyphosa Moskva V | <50 | 100 |
| Animal isolate AM3 | <50 | 800 | |
| Animal isolate AM1 | <50 | 800 | |
| 2 | Serovar Grippotyphosa Moskva V | <50 | 200 |
| Animal isolate AM3 | <50 | 200 | |
*Samples were not reactive to a panel of representative serovars, Australis, Autumnalis, Carlos, Bataviae, Cynopteri, Hebdomadis, Copenhageni, Icterohemorrhagiae, Javanica, Pomona, Pyrogenes, Hardjo, Sejroe, Wolffi, and Tarassovi; serovars Canalzonae, Huanuco, Muelleri, and Valbuzzi belong to serogroup Grippotyphosa.
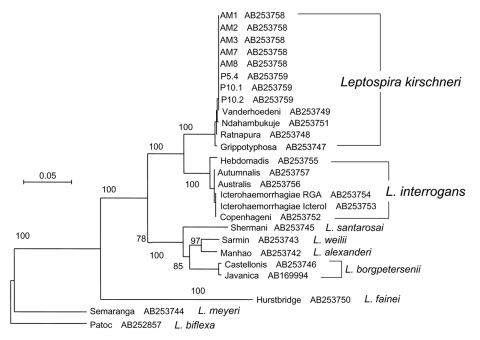
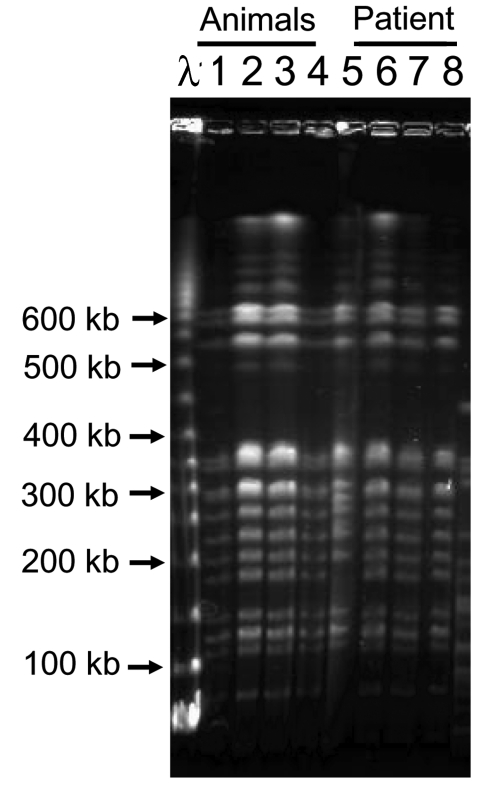
To determine the validity of the association between animals held by the company and the illness, exotic animals (75 animals, 7 species) housed in the company were tested. Leptospira was isolated from 5 of 10 kidney cultures (isolates AM1, AM2, AM3, AM7, AM8) from southern flying squirrels. DNA from the urinary bladders, including the animals' urine, was extracted by using proprietary DNA extraction kits (Quick gene, Fuji Film Co., Tokyo, Japan). Five of 10 southern flying squirrels were flaB PCR-positive (Table 2). Species of the isolates were identified by using flaB and DNA gyrase B subunit gene (gyrB) sequencing analysis. We amplified 1.2-kb partial sequences of gyrB by using primers UP1TL (5´-CAyGCnGGnGGnAArTTyGA-3´; n: A, G, T, or C; r: A or G; y: C or T) and UP2rTL (5´-TCnACrTCnGCrTCnGTCAT-3´; n: A, G, T, or C; r: A or G) (6). The isolates obtained from patient 2 and southern flying squirrels had identical flaB (data not shown) and gyrB (Figure 1) DNA sequences and were identified as L. kirschneri. The flaB sequences from the serum of patient 1 and whole blood of patient 2 were identical to those of isolates from patient 2 and animals. Additionally, restriction fragment length polymorphism (RFLP) analysis based on pulse-field gel electrophoresis was conducted (7). These isolates showed identical RFLP patterns (Figure 2), which suggests that patients were infected with L. kirschneri from southern flying squirrels.
Table 2. Detection and isolation of Leptospira from imported animals in the company.
| Animal | No. samples positive/no. samples tested |
|
|---|---|---|
| Kidney culture | flaB PCR | |
| Spiny mouse (Acomys cahirinus) | 0/9 | 0/9 |
| House mouse (species unknown) | 0/4 | 0/4 |
| Golden spiny mouse (Acomys russatus) | 0/13 | 0/13 |
| Mongolian gerbil (Meriones unguiculatus) | 0/9 | 0/9 |
| Southern flying squirrel (Graucomys volans) | 5/10* | 5/10* |
| Baluchistan pygmy jerboa (Salpingotulus michaelis) | 0/20 | 0/20 |
| Siberian chipmunk (Tamias sibiricus) | 0/10 | 0/10 |
*Four of 5 culture-positive animals were positive by polymerase chain reaction (PCR). Remaining culture-positive animal was PCR negative, whereas 1 culture-negative animal was PCR positive.
Figure 1.
Phylogenetic tree based on the Leptospira DNA gyrase B subunit gene (gyrB) sequence. The sequences obtained have been deposited in DDBJ/GenBank/EMBL with accession numbers indicated.
Figure 2.
Pulsed-field gel electrophoresis analysis of NotI restriction fragment of Leptospira isolates from patient 2 and southern flying squirrels. Leptospira cells were lysed, and DNA was digested with restriction enzyme NotI in agarose gels. DNA in the gel was electrophoresed with 1% pulsed-field certified agarose in 0.5× Tris-borate-EDTA buffer under a pulse time of 10 s for 5 h and 30 s for 12 h, followed by 60 s for 7 h at 200 V. Lane 1, AM1; lane 2, AM2; lane 3, AM3; lane 4, AM7; lane 5, serovar Grippotyphosa strain Moskva V; lane 6, P10.2; lane 7, P10.1; lane 8, P5.4; λ phage DNA concatemer is used as a DNA size marker. Isolate AM8 showed an identical restriction fragment length polymorphism pattern to that of others.
To determine serovar of the isolates, a cross-agglutination test was performed with a panel of hyperimmune rabbit serum raised to representative serovars Icterohemorrhagiae, Copenhageni, Autumnalis, Hebdomadis, Australia, Grippotyphosa, Javanica, and Castellonis, which are present in Japan. These isolates reacted with anti-Grippotyphosa serum but not with the others (data not shown). Convalescent-phase sera from patients reacted with Leptospira isolates from the squirrels and also with serovar Grippotyphosa strain Moskva V (Table 1).
On April 24, the local health government prohibited the company from trading animals and directed them to use protection, such as latex gloves and disinfection of the floor with sodium hypochlorite, against infection. On June 2, all southern flying squirrels were euthanized by carbon dioxide, and the animal house was disinfected by the local health government. PCR detected flaB DNA on the surface of the squirrels' bodies and in urine on the soaked paper in the cages; the sequences were identical to those of the isolates. Before the first case was detected, 27 southern flying squirrels had been distributed to retail pet shops. Sixteen were returned, 2 died, 7 remained at pet shops, and 2 had been sold. The 2 sold animals and 7 remaining at the pet shops were recovered and euthanized. No illness was reported among persons in contact with these animals.
Conclusions
Serovar Grippotyphosa commonly causes canine leptospirosis (8,9) and infects a variety of domestic and wild animals in the United States (10–13). In Japan, serovar Grippotyphosa is distributed in the southernmost islands, the Okinawa archipelago (14), but not on Honshu Island, the main island. Patients did not travel to Okinawa or foreign countries before disease onset. Our findings support the conclusion that the patients were infected with L. kirschneri serovar Grippotyphosa by contact with southern flying squirrels. Similarly, in the United States, humans have acquired monkeypox infection from pet prairie dogs, which had themselves been infected by exotic African rodents (15); these findings show that exotic pets represent a substantial hazard. The outbreak demonstrated how new infectious diseases could be emerging because of importation from overseas. If, during shipping and housing of the animals, the infection were to have expanded among southern flying squirrels, the infection rates and risk for humans would have increased. The leptospirosis cases reported here warn against importing exotic animals.
Acknowledgments
This work was supported in part by grant H15-Shinkou-14 and H15-Shinkou-12 for Research on Emerging and Reemerging Infectious Disease from the Ministry of Health, Labour and Welfare.
Biography
Dr Masuzawa is a professor at the Faculty of Pharmaceutical Sciences, Chiba Institute of Science, Choshi, Japan. His primary research interests are molecular epidemiology and the ecology of zoonotic and tickborne pathogens, such as Leptospira, Borrelia, and Anaplasma.
Footnotes
Suggested citation for this article: Masuzawa T, Okamoto Y, Une Y, Takeuchi T, Tsukagoshi K, Koizumi N, et al. Leptospirosis in squirrels imported from United States to Japan. Emerg Infect Dis [serial on the Internet]. 2006 Jul [date cited]. http://dx.doi.org/10.3201/eid1207.060370
References
- 1.Faine S. Leptospira and leptospirosis. Boca Raton (FL): CRC Press; 1994. [Google Scholar]
- 2.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpstra WJ. Human leptospirosis: guidance for diagnosis, surveillance and control. Geneva: World Health Organization and International Leptospirosis Society; 2003. [Google Scholar]
- 4.Kobayashi Y. Human leptospirosis: management and prognosis. J Postgrad Med. 2005;51:201–4. [PubMed] [Google Scholar]
- 5.Kawabata H, Dancel LA, Villanueva SY, Yanagihara Y, Koizumi N, Watanabe H. flaB-polymerase chain reaction (flaB-PCR) and its restriction fragment length polymorphism (RFLP) analysis are an efficient tool for detection and identification of Leptospira spp. Microbiol Immunol. 2001;45:491–6. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor KA, Barbour AG, Thomas DD. Pulsed-field gel electrophoretic analysis of leptospiral DNA. Infect Immun. 1991;59:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward MP, Guptill LF, Prahl A, Wu CC. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002). J Am Vet Med Assoc. 2004;224:1958–63. 10.2460/javma.2004.224.1958 [DOI] [PubMed] [Google Scholar]
- 9.Harkin KR, Roshto YM, Sullivan JT, Purvis TJ, Chengappa MM. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J Am Vet Med Assoc. 2003;222:1230–3. 10.2460/javma.2003.222.1230 [DOI] [PubMed] [Google Scholar]
- 10.Richardson DJ, Gauthier JL. A serosurvey of leptospirosis in Connecticut peridomestic wildlife. Vector Borne Zoonotic Dis. 2003;3:187–93. 10.1089/153036603322662174 [DOI] [PubMed] [Google Scholar]
- 11.Stamper MA, Gulland FM, Spraker T. Leptospirosis in rehabilitated Pacific harbor seals from California. J Wildl Dis. 1998;34:407–10. [DOI] [PubMed] [Google Scholar]
- 12.Gese EM, Schultz RD, Johnson MR, Williams ES, Crabtree RL, Ruff RL. Serological survey for diseases in free-ranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. J Wildl Dis. 1997;33:47–56. [DOI] [PubMed] [Google Scholar]
- 13.Williams DM, Smith BJ, Donahue JM, Poonacha KB. Serological and microbiological findings on 3 farms with equine leptospiral abortions. Equine Vet J. 1994;26:105–8. 10.1111/j.2042-3306.1994.tb04345.x [DOI] [PubMed] [Google Scholar]
- 14.Narita M, Fujitani S, Haake DA, Paterson DL. Leptospirosis after recreational exposure to water in the Yaeyama Islands, Japan. Am J Trop Med Hyg. 2005;73:652–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Guarner J, Johnson BJ, Paddock CD, Shieh WJ, Goldsmith CS, Reynolds MG, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]