Abstract
Aims
Clinical markers of cardiac autonomic function, such as heart rate and response to exercise, are important predictors of cardiovascular risk. Tetrahydrobiopterin (BH4) is a required cofactor for enzymes with roles in cardiac autonomic function, including tyrosine hydroxylase and nitric oxide synthase. Synthesis of BH4 is regulated by GTP cyclohydrolase I (GTPCH), encoded by GCH1. Recent clinical studies report associations between GCH1 variants and increased heart rate, but the mechanistic importance of GCH1 and BH4 in autonomic function remains unclear. We investigate the effect of BH4 deficiency on the autonomic regulation of heart rate in the hph-1 mouse model of BH4 deficiency.
Methods and results
In the hph-1 mouse, reduced cardiac GCH1 expression, GTPCH enzymatic activity, and BH4 were associated with increased resting heart rate; blood pressure was not different. Exercise training decreased resting heart rate, but hph-1 mice retained a relative tachycardia. Vagal nerve stimulation in vitro induced bradycardia equally in hph-1 and wild-type mice both before and after exercise training. Direct atrial responses to carbamylcholine were equal. In contrast, propranolol treatment normalized the resting tachycardia in vivo. Stellate ganglion stimulation and isoproterenol but not forskolin application in vitro induced a greater tachycardic response in hph-1 mice. β1-adrenoceptor protein was increased as was the cAMP response to isoproterenol stimulation.
Conclusion
Reduced GCH1 expression and BH4 deficiency cause tachycardia through enhanced β-adrenergic sensitivity, with no effect on vagal function. GCH1 expression and BH4 are novel determinants of cardiac autonomic regulation that may have important roles in cardiovascular pathophysiology.
Keywords: Tetrahydrobiopterin, Autonomic function, Exercise, Heart rate
1. Introduction
The autonomic nervous system plays a pivotal role in haemodynamic regulation in health and in disease states.1,2 Measures of autonomic function such as heart rate during rest and exercise are important quantitative predictors of cardiovascular risk and prognosis.3–6 Regular physical exercise is associated with improved autonomic balance and is an important protective factor in long-term cardiovascular health.7,8
BH4 is critical for synthesis of biogenic amines, as a required cofactor for tyrosine hydroxylase that catalyse required steps in the production of catecholamines.9–11 Alterations in tyrosine hydroxylase activity in humans lead to differences in urinary catecholamine secretion and haemodynamic stress responses.11 BH4 is also an essential cofactor for nitric oxide synthase (NOS) enzymes.12,13 These enzyme systems play important roles in the autonomic nervous system.14–20 BH4-dependent enzymes appear to have pivotal functional roles in cardiovascular autonomic control, suggesting that regulation of BH4 through GTPCH may be a common upstream mediator of autonomic function.
Genetic polymorphisms of genes involved in autonomic signalling have been associated with altered autonomic control in humans and in experimental models.21 In particular, genetic variants in GCH1, encoding GTP-cyclohydrolase 1 (GCH1), the rate limiting step in biosynthesis of the enzymatic cofactor tetrahydrobiopterin (BH4), have recently been associated with alterations in markers of autonomic activity and cardiovascular risk.22,23 Patients with DOPA-responsive dystonia (DRD) due to mutations affecting GCH1 expression or function were found to be normotensive but had a higher resting heart rate. Plasma catecholamines were reduced at baseline and the catecholamine response to 60° head-up tilt was blunted.23 Furthermore, individuals carrying a common polymorphism in the 3′ untranslated region (UTR) of the GCH1 gene (C + 243T variant) were also found to have impaired autonomic control of cardiac function, leading to an increased minimum heart rate and altered barorecptor responsiveness. These GCH1 variants appear to be functionally associated with altered GCH1 expression,22,24 the key determinant of BH4 levels,25 raising the possibility that constitutive variation in BH4 biosynthesis may be important in modulating cardiovascular autonomic regulation. However, the mechanistic importance of GCH1 and BH4 in cardiovascular autonomic regulation remains unknown.
We sought to investigate the mechanistic role of altered GCH1 expression in cardiovascular autonomic function, using the hph-1 mouse, a genetic mouse strain with reduced GCH1 expression,25,26 as a model of the observed genetic variants in human GCH1. We used radiotelemetric analysis of haemodynamic changes during rest, activity and physical exercise-training, combined with in vitro assessment of vagal and sympathetic cardiac regulation, in order to establish the impact of altered GCH1 expression and BH4 levels on cardiac autonomic regulation.
2. Methods
An expanded Materials and Methods section is available in the Supplementary material online.
2.1. Animals
The generation of the hph-1 mouse model of BH4 deficiency has been previously described.27 Animals were back crossed onto C57bl/6 and heterozygous breeding was used to generate matched hph-1 homozygous and wild-type (WT) littermates. Animals were genotyped as previously described26 (see Supplementary material online). Studies were performed in accordance with both the UK Home Office Animals (Scientific Procedures) Act 1986 and the guidelines for the care and use of experimental animals of the US National Institutes of Health. The study was approved by the Local University Ethical Approval Panel. Animals were 10–16 weeks at the time of sacrifice and were euthanized by cervical dislocation unless otherwise indicated below.
2.2. Western blot analysis
Western blot analysis of homogenized hph-1 and WT heart tissue was used to compare levels of eNOS, nNOS, GTPCH, β1 adrenoceptor, GRK2, β-arrestin, and tyrosine hydroxylase protein levels. GAPDH used to ensure equal protein loading (see Supplementary material online).
2.3. Measurement of eNOS, nNOS, and GCH1 expression by RT–PCR
RNA was isolated by homgenizing tissue samples in Trizol (Invitrogen) and then purifying RNA with the RNeasy micro column method (Qiagen). RNA was quantified by nano-drop and gene expression was measured by synthesizing cDNA using the QuantiTect Reverse Transcription Kit (Invitrogen, UK) and real-time RT–PCR using taqman probes (Applied Biosystems, USA). Gene expression levels of eNOS, nNOS, and GCH1 were normalized to the housekeeping gene GAPDH (see Supplementary material online).
2.4. Measurement of biopterins by HPLC
BH4, BH2 (7,8-dihydrobiopterin) and B (biopterin) from homogenized hph-1 and WT tissues were separated by reverse-phase high pressure liquid chromotography (HPLC) and quantified using electrochemical detection (for BH4) and fluorescence detection (for BH2 and B).28
2.5. Measurement of GTPCH enzymatic activity
GTPCH activity was measured in heart extracts as described previously.29 In brief following homogenization in Lysis buffer, lysates were incubated in the dark for 1 h with 10 mMol GTP. Samples then underwent iodine oxidation and deproteination. The reaction was stopped with 0.1 M ascorbic acid and alkaline phosphatase. Neopterin quantification was performed by HPLC analysis (see Supplementary material online).
2.6. Measurement of heart rate and blood pressure by tail-cuff plethysmography
Baseline blood pressure and heart rate were measured using the VisitechR tail-cuff plethysmography system30 (see Supplementary material online).
2.7. Telemetry implantation operation
PAC10 radiotelemeters (DSI, Transoma Medical Inc.) were implanted in 10–16-week-old mice under carefully titrated isofluorane anaesthesia (3–4% for induction, 1.5–2% for maintenance) adjusted to ensure abolition of established murine pain reflexes, including the pedal withdrawal reflex. Mice were kept on a warming blanket and eye protection provided with Viscotears. The surgical field was sterilized with chlorhexidine and the procedure performed under an extraction hood in full sterile conditions. Buprenorphine 0.02 mg was administered to provide post-operative analgesia. Telemeter catheters were implanted in the left carotid artery with the body of the telemeter placed in a subcutaneous pocket equidistant from the fore and hind paw.31,32 The wound was then closed with 4.0 vicryl. Post-operatively, mice were held in a recovery chamber at 37° until mobile and subsequently moved to a recovery cabinet at 28° for a further 4 h. Animals were kept under close observation for the duration of the experiment both by the experimental team and the veterinary officer. Additional buprenorphine and subcutaneous 0.9% normal saline were given where necessary although recovery was usually rapid. All mice underwent 14 days post-operative recovery before commencement of voluntary running. Pressure traces were checked and subjects with damping of the haemodynamic profile were excluded from the analysis. On completion of the experiment, animals were euthanized by terminal anaesthesia with an overdose of isofluorane.
2.8. Haemodynamic measurements during exercise training by radiotelemetry
PAC10 radiotelemeters (DSI Inc.) were implanted via the left carotid artery and used to continuously measure arterial pressure waveforms and cage activity. After a period of post-operative recovery, a freely rotating running track was introduced to facilitate voluntary wheel running over a period of 5 weeks exercise training.33 Daily data were analysed on a beat-to-beat basis according to three activity states; wheel running, cage activity, and rest. Changes in heart rate recovery at the end of exercise bouts were also assessed. A rolling average was obtained for each subject for the period of exercise training (see Supplementary material online).
2.9. In vitro measurement of heart rate response to vagal nerve stimulation, stellate stimulation, carbamylcholine, isoproterenol, and forskolin
Isolated preparations of atria with intact mediastinal structures including the right vagus nerve and stellate gangion were mounted in organ baths. Heart rate was transduced during direct electrical stimulation of the vagus nerve or stellate ganglion by an external electrode, and during direct organ bath application of carbamylcholine, isoproterenol, or forskolin in separate experiments (see Supplementary material online).
2.10. Cyclic nucleotide measurements
cAMP and cGMP were measured by enzyme immunoassay from snap frozen homogenized isolated atria at baseline and following organ bath-applied isoproterenol (see Supplementary material online).
2.11. In vivo effect of chronic administration of propranolol on heart rate and blood pressure
Heart rate and blood pressure were measured by tail-cuff plethysmography as described above before, during, and after administration of 0.5 mg/mL propranolol to the drinking water for 5 days (see Supplementary material online).
2.12. Measurement of urinary and tissue catecholamines
Dopamine, epinephrine, and norepinephrine levels were measured by HPLC from urine obtained by direct percutaneous bladder aspiration and normalized for urinary creatinine. Dopamine and norepinephrine levels were measured by HPLC from homogenized adrenal and whole heart (see Supplementary material online).
2.13. Statistics
Data are presented as mean ± SEM. Groups were compared using the Mann–Whitney U-test for non-parametric data or the Student's t-test for parametric data. When comparing multiple groups, data were analysed by analysis of variance (ANOVA). A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Expression of GCH1 and NOS mRNA and protein, GTPCH activity and effects on biopterin concentrations
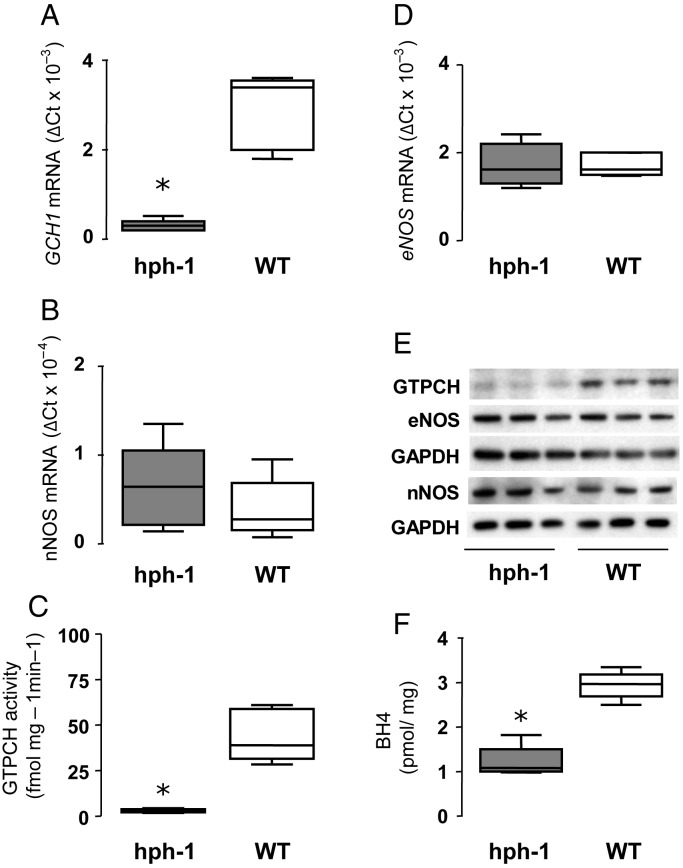
We first quantified the changes in GCH1 expression in the hph-1 mouse, using qRT–PCR, and determined the effects of altered GCH1 expression on GTPCH protein levels, GTPCH enzymatic activity, and tissue BH4 levels. Using matched hph-1 and WT littermates derived from hph-1 heterozygote matings, we observed that GCH1 mRNA was reduced by 90% in heart tissue from hph-1 mice compared with WT littermates (Figure 1A). The GTPCH protein was significantly reduced in hph-1 compared with WT, whereas eNOS and nNOS mRNA and protein were not different (Figure 1E). In keeping with the observed reduction in GCH1 expression and GTPCH protein, GTPCH enzymatic activity was reduced by 90% in hph-1 heart tissue compared with WT littermates (Figure 1C; n = 5, P < 0.001), leading to a 60% reduction in steady-state BH4 levels (Figure 1F). These data confirm that the hph-1 mouse has reduced GCH1 expression, leading to reduced cardiac BH4 levels.
Figure 1.
Reduced expression, activity, and transcription of GCH1 in hph-1 mice leading to reduced BH4 without changes in eNOS or nNOS. GCH1, eNOS, and nNOS mRNA levels from heart homogenates were quantified by real-time RT–PCR correcting for GAPDH hph-1 and WT littermate five animals per group. (A) GCH1 mRNA levels demonstrated a significant reduction in hph-1 mice compared with WT (n = 5, *P < 0.001). (B) No difference was observed in nNOS (n = 5) or (D) eNOS (n = 5) mRNA levels. (C) GTPCH activity, measured by HPLC, was significantly reduced in hph-1 compared with WT mice (n = 5, *P < 0.001). (E) Protein levels were determined in heart and cerebellum homogenates by immunoblotting with antibodies for mouse GTPCH, eNOS, nNOS, and GAPDH (n = 3 animals per group). GTPCH protein levels were reduced in heart homogenates of hph-1 mice compared with the WT. No difference was observed in heart eNOS or cerebellar nNOS with GAPDH used to ensure equal protein loading. (F) BH4 levels were measured by reverse-phase HPLC. There was a significant reduction in BH4 levels in hph-1 compared with WT littermates (n = 5, *P < 0.001).
3.2. Effects of BH4 deficiency on haemodynamic regulation in the hph-1 mouse
We next determined the effects of BH4 deficiency in the hph-1 mouse on systolic blood pressure and heart rate. Using tail-cuff plethysmography, there was no difference in systolic blood pressure, but a significant tachycardia in hph-1 animals compared with WT littermates (Figure 2). Continuous radiotelemetry confirmed that resting heart rates were higher in hph-1 mice compared with WT (P < 0.01), whereas systolic and diastolic blood pressures were not different between hph-1 and WT animals.
Figure 2.
Resting tachycardia with normal blood pressure in hph-1 mice by tail-cuff and by telemetry during voluntary exercise training. (A) Heart rate and systolic blood pressure were measured in hph-1 and WT littermates by tail-cuff plethysmography (VisitechR). There was a significant tachycardia (n = 19, *P < 0.05) in hph-1 animals but no difference in systolic blood pressure (B). (C) Heart rates of hph-1 and WT littermates were obtained by telemetry during a period of voluntary wheel running. hph-1 mice were significantly tachycardic compared with WT littermates during rest (n = 6–9, *P < 0.01) but not when active or during wheel exercise. Both hph-1 and WT littermates showed a significant fall in heart rate at rest (P < 0.05 for WT and hph-1 days 1–5 vs. days 18–32) and during activity (WT P < 0.05, hph-1 P < 0.01 days 1–5 vs. days 18–32) and a rise in the heart rate during wheel running (P < 0.01 for WT days 1–3 vs. days 18–32, P = 0.16 for hph-1 NS). Symbols are means ± SEM. (D) Blood pressure (systolic, mean, and diastolic) in the same animals showed a rise in blood pressure with activity and wheel exercise, but no significant difference between hph-1 and WT littermates.
In order to investigate the haemodynamic effects of physical exercise training, mediated by the autonomic nervous system, we undertook continuous telemetric heart rate and blood pressure monitoring in hph-1 and WT mice, during a 40-day period of spontaneous exercise training, using a computerized exercise wheel system. Each individual beat of haemodynamic data (i.e. heart rate and blood pressure) was analysed according to the activity state of the animal—either at rest (no movement, no wheel activity), during cage activity (movement but no wheel activity), or during wheel running.
Wheel-running activity did not differ between genotypes. In both groups, there was an increase in daily time spent wheel running, daily running distance, and in running speeds with voluntary exercise training, as previously described.33 Throughout the 40-day period of exercise training, resting heart rates progressively decreased in both hph-1 and WT mice (Figure 2C). Heart rate during cage activity did not change significantly throughout the training period, although heart rate during wheel-running increased to a maximum in the first week in both groups, reflecting increased maximum heart rates. There were no genotype-specific differences in heart rate during these activity states. Exercise training had no effect on blood pressure (either diastolic, mean, or systolic) in either genotype (Figure 2D).
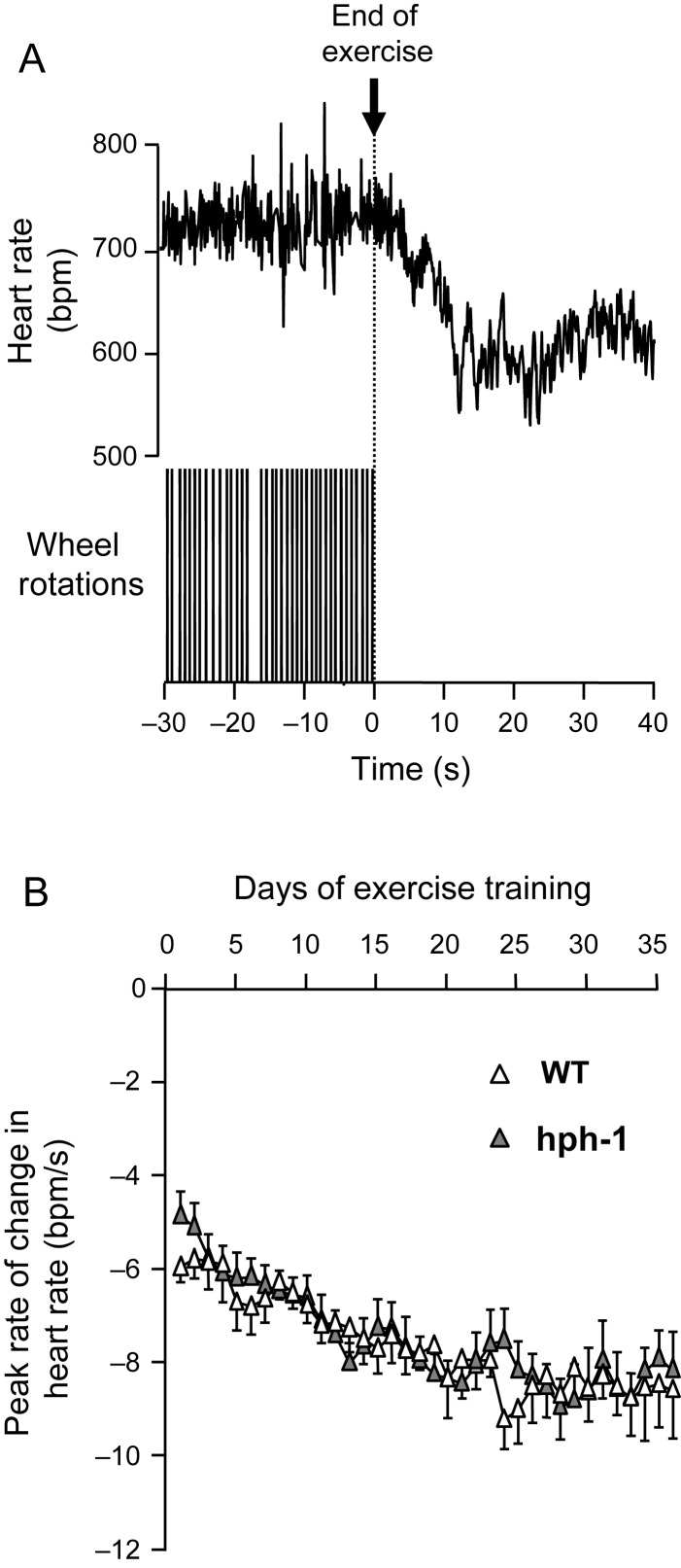
As a further measure of cardiac autonomic regulation, we next evaluated the rate of change of heart rate (heart rate recovery) at the end of individual episodes of wheel exercise, in both WT and hph-1 mice (Figure 3). Mice exercised almost exclusively during the night time period (2000–0800 h), in short bouts of wheel running. Heart rate recovery in individual animals was determined by analysis of an average of 11 exercise bouts each night (range 2–37) in both WT and hph-1 mice, corresponding to a total of 2875 bouts of exercise analysed in 14 mice over 35 days of exercise training. Bouts of wheel exercise were followed by a decrease in heart rate over ∼12 s (Figure 3A). Exercise training significantly increased the rate of heart rate recovery, as measured by the maximum rate of change of heart rate, in both WT and hph-1 mice (Figure 3B). The rate of heart rate recovery increased to a maximum by day 20 of exercise training and remained stable thereafter, but there were no differences between hph-1 and WT mice in the rate of heart rate recovery.
Figure 3.
Normal heart rate recovery following voluntary exercise bouts in telemetered hph-1 mice. (A) Typical heart rate recording in a WT mouse at the end of a bout of voluntary exercise. Changes in exercise state were associated with an extremely rapid change in heart rate that is complete within 20 s. Wheel rotations are shown as blocks of individual wheel rotation spikes that are not individually resolved on the time scale shown. (B) Effects of exercise training on heart rate recovery. There was no difference in heart rate responsiveness between the WT and hph-1 mouse (n = 7). Exercise training was associated with increased heart rate recovery at the end of exercise measured using the peak rate of change in heart rate (peak rate of change in heart rate in WT mice: days 1–7: 6.2 ± 0.5, days 21–35: 8.5 ± 0.6, P = 0.001; in hph-1 mice: days 1–7: 6.0 ± 0.4, days 21–35: 8.5 ± 0.4, P < 0.0001). The effects of exercise training on heart rate recovery were not different in WT and hph-1 mice.
3.3. Effects of BH4 deficiency on atrial responsiveness to vagal and muscarinic stimulation
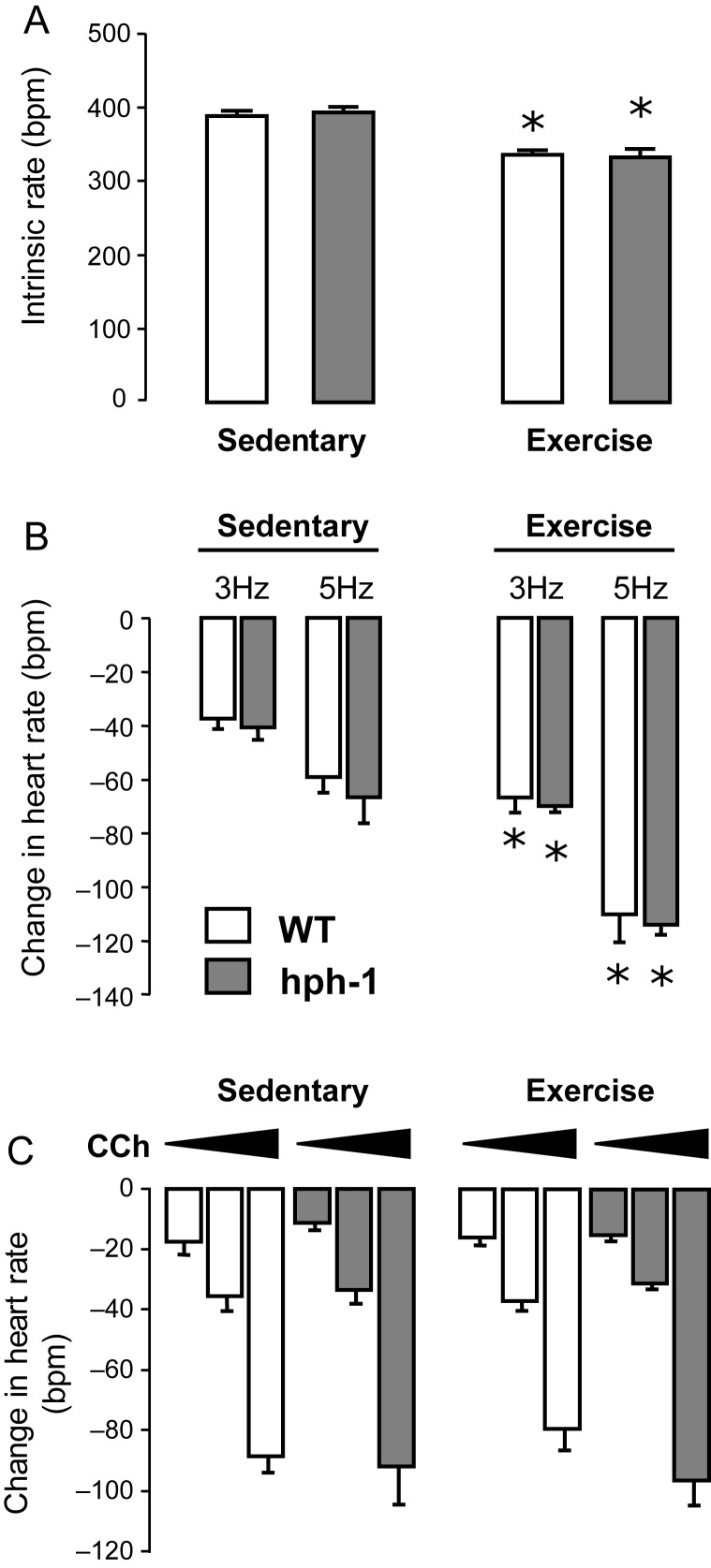
To investigate in more detail the autonomic mechanisms underlying the resting tachycardia in hph-1 mice, we measured cardiac vagal nerve function in hph-1 and WT mice in vitro. Following isolation of cardiac atria with intact right vagal nerves, the rate of intrinsic spontaneous atrial contraction was equal in both hph-1 and WT animals (Figure 4A). Heart rate responses to vagal activation via direct electrode stimulation (10 V, 1 ms pulse interval, 3–5 Hz) were similar in hph-1 and WT, as were responses to cumulative doses of the stable acetylcholine analogue, carbamyl choline, applied directly to the organ chamber (Figure 4B and C). In atria isolated from animals after exercise training, heart rate responses to vagal nerve stimulation were significantly enhanced, confirming the effect of exercise training on cardiac vagal function. However, there remained no difference between hph-1 and WT animals, suggesting that in both untrained and trained animals, cardiac vagal function is not significantly altered by reduction in GCH1 expression and BH4 levels.
Figure 4.
No difference in intrinsic rate or vagal responsiveness before or after exercise training in hph-1 mice. An in vitro preparation of cardiac atria with vagal nerve and stellate ganglion intact was mounted in physiological carbogen-bubbled mouse Ringer's at 36.5°C. n = 7–15 hph-1 and WT littermates both exercise trained and exercise naive were studied. (A) There was no difference in the intrinsic rate of atria from hph-1 and WT littermates despite a fall in rate in exercised subjects regardless of genotype (*P < 0.01). (B) Direct vagal nerve stimulation in vitro at 3 and 5 Hz led to a reduction in heart rate with increased responsiveness in exercised animals regardless of genotype (*P < 0.05). There was no difference in vagal responsiveness between hph-1 and WT littermates. (C) The heart rate response to dose increments of bath-applied carbamylcholine (3 × 10–8 M, 10–7 M, 3 × 10–7 M) was independent of genotype and exercise status.
3.4. Effects of BH4 deficiency on sympathetic function in the hph-1 mouse
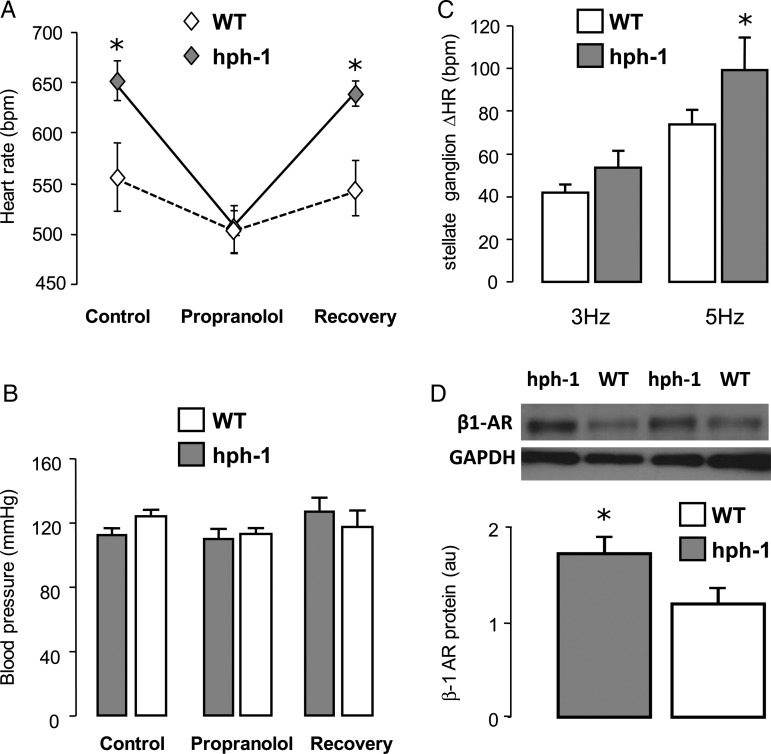
In order to test the possible role of the sympathetic nervous system in the resting tachycardia observed in hph-1 mice, we next treated hph-1 and WT littermates with propranolol (0.5 mg/mL) administered in the drinking water for 5 days. Propranolol treatment reduced heart rate in both hph-1 and WT mice (Figure 5A), without any significant effect on systolic blood pressure. However, the reduction in heart rate induced by propranolol in hph-1 mice was significantly greater than that in WT mice, such that in the presence of β-blockade the difference in resting heart rate between WT and hph-1 mice was totally abolished. Following a 5-day wash out period with propranolol removed from drinking water, the differences in heart rate between hph-1 and WT mice observed at baseline were restored (Figure 5A). There was a significant reduction in absolute urinary dopamine or urinary dopamine normalized for urinary creatinine (hph-1: 794.6 × 10−6 ± 81.7 vs. WT: 1316.2 × 10−6 ± 271, n = 6–13 per group, one-tailed t-test: P < 0.05). Urinary epinephrine and norepinephrine levels were not significantly different but showed marked inter-subject variability in both groups (see Supplementary material online). The reduction in urinary dopamine was also reflected by a reduction in adrenal dopamine (P < 0.05, n = 8–16 per group). No differences were demonstrated in adrenal norepinephrine or in cardiac dopamine or norepinephrine (n = 8–16 per group).
Figure 5.
Increased levels of β1-adrenoceptor in hph-1 mice leading to enhanced sympathetic responsiveness reversible by β-blockade. (A) The effect of propranolol administration to the drinking water (0.5 mg/mL) on haemodynamics in vivo was measured by tail-cuff plethysmography. hph-1 was significantly tachycardic at baseline (n = 6–14, *P < 0.05). This difference was completely abolished by administration of 0.5 mg/mL propranolol in the drinking water but returned when animals returned to normal drinking water. (B) Effect of propranolol administration on blood pressure in the same cohort. There were no significant blood pressure differences between genotypes. (C) An in vitro preparation of cardiac atria with stellate ganglion was mounted in physiological carbogen-bubbled mouse Ringer's at 36.5°C. In vitro direct stellate ganglion stimulation led to an increase in atrial rate. Heart rate responsiveness was significantly increased in hph-1 mice compared with WT littermates (n = 6–8, *P < 0.01). (D) β1-adrenoceptor protein levels were determined from whole-heart homogenates by western blotting (au-arbitrary units). There was a significant increase in β1-AR in hph-1 hearts compared with WT littermates (n = 4, *P < 0.05).
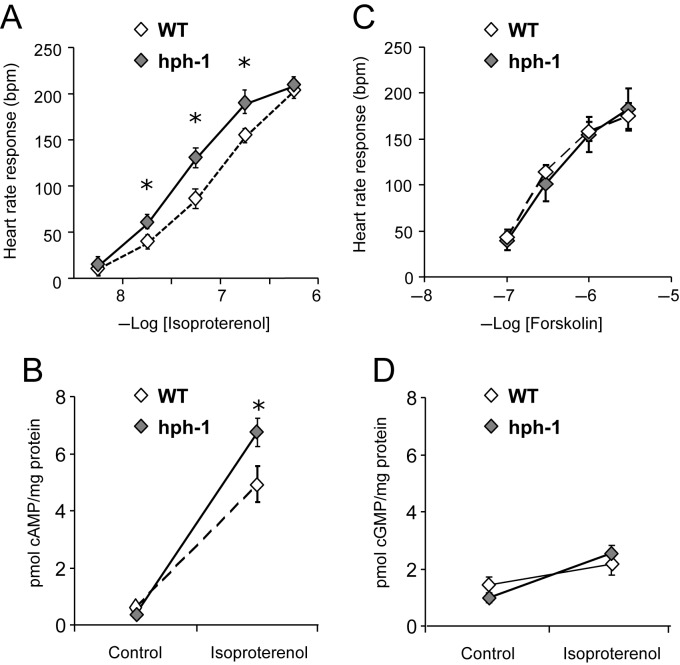
To further investigate the role of the sympathetic nervous system in the resting tachycardia observed in hph-1 mice, we compared the heart rate responses of isolated atrial preparations in vitro to stellate ganglion stimulation and to the direct application of isoproterenol (Figures 5C and 6A). The heart rate responses to stellate ganglion stimulation (10 V, 1 ms pulse interval, 3–5 Hz) were significantly increased in atria from hph-1 mice compared with WT littermates. Heart rate responses to cumulative doses of the selective β-agonist isoproterenol were enhanced throughout a range of doses in hph-1 compared with WT, such that the EC50 of the dose–response curve in hph-1 mice was significantly reduced (hph-1 vs. WT: 8.71 ± 0.9 × 10−8 vs. 19.7 ± 0.8 × 10−8 mol/L, P < 0.05), indicating enhanced β-adrenergic responsiveness. Conversely, there were no genotype differences in atrial rate response to cumulative doses of forskolin (Figure 6C). The β1-adrenoceptor protein was significantly elevated in the hearts of hph-1 mice compared with WT animals (Figure 5D, P < 0.05). There were no differences in GRK2, β-arrestin, and tyrosine hydroxylase (data not shown). Secondary signalling by cAMP but not cGMP in response to the β-receptor agonist isoproterenol was significantly elevated in hph-1 mice (Figure 6B and D, P < 0.05).
Figure 6.
Increased cAMP release following β-receptor activation in hph-1 mice leading to increased heart rate responsiveness but no difference in response to direct adenylate cyclase activation. (A) Dose response to bath-applied isoproterenol (10–8 M, 3 × 10–8 M, 10–7 M, 3 × 10–7 M, 10–6 M, 3 × 10–6 M) demonstrated a significant increased responsiveness in hph-1 mice compared with WT littermates (n = 6–14, *P < 0.05). (B) Dose response to bath-applied forskolin (10–7 M, 3 × 10–7 M, 10–6 M, 3 × 10–6 M) demonstrated no difference in heart rate response in hph-1 mice compared with WT littermates (n = 9). (C) cAMP levels measured in atria snap frozen immediately after bath application of isoproterenol (3 × 10–8 M) were significantly increased in hph-1 mice compared with WT littermates (n = 5–7, *P < 0.05). (D) There was no genotype difference in cGMP levels snap frozen after bath application of isoprenaline (3 × 10–8 M) (n = 5–7).
4. Discussion
In this study, we have demonstrated an important role for GCH1 in cardiovascular autonomic function in vivo. Using the hph-1 genetic mouse model of reduced GCH1 expression, we report, first, that reduced BH4 levels result in a resting tachycardia before and after exercise-training, despite normal blood pressure, normal heart rates during exercise, and a normal exercise performance profile. Secondly, we find that the resting tachycardia in BH4-deficient mice is normalized following β-blockade, augmented by sympathetic activation and not due to differences in the intrinsic rate of isolated, spontaneously beating atria. There is an increase in β1-adrenoceptor protein leading to increased cAMP release in response to receptor stimulation, but no difference in the response to direct adenylate cyclase activation with forskolin. Finally, we show that BH4 deficiency does not alter parasympathetic regulation of heart rate, as evidenced by the effects of chronic exercise training on heart rate, heart rate recovery after exercise, and the bradycardic response to vagal stimulation.
These findings have been enabled by the use of radiotelemetry in mice to measure real-time changes in blood pressure and heart rate during voluntary wheel running. Using this approach, combined with in vitro measurement of autonomic sensitivity, has allowed us to phenotype and quantify important new aspects of the autonomic response to both acute and chronic exercise in a mouse model that mirrors the moderate reduction in GCH1 expression and BH4 levels previously reported in humans with genetic variations in GCH1.
The haemodynamic profile of the BH4-deficient hph-1 mouse are similar to those described in humans with the BH4 deficiency, including patients with DRD and subjects with a 3′ UTR polymorphism (C243T) in GCH1.22,23 In addition to confirming the higher resting heart rate in association with reduced BH4 levels found in humans, we have now provided mechanistic evidence that implicates atrial myocardial sensitivity to sympathetic, but not parasympathetic signalling, and shown that this phenotype is normalized by β-blockade. In humans with DRD, there is a significant reduction in circulating catecholamines and a blunting of catecholamine release in response to tilt testing.22 Patients with the C243T polymorphism also showed a trend towards reduced urinary cathecholamines but with a high degree of variability.23 The hph-1 mouse is known to have reduced CNS catecholamine synthesis.34 In this study, the normalizing effect of propranolol and the augmented heart rate response to organ bath application of the β-agonist isoproterenol in hph-1 mice suggest that the major autonomic role for BH4 is in modulating β-adrenergic sensitivity. This increased sympathetic sensitivity appears to be due to an increase in β1-receptors leading to increased cAMP release in response to receptor activation but with downstream adenylate cyclase responsiveness unaffected. In humans with the C243T polymorphism, there is also a trend towards a reduction in urinary catecholamines but with a high degree of variability. In parallel, we have demonstrated a reduction in urinary and adrenal dopamine in the hph-1 mouse. This finding may suggest that the increase in β1-receptor activity may represent an adaptive response to chronic reductions in catecholamines in both human and mice with BH4 deficiency, an effect which has been previously described.35 This adaptive explanation may not, however, explain the apparent overcompensation of cardiac sympathetic sensitivity resulting in the tachycardia demonstrated. Furthermore, the differences reported in dopamine levels were not reflected in other catecholamines measured and there were no differences in catecholamines isolated from cardiac tissue. This may reflect the well-recognized technical challenge of interpreting catecholamines from samples taken immediately after sacrifice which show considerable variability which could mask an experimental difference and may not truly reflect either baseline sympathetic tone or cardiac sympathetic neuronal activity.36 An alternative explanation relates to BH4 deficiency affecting NOS-dependent signalling pathways. However, while NO signalling has been shown to couple to the β-receptor, this had the opposite effect on sympathetic-mediated tachycardia to that observed here.37 We also detect no differences in the expression of NOS enzymes in our model and no differences in the cGMP response to isoproterenol. It may require a model with a more severe form of BH4 deficiency to investigate this further.
The hph-1 mouse has been reported to have elevated blood pressure by tail-cuff.38 In our study, repeated measurements using different measurement techniques show no significant difference in blood pressure. This is in keeping with the findings in man.22,23
Our findings, taken together with those of Zhang et al.22 and Mayahi et al.23 demonstrate that deficiency of the critical catecholamine synthetic cofactor BH4 in vivo causes a resting tachycardia in both humans and mice, due to increased cardiac sympathetic sensitivity but not due to an impairment of parasympathetic function. These new insights have important clinical implications. Autonomic regulation of heart rate has fundamental roles in cardiovascular health, in the response to acute and chronic exercise training, and in the pathophysiological response to cardiovascular disease states, such as heart failure, myocardial infarction, arrhythmia, and hypertension.6,39 Thus, alterations of BH4 availability may be an important aspect of disease pathophysiology that may be amenable to pharmacologic intervention. Increased BH4 levels have been shown to restore NO-dependent functions in human diseased states.40–44 Increased BH4 levels might also be expected to reduce cardiac sympathetic sensitivity. However, further studies are required to determine the specific tissues and cells in which GCH1 and BH4 exert their regulatory effects on cardiac autonomic function in human cardiovascular disease, and to test the utility of pharmacologic strategies to modulate BH4 levels in vivo.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the British Heart Foundation (grants RG/07/003/23133 and PG/05/076) and by the Medical Research Council. D.A. was a Wellcome Trust Cardiovascular Initiative clinical training fellow and J.P.B. was a Bristol Myers Squibb research fellow. The authors acknowledge support from the BHF Centre of Research Excellence at the University of Oxford.
References
- 1.Prakash ES, Madanmohan, Sethuraman KR, Narayan SK. Cardiovascular autonomic regulation in subjects with normal blood pressure, high-normal blood pressure and recent-onset hypertension. Clin Exp Pharmacol Physiol. 2005;32:488–494. doi: 10.1111/j.1440-1681.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 2.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 3.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004;292:1462–1468. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- 4.Balady GJ, Larson MG, Vasan RS, Leip EP, O'Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004;110:1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 5.Erikssen G, Bodegard J, Bjornholt JV, Liestol K, Thelle DS, Erikssen J. Exercise testing of healthy men in a new perspective: from diagnosis to prognosis. Eur Heart J. 2004;25:978–986. doi: 10.1016/j.ehj.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Fox KM, Ferrari R. Heart rate: a forgotten link in coronary artery disease? Nat Rev Cardiol. 2011;8:369–379. doi: 10.1038/nrcardio.2011.58. [DOI] [PubMed] [Google Scholar]
- 7.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 8.Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342–362. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RS, Parniak MA, Kaufman S. The interaction of aromatic amino acids with rat liver phenylalanine hydroxylase. J Biol Chem. 1984;259:271–277. [PubMed] [Google Scholar]
- 10.Serova L, Nankova B, Rivkin M, Kvetnansky R, Sabban EL. Glucocorticoids elevate GTP cyclohydrolase I mRNA levels in vivo and in PC12 cells. Brain Res Mol Brain Res. 1997;48:251–258. doi: 10.1016/s0169-328x(97)00098-3. [DOI] [PubMed] [Google Scholar]
- 11.Rao F, Zhang L, Wessel J, Zhang K, Wen G, Kennedy BP, et al. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation. 2007;116:993–1006. doi: 10.1161/CIRCULATIONAHA.106.682302. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez-Vivar J, Martasek P, Hogg N, Karoui H, Masters BS, Pritchard KA, Jr, et al. Electron spin resonance spin-trapping detection of superoxide generated by neuronal nitric oxide synthase. Methods Enzymol. 1999;301:169–177. doi: 10.1016/s0076-6879(99)01080-0. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol. 2003;284:R628–R638. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- 15.Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- 16.Herring N, Danson EJ, Paterson DJ. Cholinergic control of heart rate by nitric oxide is site specific. News Physiol Sci. 2002;17:202–206. doi: 10.1152/nips.01386.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma S, Katsura M. Nitric oxide and peroxynitrite as factors to stimulate neurotransmitter release in the CNS. Prog Neurobiol. 2001;64:97–108. doi: 10.1016/s0301-0082(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 18.Danson EJ, Paterson DJ. Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise-trained mice. J Physiol. 2003;546:225–232. doi: 10.1113/jphysiol.2002.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan RM, Choate JK, Golding S, Herring N, Casadei B, Paterson DJ. Peripheral pre-synaptic pathway reduces the heart rate response to sympathetic activation following exercise training: role of NO. Cardiovasc Res. 2000;47:90–98. doi: 10.1016/s0008-6363(00)00066-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Li D, Plested CP, Dawson T, Teschemacher AG, Paterson DJ. Noradrenergic neuron-specific overexpression of nNOS in cardiac sympathetic nerves decreases neurotransmission. J Mol Cell Cardiol. 2006;41:364–370. doi: 10.1016/j.yjmcc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Jacob G, Garland EM, Costa F, Stein CM, Xie HG, Robertson RM, et al. Beta2-adrenoceptor genotype and function affect hemodynamic profile heterogeneity in postural tachycardia syndrome. Hypertension. 2006;47:421–427. doi: 10.1161/01.HYP.0000205120.46149.34. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Rao F, Zhang K, Khandrika S, Das M, Vaingankar SM, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayahi L, Mason L, Bleasdale-Barr K, Donald A, Trender-Gerhard I, Sweeney MG, et al. Endothelial, sympathetic, and cardiac function in inherited (6R)-L-erythro-5,6,7,8-tetrahydro-L-biopterin deficiency. Circ Cardiovasc Genet. 2010;3:513–522. doi: 10.1161/CIRCGENETICS.110.957605. [DOI] [PubMed] [Google Scholar]
- 24.Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lotsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008;12:1069–1077. doi: 10.1016/j.ejpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Tatham AL, Crabtree MJ, Warrick N, Cai S, Alp NJ, Channon KM. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J Biol Chem. 2009;284:13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoo JP, Nicoli T, Alp NJ, Fullerton J, Flint J, Channon KM. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol Genet Metab. 2004;82:251–254. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Bode VC, McDonald JD, Guenet JL, Simon D. hph-1: a mouse mutant with hereditary hyperphenylalaninemia induced by ethylnitrosourea mutagenesis. Genetics. 1988;118:299–305. doi: 10.1093/genetics/118.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howells DW, Smith I, Hyland K. Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J Chromatogr. 1986;381:285–294. doi: 10.1016/s0378-4347(00)83594-x. [DOI] [PubMed] [Google Scholar]
- 29.Werner-Felmayer G, Gross SS. Analysis of tetrahydrobiopterin and its role in nitric oxide synthesis. In: Feelish M, Stamler JS, editors. Methods in Nitric Oxide Research. John Wiley & Sons Ltd; 1996. pp. 271–294. [Google Scholar]
- 30.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 31.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, et al. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- 32.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 33.De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hyland K, Gunasekera RS, Engle T, Arnold LA. Tetrahydrobiopterin and biogenic amine metabolism in the hph-1 mouse. J Neurochem. 1996;67:752–759. doi: 10.1046/j.1471-4159.1996.67020752.x. [DOI] [PubMed] [Google Scholar]
- 35.Brodde OE, Daul A, Michel MC. Subtype-selective modulation of human beta 1- and beta 2-adrenoceptor function by beta-adrenoceptor agonists and antagonists. Clin Physiol Biochem. 1990;8(Suppl. 2):11–17. [PubMed] [Google Scholar]
- 36.Grouzmann E, Cavadas C, Grand D, Moratel M, Aubert JF, Brunner HR, et al. Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch. 2003;447:254–258. doi: 10.1007/s00424-003-1140-x. [DOI] [PubMed] [Google Scholar]
- 37.Choate JK, Murphy SM, Feldman R, Anderson CR. Sympathetic control of heart rate in nNOS knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H354–H361. doi: 10.1152/ajpheart.00898.2007. [DOI] [PubMed] [Google Scholar]
- 38.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, et al. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 39.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 40.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 41.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 42.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 44.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]