Abstract
Mitochondrial dysfunction and autophagy are centrally implicated in Parkinson’s disease (PD). Mutations in ATP13A2, which encodes a lysosomal P-type ATPase of unknown function, cause a rare, autosomal recessive parkinsonian syndrome. Lysosomes are essential for autophagy, and autophagic clearance of dysfunctional mitochondria represents an important element of mitochondrial quality control. In this study, we tested the hypothesis that loss of ATP13A2 function will affect mitochondrial function. Knockdown of ATP13A2 led to an increase in mitochondrial mass in primary mouse cortical neurons and SH-SY5Y cells forced into mitochondrial dependence. ATP13A2-deficient cells exhibited increased oxygen consumption without a significant change in steady-state levels of ATP. Mitochondria in knockdown cells exhibited increased fragmentation and increased production of reactive oxygen species (ROS). Basal levels of the autophagosome marker LC3-II were not significantly changed, however, ATP13A2 knockdown cells exhibited decreased autophagic flux, associated with increased levels of phospho-mTOR, and resistance to autophagy induction by rapamycin. The effects of ATP13A2 siRNA on oxygen consumption, mitochondrial mass and ROS production could be mimicked by inhibiting autophagy induction using siRNA to Atg7. We propose that decreased autophagy associated with ATP13A2 deficiency affects mitochondrial quality control, resulting in increased ROS production. These data are the first to implicate loss of ATP13A2 function in mitochondrial maintenance and oxidative stress, lending further support to converging genetic and environmental evidence for mitochondrial dysregulation in PD pathogenesis.
Keywords: mitochondrial quality control, autophagy, recessive parkinsonism, Kufor-Rakeb syndrome
INTRODUCTION
Kufor-Rakeb syndrome was originally described as an early onset parkinsonian syndrome with supranuclear gaze paresis and widespread cortical atrophy (Najim al-Din et al., 1994). Mutations in ATP13A2 (PARK9) were recently identified as causative in Kufor-Rakeb syndrome (Ramirez et al., 2006). ATP13A2 encodes a P-type ATPase (Axelsen and Palmgren, 1998; Kuhlbrandt, 2004), which is localized to the lysosomal membrane with ten transmembrane domains. However, its substrate specificity and function remain unknown. Putative loss-of-function frameshift or exon skipping mutations in ATP13A2 were shown to result in a truncated protein that was retained in the endoplasmic reticulum and degraded by the proteasome (Ramirez et al., 2006).
Autophagy, the process by which intracellular cargo is delivered to lysosomes for degradation, has been recently implicated in the pathogenesis of Parkinson’s and related diseases (Cherra et al., 2010a; Wong and Cuervo, 2010). Alterations in lysosomal function can affect upstream autophagic functions. A variety of enzyme defects associated with lysosomal storage diseases elicit accumulation of autophagosomes (Cao et al., 2006; Fukuda et al., 2006; Jennings et al., 2006; Ko et al., 2005; Koike et al., 2005; Pacheco et al., 2007; Settembre et al., 2008; Takamura et al., 2008; Tanaka et al., 2000; Vergarajauregui et al., 2008), with some showing increased autophagic induction (Cao et al., 2006; Ko et al., 2005; Koike et al., 2005; Pacheco et al., 2007; Takamura et al., 2008; Vergarajauregui et al., 2008) and others showing a block in autophagosome maturation (Jennings et al., 2006; Settembre et al., 2008; Tanaka et al., 2000; Walls et al., 2007). Lysosomal degradation is also recognized as the primary mode of mitochondrial clearance, as damaged or unneeded mitochondria are delivered to the lysosome via autophagy (Kim et al., 2007). In fibroblasts from patients with mucolipidosis, a lysosomal storage disease, mitochondria exhibit fragmentation and impaired calcium buffering (Jennings et al., 2006). Similarly, autophagy-deficient yeast accumulate dysfunctional mitochondria with increased reactive oxygen species production (Zhang et al., 2007).
Autophagy plays a key role in neurons, as neural specific knockouts of the key autophagy proteins Atg7 or Atg5 result in early onset neurodegeneration (Hara et al., 2006; Komatsu et al., 2006). Several genes whose mutations cause familial PD (SNCA, PARK2, PINK1, LRRK2, and DJ-1) have been shown to modulate autophagy (Dagda et al., 2009; Herman and Moussa, 2011; Krebiehl et al., 2010; Martinez-Vicente et al., 2008; Plowey et al., 2008; Winslow et al., 2010). Indeed, improper clearance of mitochondria by autophagy may lie at the crossroads of several pathogenic pathways for PD (Batlevi and La Spada, 2011). PINK1 and Parkin (gene product of PARK2) are implicated as partners in the detection and autophagic clearance of dysfunctional mitochondria (Narendra et al., 2008), and altered basal mitophagy is observed in DJ-1 deficient cells (Krebiehl et al., 2010). Expression of LRRK2 mutants is associated with decreased mitochondrial membrane potential (Mortiboys et al., 2010) and increased autophagy (Plowey et al., 2008). α-Synuclein (gene product of SNCA) may interact with mitochondrial complex I and subsequently increase mitochondrial autophagy (Chinta et al., 2010), although this protein may also impair autolysosomal degradation in general (Martinez-Vicente et al., 2008; Winslow et al., 2010).
The location of ATP13A2 within the lysosomal membrane places it in a potential position to modulate autophagy, and therefore, proper mitochondrial maintenance. In this study, we used RNAi mediated knockdown of ATP13A2 in cultured neuronal cells and cortical neurons. We show that ATP13A2-deficiency increases mitochondrial mass resulting in increased respiration and reactive oxygen species production. We dissect the role of ATP13A2 on autophagy and show that knockdown results in decreased autophagic flux due to decreased induction and a trend towards delayed maturation. These results are the first to implicate ATP13A2 in the growing body of evidence supporting the convergence of autophagy and mitochondrial dysregulation in parkinsonian neurodegeneration.
MATERIALS AND METHODS
SH-SY5Y and primary neuron cultures
SH-SY5Y cells (ATCC) were maintained at 37°C, 5% CO2 in antibiotic-free Dulbecco's Modified Eagle Medium (DMEM) (Lonza, Walkersville, MD) with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 15 mM HEPES, and 2 mM glutamine. To promote reliance on oxidative phosphorylation for ATP, cells were switched to glucose-free DMEM with 10% heat-inactivated fetal bovine serum, 10 mM galactose, 2 mM glutamine, 1 mM sodium pyruvate, and 10 mM HEPES two days prior to transfection. All results in SH-SY5Y cells were obtained 3 days after transfection, after 5 days in glucose-free, galactose/glutamine containing medium.
Cortices were dissected from 15-day in utero C57BL/6 mouse embryos (Hilltop Laboratory Animals, Scottdale, PA) as previously described (Cherra et al., 2010b; Dagda et al., 2011), and plated at 3×105 cells/cm on poly-L-lysine coated 4-well chamber slides, or 6-well plates in Neurobasal media supplemented with 2% B27 and Glutamax.
See Supplementary Methods for cell death and cell survival assays.
RNA interference and Transfections
Small interfering RNA (siRNA) was used to knock down the expression of ATP13A2 or of ATG7 in SH-SY5Y cells and of Atp13a2 in mouse cortical neurons, using a pool of three siRNA sequences (5’-CCAACGUGAUCAGCAUACCGGUCAA-3’, 5’-GGACUUGAAGAUGGUGGAGUCUACU-3’, and 5’-CAGAGCUGGUGUGCGAGCUAGAGAA-3’; 20 nM; Invitrogen) targeting human ATP13A2 (siATP13A2), the Dharmacon smartpool siRNA (20 nM, Dharmacon, Layfayette, CO) targeting human ATG7 (siATG7), or a pool of three siRNA sequences (5’-CCGCCAUGACGGUGUGCACACUCUA-3’, 5’-GGCAAAGCAGGUACUGCGCUAUUAU-3’, and 5’-UCACAGAGGACGGCUUGGACGUAAU-3’; 20 nM; Invitrogen) targeting mouse Atp13a2. The siRNAs were transfected in 0.25% Lipofectamine 72 hr prior to further experimentation (Plowey et al., 2008). A non-silencing siRNA pool (siCtrl, Dharmacon, Lafayette, CO) was used as a control. For primary cortical neuron imaging studies, Atp13a2 was knocked down at 7 DIV using a GIPZ lentiviral shRNAmir (shAtp13a2; Sense 5’-CAGAGCTGCTGGCACTATA-3’; Open Biosystems, Huntsville, AL) expressing TurboGFP as a marker, or non-targeting shRNAmir (shCtrl, 1 µg/mL in 0.25% Lipofectamine), with media change three days later. Efficiency of gene silencing was determined by Western blot for SH-SY5Y cells and RT-PCR for primary cortical neurons (Applied Biosystems, Carlsbad, CA).
Primary cortical neurons or SH-SY5Y cells were transfected with RFP-LC3 or GFP-RFP-LC3 (courtesy of Dr. Tomatsu Yoshimori, Osaka University, Japan), mito-RFP (courtesy of Dr. Ian J. Reynolds, Merck), mito-GFP (courtesy of Dr. Stefan Strack, University of Iowa), human ATP13A2 (courtesy of Dr. Christian Kubisch, University of Ulm, Germany), pHyPer-dMito or pHyPer-cyto (Evrogen, Moscow, Russia, at 1.5 µg/mL in 0.25% Lipofectamine 200 (Invitrogen). For rescue experiments, primary neuron cultures were co-transfected with human ATP13A2 and shAtp13a2 at a 15:1 mass ratio.
Detection of Reactive Oxygen Species
SH-SY5Y cells or primary cortical neurons were stained with 0.5 µM MitoSOX Red (Invitrogen) in HBSS for 10 min at 37°C. Cells were washed three times in HBSS. Nuclei were stained with 10 µM DRAQ5 (Biostatus Limited, Leicestershire, UK) prior to imaging. Sequential individual channel images were collected at 60× magnification using the same exposure times with an Olympus IX71 microscope. Integrated intensity for MitoSOX was calculated using Metamorph and divided by the number of cells per field (Dagda et al., 2009).
Alternatively, cells were transfected with either a cytosolic or a mitochondrially targeted Hydrogen Peroxide detection vector (HyPer; Evrogen) one day after transfection with the indicated siRNA. Fluorescence was quantified on a SpectraMax A2 plate reader (Molecular Devices, Sunnyvale, CA) with Excitation = 500 nm and Emission = 516 nm.
Measurement of autophagic flux, induction and maturation
Autophagic flux was assessed by LC3-II band densitometry or increase in RFP-LC3 puncta in SH-SY5Y cells or primary neuron cultures after treatment with bafilomycin (5 nM) for 1 hr. Autophagy induction was assessed by increases in LC3-II band intensity after treatment with rapamycin (5 µM) for 1 hr. Autophagic vacuole (AV) maturation was assessed by transfecting SH-SY5Y cells with a GFP-RFP-LC3 construct 24 h after transfection with siCtrl or siATP13A2. Under these conditions, ~94% of DNA-transfected cells also express cytoplasmic siRNA (Plowey et al., 2008). Cells were treated with DMSO or rapamycin (5 µM) for 1 or 3 hr. Transfected cells were imaged (12-bit pixel memory) using a Zeiss LSM 510 meta inverted laser scanning confocal microscope with Plan-Apochromat 63×/1.4 oil immersion objection, and 488-nmAr and 543-nm HeNe lasers. The number of puncta (green and red vs. red only) were counted for each cell, and independently confirmed by an individual not involved in the study. Dual green and red positive puncta indicate early AVs while singly red positive puncta indicate late AVs after lysosomal acidification quenches the green fluorescence (Klionsky et al., 2008).
See Supplemental Methods for Lysosomal Function Assays.
Western blotting
Western blotting was performed as described (Zhu et al., 2007), with equal loading of total protein determined by Coomassie Blue Protein Assay (Pierce, Rockford, IL), employing the following primary antibody dilutions: 1:1000 rabbit anti-ATP13A2 (Sigma, St. Louis, MO), 1:1000 rabbit anti-ATG7 (Rockland, Gilbertsville, PA), 1:500 mouse anti-LC3 (Nanotools (clone 5F10), Teningen, Germany), 1:1000 Mitoprofile (mixture of 5 monoclonal antibodies, Mitosciences, Eugene, OR), 1:1000 rabbit anti-Rubicon (courtesy of Dr. Zhenyu Yue, Mount Sinai School of Medicine), 1:1000 mouse anti-Beclin-1 (BD, Franklin Lakes, NJ), or 1:1000 rabbit anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA),.followed by ECL detection (GE Healthcare, Little Chalfont). Membranes were stripped and reprobed with 1:10000 mouse anti-β-actin (Sigma). Densitometry was performed using ImageJ’s Gel analyzer (NIH, Bethesda, MD).
Mitochondrial respiration and steady-state ATP level measurements
Mitochondrial respiration was assessed using a Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). SH-SY5Y cells were transfected with siCtrl or siATP13A2 3 days before each experiment and were plated (100,000 cells/well) in a 24-well Seahorse cell culture microplate 24 hr prior to each experiment. Cells cultured in galactose showed no significant change in cell number 24 hours after plating, and no significant difference in cell number comparing siCtrl with siATP13A2 treated cells. The sensor cartridge was hydrated with calibration buffer one day prior to each experiment. Each well was washed with unbuffered DMEM, and a final volume of 700 µL DMEM added to each well and incubated at 37°C × 60 min before the start of the experiment. After cartridge calibration, cellular oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured basally and after the sequential addition of oligomycin (1 µM), FCCP (300 nM), and rotenone (1 µM) / antimycin A (1 µM), as described previously (Choi et al., 2009; Qian and Van Houten, 2010). Three measurements were recorded basally and following the addition of each compound. The mean of 8–10 replicate wells per experiment were averaged across independent experiments, and a Student’s t-test was performed to compare control and ATP13A2 deficient cells. OCR and ECAR values were normalized by collecting protein from each well after experimental completion and dot blotting for β-actin.
Steady-state ATP levels were determined in a parallel plate using an ATPlite Luminescence Assay (PerkinElmer, Waltham, MA). 100,000 cells/well were transfected in a 96-well, black-sided plate. Each group consisted of 4–6 replicate media-treated wells and 3 antimycin A-treated (1 µM × 30 min) wells per experiment. Mitochondrial specific ATP levels were derived by subtracting the ATP levels after antimycin A from ATP levels in the absence of antimycin A.
Mitochondrial membrane potential measurements
Mitochondrial membrane potential was determined using 50 nM tetramethylrhoadamine (TMRM, Invitrogen) or 2 µM JC-1 (Invitrogen) as previously described (Hu et al., 2009; Lemasters and Ramshesh, 2007). The TMRM fluorescence was normalized to background cellular fluorescence of the nuclear-cytosolic compartment (Lemasters and Ramshesh, 2007). See Supplemental Methods for details.
Mitochondrial morphology, area and volume measurements
SH-SY5Y cells transfected with mito-GFP were fixed in 4% paraformaldehyde after 72 hr and imaged using an Olympus IX71 microscope. Mitochondrial fragmentation was assessed using an ImageJ macro measuring the circularity of each mitochondrial particle as previously described (Dagda JBC paper). Mitochondrial area was determined using an ImageJ macro that calculates the fraction of total cytoplasmic area that is occupied by mitochondrial particles.
Primary cortical neurons transfected with shCtrl or shAtp13a2 were fixed in 4% paraformaldehyde, and stained using a rabbit anti-Tom20 primary antibody (1:200, Santa Cruz) and a goat anti-rabbit IgG Alexa Fluor 546 secondary antibody (Invitrogen). Transfected neurons were imaged (12-bit pixel memory) using a Zeiss LSM 510 Meta inverted laser scanning confocal microscope with a Plan-Apochromat 63×/1.4 oil immersion objective and exciting with a 488-nm Ar laser (10% laser power). Mitochondrial were visualized by exciting Tom20 staining with a 543-nm HeNe laser (1% laser power). High-resolution z-stacks were taken of the soma of individual neurons at 0.5 µm steps. The mitochondrial volume of a random portion of the somatic cytoplasm was determined as a fraction of GFP positive cytoplasm using ImageJ’s 3D Object Counter macro (Fabrice Cordelires & Jonathan Jackson).
Statistical analysis
Results are displayed as mean ± SD from at least three independent experiments. All graphics and statistical analyses were generated with R (R Project for Statistical Computing). Two-tailed Student’s t-test (p < 0.05) was used to calculate significance for experiments comparing two groups, employing the Bonferroni correction for multiple group comparisons.
RESULTS
When cultured in standard glucose-containing media, human SH-SY5Y neuroblastoma cells showed a sharp decline in ATP following treatment with 2-deoxyglucose, but were unaffected by the oxidative phosphorylation (OXPHOS) inhibitors oligomycin or rotenone (Fig. S1A), indicating glycolytic metabolism. Culturing SH-SY5Y cells in galactose and glutamine in the absence of glucose forces immortalized cell lines to rely on OXPHOS for energy (Gohil et al., 2010; Reitzer et al., 1979; Rossignol et al., 2004). When SH-SY5Y cells were cultured in galactose/glutamine, greater than 90% of the basal ATP level was sensitive to treatment with OXPHOS inhibitors (Fig. S1B). Basal and FCCP induced mitochondrial OCR was also increased in cells cultured in galactose/glutamine compared with cells cultured in glucose (data not shown). To study the effects of ATP13A2 on mitochondrial metabolism under conditions mimicking the dependence of neurons on mitochondrial metabolism, all experiments using SH-SY5Y cells were performed in galactose/glutamine.
ATP13A2 Knockdown
ATP13A2 was knocked down by approximately 80% in SH-SY5Y cells treated with siATP13A2 as assessed by Western blotting 3 days (Fig. S2A). Atp13a2 gene expression was reduced by 50% in primary cortical neurons treated with siAtp13a2 for 3 days (Fig. S2B). We further confirmed the ability of the GIPZ shRNAmir to knockdown mouse Atp13a2 in HT-22 cells after 1 week in puromycin (10 µg/mL), resulting in > 60% knockdown as assessed by RT-PCR (Fig. S2C). There were no significant effects of ATP13A2 knockdown on viability in either SH-SY5Y cells or in primary neurons, as assessed by propidium iodide staining or caspase-3 activity (Supplemental Table 1).
ATP13A2 knockdown increases oxygen consumption
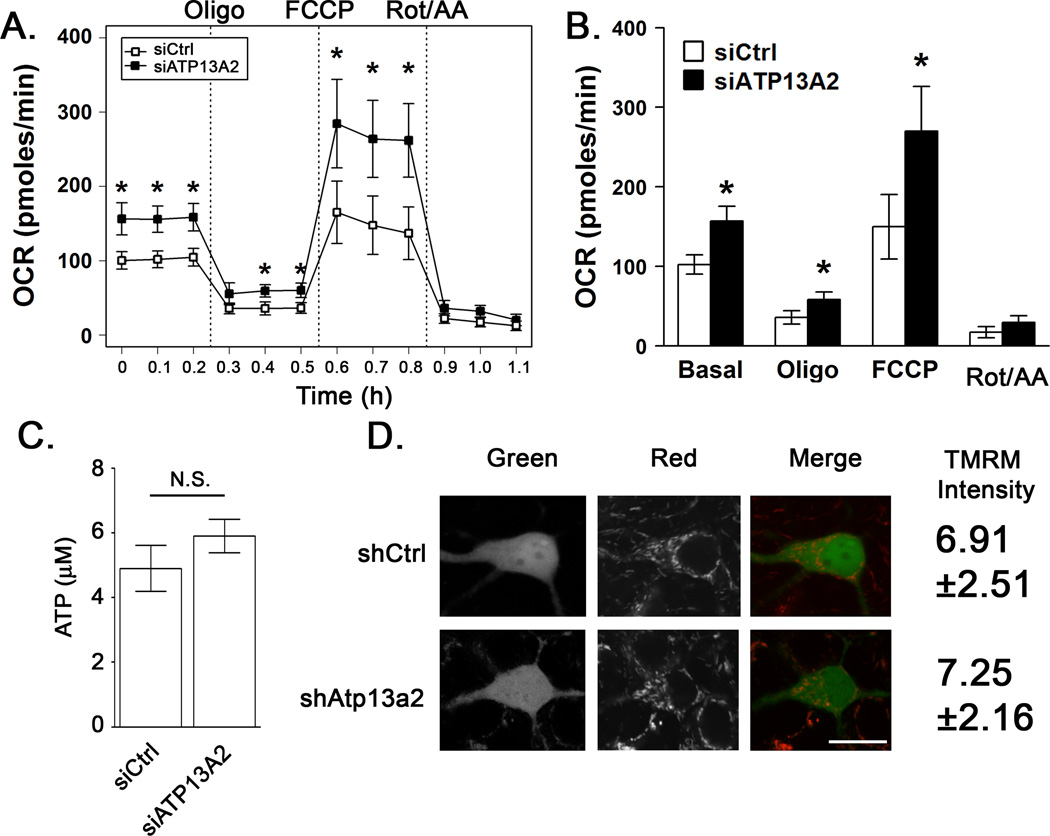
Oxygen consumption is a critical readout of mitochondrial function. The OCR was assessed in SH-SY5Y cells after 3 days of ATP13A2 RNAi using a Seahorse Extracellular Flux Analyzer, which can rapidly screen the bioenergetic profile of in situ mitochondria (Beeson et al., 2010; Dagda et al., 2011; Nicholls et al., 2010). A compiled trace of the bioenergetic profiles of 7 replicate experiments is displayed in Fig. 1A. Basal oxygen consumption and the FCCP induced maximal respiratory capacity were increased in ATP13A2-deficient cells (Fig. 1B). However, steady-state ATP levels were not significantly different (Fig. 1C). Cells cultured in galactose and glutamine have very low levels of flux through glycolysis, and as expected there were no significant changes in ECAR between siCtrl and siATP13A2 treated cells (data not shown).
Figure 1. ATP13A2 knockdown increases mitochondrial oxygen consumption without significant increase in steady-state levels of ATP.
SH-SY5Y cells cultured in glucose-free, galactose/glutamine containing media were treated with non-targeting siCtrl or siATP13A2 for 72 hours prior to assay. A) Cellular oxygen consumption was assessed using a Seahorse XF24 extracellular flux analyzer. Basal oxygen consumption (OCR) and OCR following sequential treatments with oligomycin (1 µM), FCCP (300 nM), and rotenone (1 µM)/antimcyin A (1 µM). Error bars represent the average of N=7 replicate experiments, with 8–10 replicate wells per experiment. B) The rates at each time point after a given inhibitor treatment were averaged across the 7 independent experiments. C) Steady-state ATP levels were measured using a luminescence-based assay. Averages are from N=7 replicate experiments, with 4–6 replicate wells per experiment. D) Primary cortical neuron cultures transfected with shRNA 72 h prior were treated with TMRM (50 nM) for 30 min at 37°C. Cells were washed and maintained in Krebs-Ringer buffer containing TMRM (12.5 nM) and imaged using confocal microscopy (Nikon A1). Bar, 20 µm. N=5 with 15–20 cells imaged per experiment. All values are represented as mean ± SD. * p < 0.05 versus respective siCtrl.
Atp13a2 knockdown does not affect coupling efficiency or ΔΨm
Mitochondrial membrane potential (ΔΨm) is altered in some recessive genetic models of PD (Guzman et al., 2010; Mortiboys et al., 2010) and may play an important role in signaling the clearance of damaged mitochondria by autophagy (Matsuda et al., 2010; Narendra et al., 2008; Narendra et al., 2010). To assess ΔΨm, cultures were loaded with TMRM or JC-1 and imaged by confocal microscopy. Cortical neuron cultures treated with shAtp13a2 showed no significant changes in ΔΨm compared with cultures treated with shCtrl (Fig. 1D). Likewise, there was no significant change in SH-SY5Y cells (JC-1 red:green fluorescence: siCtrl - 134.4±24.8 vs. siATP13A2 -144.9±30.4, N=3).
After subtracting non-mitochondrial rates, mitochondrial coupling efficiency can be estimated by taking the ratio of OCR that is sensitive to inhibition with oligomycin to basal OCR, although this underestimates the true coupling efficiency due to increased membrane potential induced by oligomcyin (Brand, 1990). Using basal OCR rates from Seahorse experiments (Fig. 1B), we observed no apparent difference in coupling efficiency (siCtrl – 80.53%±4.58, siATP13A2 – 77.6%±4.17).
ATP13A2 knockdown alters mitochondrial morphology and increases mitochondrially derived reactive oxygen species
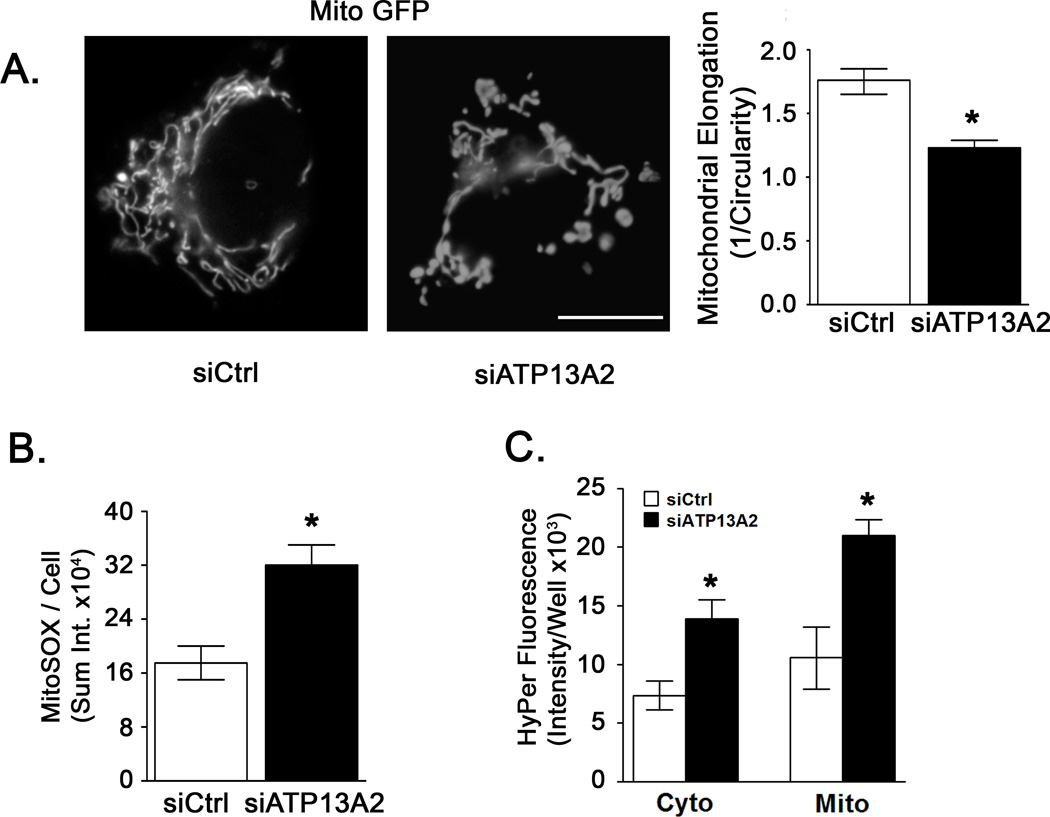
Changes in mitochondrial morphology have been observed in genetic and toxin models of parkinsonian injury (Dagda et al., 2009; Mortiboys et al., 2010; Mortiboys et al., 2008; Yang et al., 2006). In particular, loss of mitochondrial interconnectivity and increased fragmentation are observed in human neuronal cells (Dagda et al., 2009) (Thomas et al., 2011). Using a particle analysis algorithm, we found that ATP13A2 siRNA-treated SH-SY5Y cells exhibited more fragmented and circular mitochondrial profiles, accounting for a 43% decrease in mitochondrial elongation (Fig. 2A).
Figure 2. ATP13A2 knockdown alters mitochondrial morphology and increases reactive oxygen species production.
A) One day following siCtrl or siATP13A2 treatment, cells were transfected with mito-GFP. After an additional 48 hr, mitochondria were imaged on an Olympus IX71 fluorescent microscope. Mitochondrial fragmentation was assessed using an ImageJ macro that estimates mitochondrial elongation as inverse circularity. N=5 with 5–15 cells imaged per condition per experiment. Bar, 20 µm. B) Three days after siRNA transfection, cells were treated with MitoSOX Red (0.5 µM) for 10 min followed by addition of the DRAQ5 (10 µM) nuclear stain. MitoSOX Red staining was imaged for no longer than 20 min after treatment. Superoxide levels were quantified using a custom designed macro for Metamorph that measured the integrated intensity of particulate mitochondrial staining divided by the total number of DRAQ5 positive nuclei per field (Dagda et al., 2009). N=5 with 10–15 cells per experiment. C) Hydrogen peroxide levels were quantified using targeted hydrogen peroxide (HyPer) sensing vectors. One day following siCtrl or siATP13A2 treatment, cells were transfected with with cytosolically or mitochondrially targeted HyPer vectors and analyzed after an additional 48 h using a 96-well fluorescence reader. Background fluorescence of cells not transfected with HyPer vectors was subtracted to obtain specific fluorescence signals. N=4. Values are means ± SD. * p < 0.05 versus respective siCtrl.
Mitochondria are generally attributed with producing the majority of intracellular ROS (Skulachev, 1996; St-Pierre et al., 2002), and mitochondrial fragmentation can be triggered by ROS signals (Dagda et al., 2009; Koopman et al., 2007; Scott and Logan, 2008). We assessed intracellular oxidant formation using the mitochondrially targeted hydroethidine indicator MitoSOX Red. SH-SY5Y cells treated with siRNA targeting ATP13A2 exhibited an 83% increase in MitoSOX oxidation compared with non-targeting siRNA treated cells (Fig. 2B). As an independent verification of increased ROS flux, we used a set of vectors based on the hydrogen peroxide (HyPer) sensing regulatory domain of OxyR, targeted to cytoplasmic or mitochondrial compartments (Belousov et al., 2006). The mitochondrially targeted HyPer demonstrated an 87% increase in hydrogen peroxide levels, while the cytoplasmic HyPer showed a 70% increase (Fig. 2C).
ATP13A2 knockdown increases mitochondrial mass
The fraction of cytoplasmic area occupied by mitochondria was determined using cells expressing COX8-GFP. The mitochondrial area was increased by 44% in cells treated with ATP13A2 siRNA (Fig. 3A). Mitochondrial content was also studied by Western blot using an antibody cocktail against subunits of Complexes I–V. Protein levels of each electron transport chain complex were significantly increased in SH-SY5Y cells treated with ATP13A2 siRNA (Fig. 3B). In primary cortical neurons, 3-D confocal analysis was used to calculate the percent of GFP-transfected neuron cytoplasm occupied by Tom20-immunostained mitochondria. Neurons treated with shAtp13a2 showed a significantly increased mitochondrial fraction that was reversed by transfection with a plasmid for human ATP13A2 (Fig. 3C).
Figure 3. ATP13A2 knockdown increases mitochondrial mass.
A) Control and ATP13A2 siRNA-expressing cells transfected with mito-GFP were analyzed for the % cytoplasmic area occupied by mitochondria using an ImageJ macro. Images were collected by epifluorescent microscopy, which captures mitochondrial content of the whole cell. Mitochondrial content is expressed as a fraction of the total cytoplasmic area defined by the borders of the plasma membrane. N=5 with 15–20 cells per experiment. B) Cells were collected 72 hr following siRNA transfection and Western blots for components of mitochondrial complexes I–IV performed. Protein band intensities were normalized to β-actin. Data were normalized to siC treated cells. siC, non-targeting siRNA; siA, ATP13A2 targeting siRNA. N=7. C) C57BL/6 primary cortical neuron cultures expressing shCtrl or shAtp13a2 for 72 hr were fixed and immunostained using a Tom20 primary antibody and anti-rabbit Cy3 secondary antibody. Some shAtp13a2 cultures were rescued by expression of human ATP13A2. Due to the more compact, three dimensional nature of neuronal soma, the cells were imaged using confocal microscopy (Zeiss LSM 510 meta) with high resolution 12-bit z-stack images taken at 0.5 µm increments. Ctyoplasmic (green) and mitochondrial (red) volumes were calculated using ImageJ in a non-nuclear containing region of cytoplasm, and mitochondrial volume was expressed as a percent of cytoplasmic volume. Bar, 20 µm. Molecular weight markers = kDa. N=5 with 10–15 cells imaged per experiment. Values are means ± SD. * p < 0.05 versus respective siCtrl.
ATP13A2 knockdown results in reduced autophagic flux
Given the lysosomal location of ATP13A2 and the accumulation of mitochondria with ATP13A2 knockdown, we investigated whether or not basal or induced autophagic flux is altered by ATP13A2 deficiency. Autophagosome content was assessed in SH-SY5Y cells by monitoring LC3-II levels by Western blot and in primary neurons by co-transfection of RFP-LC3 with either shCtrl or shAtp13a2 to allow quantification of RFP-LC3 puncta as a marker of AVs.
Basal levels of LC3-II and RFP-LC3 puncta were not significantly different comparing cells treated with non-targeting or ATP13A2/Atp13a2 targeting RNAi in SH-SY5Y cells or primary cortical neurons, respectively (Fig. 4A,B). As these static measurements of AV content are affected by the dynamic interaction between formation and degradation of AVs (Klionsky et al., 2008), addition of the lysosomal H+-ATPase inhibitor bafilomycin A1 to block the fusion of autophagosomes with lysosomes is used to monitor levels of autophagic flux (Fass et al., 2006). The use of 5 nM bafilomycin A1 results in the optimal neutralization of lysosomal pH (Ji et al., 2006), without eliciting signs of stress or injury. After 1 hr in the presence of bafilomycin A1, SH-SY5Y cells and primary cortical neurons treated with non-targeting RNAi showed a significant increase in LC3-II levels and RFP-LC3 puncta, respectively (Fig. 4A,B), indicating a level of ongoing basal autophagic degradation. However, SH-SY5Y cells and cortical neurons treated with ATP13A2/Atp13a2 targeting RNAi showed no consistent increase in LC3-II levels or RFP-LC3 puncta, respectively, after bafilomcyin A1 treatment (Fig. 4A,B), indicating decreased basal activity. Reversal studies confirm that co-transfection with ATP13A2 is able to significantly restore autophagic flux in shAtp13a2 knocked down neurons. (Fig. 4B).
Figure 4. ATP13A2 knockdown decreases autophagic flux.
A) Three days after transfection, SH-SY5Y cells were treated with DMSO or bafilomyin (5 nM) for 1 hr and lysates were collected for Western blotting against LC3. LC3-II band intensities were normalized to β-actin, and expressed as fold-change relative to siCtrl treated cells. N=10. B) Primary cortical neuron cultures were co-transfected with the indicated shRNA and RFP-LC3 at 6 DIV. Some shAtp13a2 cultures were rescued by expression of human ATP13A2. Three days later, cultures were treated with DMSO or bafilomycin (5 nM) for 1 hr. Cells were imaged and the number of somatic RFP positive puncta were counted per cell. Bar, 20 µm. Molecular weight markers = kDa. N=7 with 5–10 cells imaged per experiment. Values are means ± SD. * p < 0.05 versus respective siCtrl.
Given the decreased basal autophagic flux in ATP13A2-deficient cells, we assessed indices of global lysosomal function and integrity. There was no significant effect of ATP13A2 knockdown on either lysosomal pH or Acridine orange staining in either SH-SY5Y cells or cortical neurons (Table S2).
ATP13A2 knockdown results in impaired induction of autophagy
Given that decreased autophagic flux was not due to global lysosomal failure, we assessed the capacity for autophagy induction using the mTOR inhibitor rapamycin (Kamada et al., 2000). SH-SY5Y cells treated with non-targeting RNAi showed a significant increase in LC3-II levels after rapamycin treatment, while cells treated with ATP13A2 targeting RNAi showed no significant increase in LC3-II levels (Fig. 5A), whether or not bafilomycin was also present to inhibit degradation (not shown).
Figure 5. ATP13A2 knockdown decreases induction of autophagy.
A) Three days after transfection, SH-SY5Y cells were treated with DMSO or rapamycin (5 µM) for 1 hr and lysates were collected for Western blotting against LC3. Band intensities were normalized to β-actin, and graphed below as fold change relative to siCtrl treated cells. N=7. B–F) For maturation studies, SH-SY5Y cells were transfected with RFP-GFP-LC3 1 d after siRNA treatment and analyzed after an additional 48 hr. Cells were treated with DMSO or with rapamycin (5 µM) for 1 hr (B, C) or 3 hr (D, E, F), fixed and imaged with confocal microscopy (Zeiss LSM 510 meta). Early AVs were counted as dual GFP and RFP positive puncta (B, D) and late AVs were counted as single RFP positive puncta (C, E). Representative images after 3 hr rapamycin treatment are shown in (F), with arrowheads indicating early AVs and arrows indicating late AVs. Bar, 10 µm. Molecular weight markers = kDa. N=5 with 10–15 cells imaged per experiment. Values are means ± SD. * p < 0.05 versus siCtrl without Rapa. † p < 0.05 versus siCtrl without Rapa and p < 0.05 versus siCtrl with Rapa.
We utilized a tandem GFP-RFP-tagged LC3 reporter to further dissect the process of autophagosome maturation (Kimura et al., 2007). GFP fluorescence is quenched under the acidic conditions of the lysosome, while RFP fluorescence persists. Thus, co-localized GFP and RFP fluorescence indicates an early autophagosome (early AV), while RFP fluorescence alone indicates AV maturation and acidification (late AV). Treating cells with rapamycin (5 µM) for 1 hr led to a significant increase in early AVs in control cells, while siATP13A2 treated cells showed no rapamycin-induced increase in early AVs (Fig. 5B). Neither control nor siATP13A2 treated cells showed a significant change in late AVs at this time point (Fig. 5C). Treating cells with rapamycin for 3 hr again led to a significant increase in early AVs in control cells (arrowheads, Fig. 5D,F), as well as in late AVs (Fig. 5E,F), indicating autophagosome maturation. There were no corresponding rapamycin-induced increases in early or late AVs in ATP13A2-deficient cells at 3 h. (Fig. 5E,F). At both 1 hr and 3 hr time points, siCtrl cells treated with rapamycin exhibited significantly more early AVs than siATP13A2 cells (Fig. 5B,D), indicating that the lack of further increase with rapamycin in siATP13A2 cells was not due to assay saturation.
ATP13A2 knockdown elicits basal increase in phosphorylation of mTOR
Signaling through the mTOR pathway is responsive to changes in cellular energy status as reflected in amino acid, glucose, or ATP levels (Dennis et al., 2001; Hara et al., 1998; Inoki et al., 2003). mTOR also lies at the interface of nutrient availability and autophagy, functioning to inhibit autophagy under nutrient rich conditions by inhibiting Atg1 activity (Jung et al., 2010). ATP13A2 knockdown in SH-SY5Y cells led to a significant increase in phosphorylation of mTOR at Ser2448 (Fig. 6A) and at Ser2481 (Fig. S3). Rapamycin treatment resulted in dephosphorylation of mTOR in both siCtrl and siATP13A2 treated cells (Fig. 6A). There were no changes in expression of Beclin-1, a positive regulator of autophagy, or rubicon, which acts to negatively regulate Beclin-1 dependent autophagosome maturation (Fig. 6B) (Kang et al., 2011; Matsunaga et al., 2009). There was a non-significant trend toward increased expression of Bcl-2, a negative regulator of autophagy (Kang et al., 2011), in ATP13A2 deficient cells (Fig. 6B).
Figure 6. ATP13A2 knockdown increases phosphorylation of mTOR.
A) Three days after transfection, cells were treated with either rapamycin (5 µM) or DMSO vehicle for 1 hr. SH-SY5Y cell lysates were collected and Western blotting was performed for phospho-mTOR (Ser2448) followed by reprobing for total mTOR. Phospho-mTOR band intensities were normalized to total mTOR, and data expressed as fold-change relative to siCtrl treated cells. N=6. B) Western blotting was performed for Beclin-1, rubicon, and Bcl-2. Band intensities were normalized to β-Actin, and data were normalized to siCtrl treated cells. Molecular weight markers = kDa. Values are means ± SD. * p < 0.05 versus respective siCtrl.
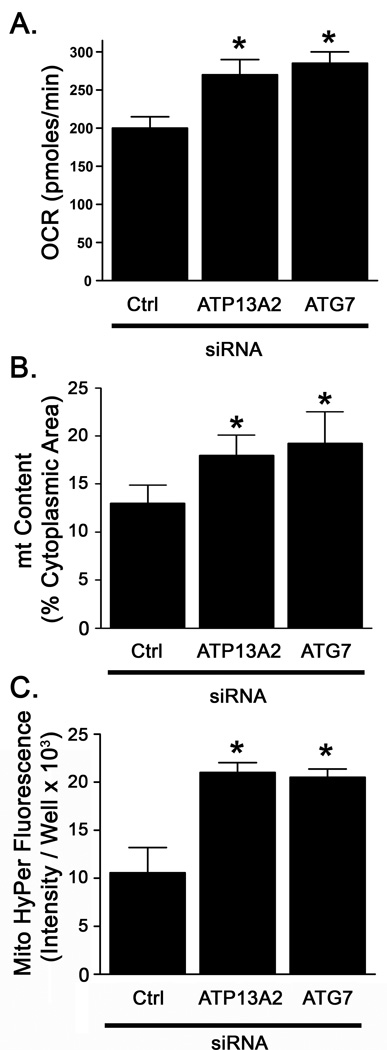
ATG7 knockdown recapitulates increased oxygen consumption, reactive oxygen species levels, and mitochondrial content
ATG7 is critical for the induction of macroautophagy (Tanida et al., 1999; Tanida et al., 2001). To determine whether impaired induction of autophagy could recapitulate mitochondrial phenotypes observed with ATP13A2 knockdown, we used siRNA to knockdown ATG7 protein by 75% (Fig. S4). ATG7 knockdown increased basal OCR by 43% over control siRNA treated cells and to a similar level as ATP13A2 knockdown (Fig. 7A). Although mitochondrial fragmentation was not significantly affected (not shown), ATG7 knockdown also elicited a 46% increase in mitochondrial content (Fig. 7B), and caused a 94% increase in mitochondrial hydrogen peroxide levels (Fig. 7C), similar to the increases observed with ATP13A2 knockdown.
Figure 7. ATG7 knockdown increases oxygen consumption and reactive oxygen species production.
SH-SY5Y cells were transfected with non-targeting, ATP13A2 targeting, or ATG7 targeting (siATG7) siRNA. A) Three days after transfection, basal oxygen consumption was measured as in Fig. 1. N=3. B) Cells were transfected with HyPer vectors one day after siRNA transfection as in Fig. 2. N=4. Values are means ± SD. * p < 0.05 versus respective siCtrl.
DISCUSSION
Putative loss of function mutations in ATP13A2 have been linked to Kufor-Rakeb syndrome, a form of early onset parkinsonism (Ramirez et al., 2006), however its basic function and the mechanism(s) by which its loss of function is linked to disease remain unknown. Given the location of ATP13A2 within the lysosomal membrane, we studied the consequence of ATP13A2 knockdown on mitochondrial function and autophagy, the pathway through which mitochondrial turnover and quality control are achieved. Given the widespread cortical involvement seen in Kurfor-Rakeb syndrome (Najim al-Din et al., 1994; Ramirez et al., 2006), the effects of Atp13a2 deficiency was studied in primary cortical neurons, using the SH-SY5Y cell line cultured under conditions that produce reliance on mitochondrial metabolism (Rossignol et al., 2004) to further dissect mechanisms underlying decreased autophagic flux.
We found that knockdown of ATP13A2 for three days resulted in accumulation of subtly altered mitochondria without major effects on viability. Although there were no deficiencies in mitochondrial coupling efficiency or membrane potential, the increased free radical production and mitochondrial fragmentation may represent initial signs of mitochondrial dysfunction. Furthermore, while we observed increased respiratory rates, we observed no significant increase in steady-state ATP levels. Thus, ATP13A2 knockdown cells may exhibit increased consumption of ATP due to increased metabolic demand, or decreased ATP generation from processes such as autophagy (Hubbard et al., 2010). Consumption of more oxygen to maintain cellular ATP, while producing increased ROS, are signs of mitochondrial dysfunction and of compensated cellular stress that could contribute to chronic neurodegeneration if sustained.
Given the lysosomal location of ATP13A2, we hypothesized that its knockdown may affect mitochondrial mass through alterations in autophagy. The bafilomycin-induced increase in autophagy markers is reflective of a population of autophagosomes that are induced and actively degraded during the period of bafilomycin treatment. The lack of accumulation observed following ATP13A2 knockdown in mitochondrially-dependent cells indicates decreased basal autophagic flux. Decreased flux can be due to changes in autophagy induction or in maturation/degradation, and the latter situation typically causes increased baseline LC3-II levels (Klionsky et al., 2008; Shintani and Klionsky, 2004; Walls et al., 2007). While the Atp13a2-deficient cortical neurons exhibited increased baseline levels of early, unacidified AVs, compatible with decreased maturation, there was only an inconsistent trend of increased LC3-II in knockdown SH-SY5Y cells. Moreover, there was no evidence of global lysosomal dysfunction or membrane permeabilization, as has been reported in other systems (Dehay et al., 2010), suggesting additional mechanisms of impaired autophagic flux.
While the ability of rapamycin to induce autophagy in primary neurons has been inconsistent (Rubinsztein and Nixon, 2010; Tsvetkov et al., 2010; Tsvetkov et al., 2009), SH-SY5Y cells respond to rapamycin with increased LC3-II levels and increased autophagic flux (Cherra et al., 2010b; Pan et al., 2009). Indeed, ATP13A2-deficient cells showed a defect in rapamycin-mediated autophagy induction (Fig. 5A) as assessed by either total LC3-II levels and by using a tandem GFP-RFP-LC3 construct capable of distinguishing early and late AVs (Klionsky et al., 2008). As a decreased propensity for autophagy induction may serve to mask effects of inefficient autophagy maturation, this may account for similar levels of baseline LC3-II in the SH-SY5Y cells.
The basal deficit in autophagy activity observed in ATP13A2-deficient cells may be attributed to increased levels of phospho-mTOR following ATP13A2 knockdown (Fig. 6), as phosphorylated, activated mTOR plays a major role in suppressing autophagy. Moreover, the ability of rapamycin to dephosphorylate mTOR in siATP13A2 treated cells suggests that loss of ATP13A2 either affects autophagy both upstream and downstream of mTOR or that additional mTOR-independent mechanisms of autophagy suppression are involved. Decreased levels of Beclin 1, which plays a central role in autophagy induction and maturation, have been reported in Alzheimer’s disease (Pickford et al., 2008). However, there was no decrease in the Beclin 1 protein in ATP13A2 knockdown cells. The autophagy-promoting functions of Beclin 1 can be negatively regulated by complex formation with rubicon or Bcl-2 (Kang et al., 2011). While there were no differences in rubicon levels, a non-significant trend towards increased Bcl-2 protein levels was observed in ATP13A2 deficient cells (Fig. 6). Thus, regulation of the Beclin 1 complex rather than its absolute levels may be altered. Additional studies will be needed to fully elucidate the mechanism(s) by which ATP13A2 loss of function contributes to reduced autophagic flux.
Knockdown of ATP13A2 affects autophagy in a different fashion than has been reported in lysosomal storage diseases exhibiting increased levels of autophagosomes (Cao et al., 2006; Ko et al., 2005; Koike et al., 2005; Pacheco et al., 2007; Takamura et al., 2008; Vergarajauregui et al., 2008). However, the possibility of decreased autophagic maturation, which may be masked by the induction deficits, may be shared with some lysosomal storage diseases (Jennings et al., 2006; Settembre et al., 2008; Tanaka et al., 2000). Mitochondrial respiratory function remains largely intact in our system, and this may reflect reduced sequestration of mitochondria into autophagosomes. Similarly, in mucolipidosis type IV, fragmented mitochondria accumulate outside of autophagosomes, suggesting feedback inhibition of mitophagy induction due to downstream lysosomal dysfunction (Kiselyov et al., 2007). Likewise, blocking autophagy induction directly by ATG7 knockdown also causes increased mitochondrial content, OCR, and ROS levels. However, the increased mass does not necessarily translate to increased mitochondrial quality, resulting in greater ROS generation (Fig. 2 & 7), or impaired calcium buffering (Jennings et al., 2006), factors that may contribute further to mitochondrial damage and enhanced susceptibility to cell death.
This is consistent with previous studies in which yeast strains harboring various autophagy gene knockouts (Zhang et al., 2007), or human THP1 cells in which ATG5 or Beclin 1 was knocked down (Zhou et al., 2011), exhibited increased mitochondrial ROS production and mass, but represents the first report of this phenomenon in primary neurons. However, the observation that ATG7 knockdown did not cause mitochondrial fragmentation indicates that ATP13A2 knockdown may have additional effects on mitochondria. Indeed, a recent study has shown that lysosomes themselves may generate ROS and contribute to subsequent mitochondrial dysfunction (Kubota et al., 2010).
In our model system we did not observe decreased membrane potential or diminished oxygen consumption as seen in yeast (Zhang et al., 2007). This discrepancy may relate to differences in cell type. The half-life of brain mitochondria has been reported to be up to 24.4 days, which is much longer than values of 17.5, 1.83 to 12.6, and 9.3 days reported for heart, liver and testes mitochondria, respectively (Menzies and Gold, 1971; Miwa et al., 2008). While stable models of ATP13A2 deficiency have not yet been described, future studies employing stable cellular or animal models will illuminate more fully the long-term effects of ATP13A2-related autophagolysosomal dysfunction on mitochondrial quality.
Increasing evidence has implicated dysfunctional autophagic mitochondrial quality control as an important mechanism in the pathogenesis of PD (Cherra et al., 2010a; Narendra et al., 2008; Zhu and Chu, 2010). In this study, we report that loss of ATP13A2 results in mitochondrial alterations in conjunction with reduced basal and rapamycin-induced autophagic flux. These data further support a convergence on impaired mitochondrial quality control as a central pathogenic mechanism among various genetic causes of PD-related syndromes.
Research Highlights.
Mutations in the ATP13A2 gene cause autosomal recessive parkinsonism with dementia.
ATP13A2 RNAi increases mitochondria mass and fragmentation in neurons & SH-SY5Y.
Cells consume more oxygen to maintain cellular ATP, while producing increased ROS.
ATP13A2 deficiency reduces basal autophagic flux and increases phospho-mTOR.
Loss of this lysosomal P-type ATPase adversely affects autophagic quality control.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Tomatsu Yoshimori, Ian J. Reynolds, Christian Kubisch and Stefan Strack for the gifts of plasmids as detailed in Methods. We thank Jason Callio for technical assistance with primary neuron cultures, and Drs. Michelle Barbi and Tao Wang for technical assistance with the Seahorse XF24. We thank Salvatore Cherra for helpful discussions and assistance with independent confirmation of puncta counts. This work was supported by funding from the National Institutes of Health (CTC: AG026389 and NS065789) and the American Parkinson Disease Association (JZ). CTC is recipient of an AFAR/Ellison Medical Foundation Julie Martin Mid-Career Award in Aging Research. AMG was supported in part by the University of Pittsburgh Department of Pathology as a Pathology Post-sophomore fellow, and is currently a member of the University of Pittsburgh Physician Scientist Training Program.
ABBREVIATIONS
- PD
Parkinson’s Disease
- ROS
reactive oxygen species
- siRNA
small interfering RNA, HyPer vector, hydrogen peroxide sensing vector
- OXPHOS
oxidative phosphorylation
- OCR
oxygen consumption rate
- ECAR
extracellular acidification rate
- oligo
oligomcyin
- siCtrl
non-targeting siRNA
- siATP13A2
human ATP13A2 targeting siRNA
- shCtrl
non-targeting shRNA
- shAtp13a2
mouse Atp13a2 targeting shRNA
REFERENCES
- Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- Batlevi Y, La Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis. 2011;43:46–51. doi: 10.1016/j.nbd.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson CC, et al. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem. 2010;404:75–81. doi: 10.1016/j.ab.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Brand MD. The proton leak across the mitochondrial inner membrane. Biochim Biophys Acta. 1990:128–133. doi: 10.1016/0005-2728(90)90232-s. [DOI] [PubMed] [Google Scholar]
- Cao Y, et al. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J Biol Chem. 2006;281:20483–20493. doi: 10.1074/jbc.M602180200. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, et al. Review: autophagy and neurodegeneration: survival at a cost? Neuropathol Appl Neurobiol. 2010a;36:125–132. doi: 10.1111/j.1365-2990.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, et al. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010b:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, et al. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, et al. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, et al. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson's disease. Cell Death Differ. 2011;18(12):1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PB, et al. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Fass E, et al. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–36316. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- Fukuda T, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- Gohil VM, et al. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol. 2010;28:249–255. doi: 10.1038/nbt.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Herman AM, Moussa CE. The ubiquitin ligase parkin modulates the execution of autophagy. Autophagy. 2011:7. doi: 10.4161/auto.7.8.15814. [DOI] [PubMed] [Google Scholar]
- Hu LF, et al. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- Hubbard VM, et al. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, et al. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jennings JJ, Jr, et al. Mitochondrial aberrations in mucolipidosis Type IV. J Biol Chem. 2006;281:39041–39050. doi: 10.1074/jbc.M607982200. [DOI] [PubMed] [Google Scholar]
- Ji ZS, et al. Reactivity of apolipoprotein E4 and amyloid beta peptide: lysosomal stability and neurodegeneration. J Biol Chem. 2006;281:2683–2692. doi: 10.1074/jbc.M506646200. [DOI] [PubMed] [Google Scholar]
- Jung CH, et al. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, et al. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, et al. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, et al. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, et al. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3:259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, et al. Cell-autonomous death of cerebellar purkinje neurons with autophagy in Niemann-Pick type C disease. PLoS Genet. 2005;1:81–95. doi: 10.1371/journal.pgen.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, et al. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease) Am J Pathol. 2005;167:1713–1728. doi: 10.1016/S0002-9440(10)61253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, et al. Human NADH:ubiquinone oxidoreductase deficiency: radical changes in mitochondrial morphology? Am J Physiol Cell Physiol. 2007;293:C22–C29. doi: 10.1152/ajpcell.00194.2006. [DOI] [PubMed] [Google Scholar]
- Krebiehl G, et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1. PLoS One. 2010;5:e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota C, et al. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem. 2010;285:667–674. doi: 10.1074/jbc.M109.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Ramshesh VK. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283–295. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- Miwa S, et al. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, et al. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- Mortiboys H, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najim al-Din AS, et al. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol Scand. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- Narendra D, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000298. e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, et al. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010 Dec 6;46 doi: 10.3791/2511. pii: 2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco CD, et al. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum Mol Genet. 2007;16:1495–1503. doi: 10.1093/hmg/ddm100. [DOI] [PubMed] [Google Scholar]
- Pan T, et al. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Pickford F, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowey ED, et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Van Houten B. Alterations in bioenergetics due to changes in mitochondrial DNA copy number. Methods. 2010;51:452–457. doi: 10.1016/j.ymeth.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ramirez A, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Reitzer LJ, et al. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- Rossignol R, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Nixon RA. Rapamycin induces autophagic flux in neurons. Proc Natl Acad Sci U S A. 2010;107:E181. doi: 10.1073/pnas.1014633107. author reply E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I, Logan DC. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 2008;177:90–101. doi: 10.1111/j.1469-8137.2007.02255.x. [DOI] [PubMed] [Google Scholar]
- Settembre C, et al. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, et al. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Takamura A, et al. Enhanced autophagy and mitochondrial aberrations in murine G(M1)-gangliosidosis. Biochem Biophys Res Commun. 2008;367:616–622. doi: 10.1016/j.bbrc.2007.12.187. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- Tanida I, et al. Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy. Mol Biol Cell. 1999;10:1367–1379. doi: 10.1091/mbc.10.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, et al. The human homolog of Saccharomyces cerevisiae Apg7p is a Protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 2001;276:1701–1706. doi: 10.1074/jbc.C000752200. [DOI] [PubMed] [Google Scholar]
- Thomas KJ, et al. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011 Jan 1;20(1):40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov AS, et al. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci U S A. 2010;107:16982–16987. doi: 10.1073/pnas.1004498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov AS, et al. Protein turnover differences between neurons and other cells. Autophagy. 2009:5. [Google Scholar]
- Vergarajauregui S, et al. Autophagic dysfunction in mucolipidosis type IV patients. Hum Mol Genet. 2008;17:2723–2737. doi: 10.1093/hmg/ddn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls KC, et al. Altered regulation of phosphatidylinositol 3-kinase signaling in cathepsin D-deficient brain. Autophagy. 2007;3:222–229. doi: 10.4161/auto.3822. [DOI] [PubMed] [Google Scholar]
- Winslow AR, et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–346. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011 Jan 13;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chu CT. Mitochondrial dysfunction in Parkinson's disease. J Alzheimers Dis. 2010;20 Suppl 2:S325–S334. doi: 10.3233/JAD-2010-100363. [DOI] [PubMed] [Google Scholar]
- Zhu JH, et al. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.