Abstract
INTRODUCTION
Primary parotid malignancies represent a rare diagnosis, making high-quality comparative research unfeasible. There is little UK-based evidence to guide practice. A review was therefore undertaken of a large series of patients treated by a multidisciplinary team in a National Health Service tertiary referral centre.
PATIENTS AND METHODS
Retrospective patient record review at the John Radcliffe Hospital in Oxford identified 401 patients who had undergone parotidectomy between 1995 and 2010, of whom 50 subjects were given a definitive diagnosis of primary parotid malignancy, treated with surgery and postoperative radiotherapy. Case notes, histology and imaging were reviewed by the study team.
RESULTS
The median follow up for the cohort was 60 months (range: 1-108 months). Facial nerve function was preserved in all patients undergoing partial or total conservative parotidectomy. Although histology showed microscopically close or positive margins in 82% of cases, all patients underwent postoperative radiotherapy and locoregional recurrence was identified in only two (4%) patients.
CONCLUSIONS
The data presented demonstrate a reasonable and practical multidisciplinary approach to a complex management problem. Facial nerve sparing surgery and postoperative radiotherapy result in good control of locoregional disease.
Keywords: Parotid neoplasms; Otolaryngology; Radiotherapy; Biopsy, fine-needle
The diagnosis of primary parotid malignancy is rare. The UK Cancer Registries recorded 365 cases of parotid cancer in 2007, along with 112 classified as unspecified major salivary gland tumour.1 Combining these classifications gives crude UK incidence rates of 1.1 per 100,000 for males and 0.8 per 100,000 for females. Their treatment is challenging because of their infrequency, their unpredictable biological behaviour and their prolonged risk of locoregional and distant recurrence. Understanding of their behaviour and management relies chiefly on publications of large series from individual institutions. Surgery has formed the mainstay of treatment2,3 and the role of postoperative radiotherapy has been established with a growing scope of application.4-15 as these data accumulate, a wider perspective can be drawn on certain aspects, for example on the prognostic factors in advanced adenocarcinoma.16
In the vast majority of patients with head and neck cancer, a tissue (cytological or histological) diagnosis has been made before arriving at a definitive management plan. This is not necessarily the case in patients with parotid malignancy, where the clinical diagnosis is obvious only in those with advanced disease. The majority of patients (60%) with parotid malignancy present with a discrete lump that is clinically indistinguishable from a benign parotid tumour.17 Preoperative imaging or fine needle aspiration (FNA) and preoperative frozen sections may provide a diagnosis in some of these patients but in many the diagnosis of malignancy is made only on definitive postoperative histology. We therefore have to tailor a ‘universal’ surgical management plan for parotid lumps, both benign and malignant, acknowledging our inability always to diagnose malignancy preoperatively.
This paper reviews the Oxford head and neck multidisciplinary team (MDT) approach to the problem of primary parotid carcinoma over the past 15 years. We would accept that our follow-up period is relatively short when considering salivary gland neoplasms (as opposed to other squamous cell cancers in the head and neck).
Patients and Methods
All patients with a diagnosis of parotid cancer in Oxford since 1995 were identified from the records of the ear, nose and throat (ENT) department as well as the radiotherapy and pathology departments. Written and computer records were interrogated for the data presented. The study covers 50 patients (27 male, 23 female; mean age: 54.3 years; age range: 19-86 years) treated between 1995 and 2010 at the ENT department at the Oxford Radcliffe Hospitals NHS Trust and who were affected by a primary malignancy of the parotid gland and treated with surgery and postoperative radiotherapy. Over that same period 401 patients underwent parotidectomy for all types of tumour of the parotid; hence, in this series 12.5% of parotid tumours proved malignant.
Figure 1.
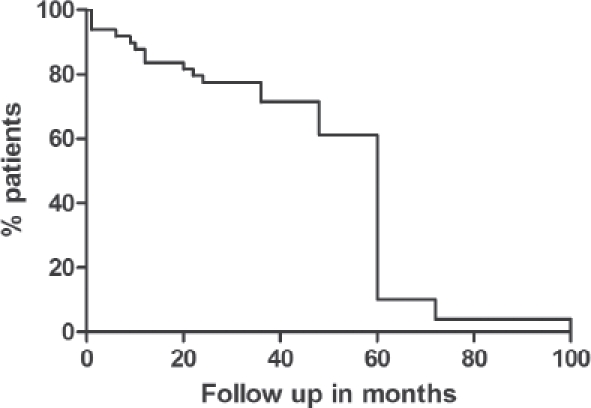
Length of follow up for patients in this report
Presentation
All patients presented with a mass in the parotid region and three patients also had facial nerve weakness at presentation (in two cases they had complete facial palsy, ie House-Brackmann grade 6).
Preoperative investigation
FNA specimens were available for 27 of the 50 patients who were investigated and treated at the Oxford Radcliffe Hospitals NHS Trust. The clinical team or one of a group of pathologists (including the author KaS) carried out the aspirates. For the other patients, FNA was either not done, especially in the early years of the study, or was carried out at other centres and material was not available for review. Preoperative imaging was by magnetic resonance imaging (MRI) unless the patient was claustrophobic.
Staging
On the basis of the clinical records and radiologic findings, patients were retrospectively classified in accordance with the International Union Against Cancer ‘tumour, nodes, metastasis’ staging for malignant salivary gland disease.18 a total of 22 (44%) patients had T1 disease, 17 (34%) had T2, 5 (10%) had T3 and 6 (12%) had T4a. One patient had N1 disease at the time of presentation.
Histology
The histopathological classification after surgery is shown in Figure 2. A total of 33 (66%) cases were classified as low grade and 10 (20%) as high grade. The remaining seven cases were adenoid cystic carcinoma, where there is no consensus on histological grading.
Figure 2.
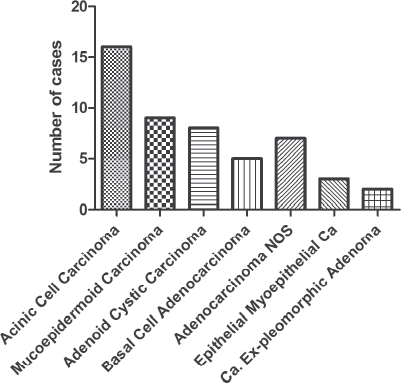
World Health Organization histopathological classification after surgery
Surgery
The extent of surgery was dictated by the site (superficial or deep lobe) and size of the tumour. Forty (80%) patients had tumours involving the superficial lobe of the gland and underwent partial parotidectomy. Seven (14%) had tumours involving both the superficial and deep lobes of the gland and underwent total conservative parotidectomy. Two (4%) had preoperative complete facial palsies and underwent radical parotidectomy. One underwent total conservative parotidectomy with ipsilateral neck dissection (this patient had a tumour involving both superficial and deep lobes of the gland with involved level 2 lymph nodes).
The definitive microscopic examination of the resected tissue revealed close or positive excision margins (<5mm)19 in 41 (82%) of the 50 patients. In the remaining nine (18%) patients, surgical margins were clear.
Radiotherapy
Postoperative radiotherapy was given in all cases in this report on the basis of the histological findings, tumour dimensions, type of surgery performed and extent of cervical lymph node metastasis. The radiotherapy dose was 60Gy in 30 fractions in all these cases, usually delivered conformally as a wedged pair of anterior oblique and posterior oblique beams.
Results
Fine needle aspiration
Of the 27 patients who had preoperative FNA, 18 (66.6%) had a definitive diagnosis of malignancy and in 12 (44.4%) of these patients, the tumour was accurately subtyped on FNA when compared with the final histology. Cytological diagnosis of a benign tumour (false negative) was made in 4 of 27 patients (14.8%). On review, the reasons for discordant diagnoses were sampling bias (lesion not adequately sampled) in one case and errors in cytological interpretation (lesion sampled but misread) in three. The test was non-diagnostic in 5 of 27 patients (18.5%) and when assessed on the basis of the number of aspirates performed, the rate was higher for clinicians (62.5%, 5 of 8) when compared with the pathologists (3.5%, 1 of 28).
Recurrence
There were only two cases of recurrence (4%) among these fifty patients. The first patient had adenoid cystic carcinoma, staged as T4 N0 at presentation. This patient underwent total conservative parotidectomy with a macroscopically positive margin. Postoperative radiotherapy was given but local recurrence occurred two years after the completion of treatment.
The second patient had T1 N0 epithelial myoepithelial carcinoma. This patient also underwent total conservative parotidectomy with positive excision margins and received postoperative radiotherapy. After two years the patient developed local recurrence (presenting with a complete facial palsy), which was managed surgically by cortical mastoidectomy with revision parotidectomy followed by brachytherapy.
Metastasis
Distant metastasis occurred in both of the patients who developed recurrence. The metastases affected the lungs in the patient with adenoid cystic carcinoma while the patient with recurrent myoepithelial carcinoma developed intracranial metastasis.
Facial nerve function
The facial nerve was anatomically intact postoperatively in all the patients (48) who underwent partial and total conservative parotidectomies. Facial nerve function in the long term (after surgery and postoperative radiotherapy) was normal in all these cases (House-Brackmann grade 1). Among this group, one patient with acinic cell carcinoma presented with partial facial nerve palsy preoperatively but recovered after undergoing total conservative parotidectomy. The two patients who underwent radical parotidectomy had a complete facial nerve palsy (House-Brackmann grade 6) preoperatively.
Discussion
There is controversy regarding the management of primary carcinoma of the parotid gland. Complete surgical excision of the tumour along with an adequate margin of his-tologically normal tissue remains the main aim of surgical treatment. However, much of the surgical literature details management plans tailored to specific histologic diagnoses although this diagnosis is usually not available definitively until the surgical excision has occurred. Different studies have also recommended different surgical approaches depending on tumour stage and grade.6-8-20
Although the aim of surgical removal with clear margins would be agreed, the extent to which this may be achieved is complicated by the relationship between the tumour and the facial nerve. In our series there was a close or positive microscopic margin19 in 82% of cases and this is largely related to performing facial nerve sparing surgery. Histological studies of such surgery for benign disease have shown focal capsular exposure in virtually all cases.21,22 In surgery for malignant parotid tumours, Bron et al found 74% of patients had microscopic positive margins and yet had similar survival and recurrence rates with postoperative radiotherapy to those patients undergoing more radical surgery.4
The consensus of opinion is that a functionally intact nerve should be preserved if there is no intraoperative finding of direct macroscopic nerve invasion.23,24 The rationale for this is that resection of an intact nerve has not been shown to improve local disease control.25 The results in our series would certainly support this approach. We report an overall recurrence rate across all histological subtypes of 4%, while historical series over several decades have reported rates around 25% with a range of 14-40%.4-6.12,26 However, we would accept that the follow-up time for our patients is not sufficient to draw firm conclusions regarding overall survival of the cohort.
There is also debate regarding the indications for postoperative (adjuvant) radiotherapy. All patients in this series received radiotherapy treatment. Radiotherapy was advised because of the close or positive margins and also in the hope of reducing the risk of locoregional recurrence entailing the need for revision surgery, which is associated with a much greater risk of facial nerve injury than primary surgery.
Clear indications for post-operative radiotherapy may include macroscopic residual tumour, high grade cancers and probably microscopic positive margins. Relative indications include perineural spread, tumour close to the nerve and cervical node metastases.27 Clear evidence of a dose response is lacking in this rare disease and the dose of 60Gy in daily 2Gy fractions represents a practical consensus. Developments in radiotherapy technique, such as intensity modulated radiotherapy, have been applied to parotid treatment28,29 and the hope is that this may permit an escalation of dose with the aim of increasing local control. The precise role and impact of newer technologies, however, remains to be defined.
Results of FNA were available for 27 patients, 5 (18.5%) of whom had non-diagnostic aspirates. The lower non-diagnostic rate of 3.5% was seen for pathologists carrying out the procedure in dedicated FNA clinics, emphasising that due attention to and experience of the aspiration technique are important for a diagnostic result. The higher rate of 62.5% for clinicians probably reflects a mixture of junior and senior doctors with varying expertise carrying out the procedure in routine outpatient clinics.
When non-diagnostic results are excluded from the analysis, preoperative FNA was found correctly to predict the tumour type in 12 of 22 patients (54.5%). In a further six patients it provided a positive diagnosis of malignancy. Thus, when the FNA sample was diagnostic, the predictive rate of malignancy in our series was 81.8% (18 of 22), indicating that 8 out of 10 patients with parotid malignancy were correctly identified on FNA (Figs 3 and 4). Direct comparison of these figures with published series on sensitivity and specificity of salivary gland FNA would be misleading as our series includes only malignant tumours. Benign tumours account for the vast majority of parotid neoplasms and their exclusion in this study results in a sampling bias.
Figure 3.
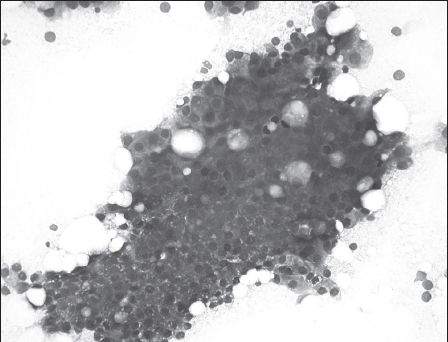
Fine needle aspirate containing uniform epithelial cells (May-Grünwald-Giemsa staining; 200x magnification)
Figure 4.
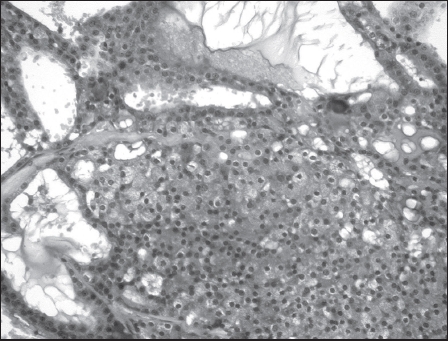
Histological section of acinic cell carcinoma with solid and cystic areas (haematoxylin and eosin staining; 200x magnification); specimen from the same patient as the FNA in Fig 3
There were four patients in whom FNA gave a false negative result of a benign tumour. In three of these patients representative material was present but not correctly interpreted and in the fourth patient the lesion was not fully sampled. Salivary gland tumours are uncommon and, as a result, exposure of pathologists to their cytological appearances can be limited. Additionally, a degree of morphological overlap exists in the cytological appearance of different tumours, which can lead to diagnostic errors even among experienced pathologists. An important message from this study, which we have incorporated in our current cytological practice, is that unless characteristic cytological features are present, a specific diagnosis of salivary gland tumour subtype is avoided. Instead, we state that the aspirate findings suggest a salivary gland neoplasm and, where possible, a list of differential diagnosis is offered so that an appropriate surgical decision can be made.
Notwithstanding these limitations, FNA has a useful role in the management of salivary gland tumours. A malignant cytology result can provide for better preoperative counselling of patients, including increased risk to facial nerve function and the likelihood of requiring postoperative radiotherapy.
Imaging is used to confirm that a palpable mass is within or arising from the parotid and to give an indication of its extent, anatomical relations and likely nature. MRI is preferred to ultrasound for its ability to demonstrate the deep lobe of the parotid and its sensitivity in demonstrating perineural tumour spread. MRI can also provide valuable information regarding:
the position of the tumour in relation to the main trunk of the facial nerve;
findings not appreciated clinically, such as a small tumour in the contralateral parotid or a second small primary tumour within the ipsilateral parotid;
the extent of any nodal disease (within the parotid itself or in the neck), whether or not it is suspected on clinical grounds;
the presence of perineural tumour spread including intracranial spread via the facial nerve (that would indicate incurable disease); and
the presence of extraparotid spread, eg invasion of the mandibular ramus, skull base or parapharyngeal space as well as encasement or occlusion of the internal carotid artery or internal jugular vein.
MRI is also the imaging modality of choice for post-treatment follow up.
It is important to realise that it is often impossible to distinguish benign from malignant lesions on imaging. Many well differentiated malignant salivary gland tumours are well circumscribed and appear similar to benign tumours on MRI, computed tomography (CT) and ultrasound (Fig. 5). Positron emission tomography (PET) combined with CT gives anatomical as well as metabolic information about a tumour. This can give an indication of malignancy and has become important in the investigation of tumours in other parts of the body, eg lung and oesophagus. Unfortunately, there is significant overlap in the PET-CT appearances of benign and malignant parotid tumours30 and it is therefore not part of our routine investigation for parotid tumours.
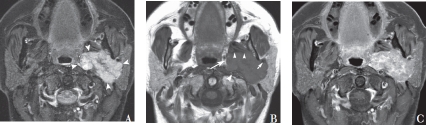
Figure 5.
Axial STIR (A), T1-weighted (B) and gadolinium enhanced, fat saturated T1-weighted (C) MRI scans showing a large, well circumscribed, avidly enhancing mass lesion of the deep lobe of the left parotid (arrowheads in A). The images clearly demonstrate infiltration into the parapharyngeal fat (large arrow in B), posterior displacement of the internal carotid artery (large arrowhead in B), anterior displacement of the medial pterygoid muscle (small arrowheads in B) and anterolateral displacement of the retromandibular vein (small arrow in B) suggesting that the facial nerve is laterally displaced by the mass although it cannot be visualised in this case. It was not possible to distinguish with certainty between a pleomorphic adenoma and a malignant tumour on the basis of this scan. Histology showed an acinic cell carcinoma.
Conclusions
This paper details how the Oxford head and neck MDT has approached the problem of primary parotid carcinoma over the past 15 years and describes the patient outcomes measured. We would accept that our follow-up period is relatively short when considering salivary gland neoplasms (as opposed to other squamous cell cancers in the head and neck). The MDT has adopted the following approach to patients presenting with a parotid mass:
Preoperative investigation includes a minimum of FNA and MRI (contrast enhanced CT is a reasonable alternative in cases where MRI is contraindicated), which inform patient counselling and discussion of adjuvant radiotherapy.
The extent of surgery is dictated by the site and size of the tumour and involves a form of superficial or total conservative parotidectomy with preservation of a functioning facial nerve.
There is a low threshold for adjuvant radiotherapy with 60Gy in 2Gy fractions in the hope of reducing the risk of local recurrence (and hence avoiding the risk of revision surgery with its greatly increased risk of facial nerve injury).
References
- 1.Office for National Statistics. Cancer statistics registrations. Series MB1 No. 38. London: ONS; 2010. [Google Scholar]
- 2.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–184. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 3.Spiro RH, Huvos AG, Strong EW. Cancer of the parotid gland. A clinicopathologic study of 288 primary cases. Am J Surg. 1975;130:452–459. doi: 10.1016/0002-9610(75)90483-3. [DOI] [PubMed] [Google Scholar]
- 4.Bron LP, Traynor SJ, McNeil EB, O'Brien CJ. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113:1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Cederblad L, Johansson S, et al. Cancer of the parotid gland; long-term follow-up. A single centre experience on recurrence and survival. Acta Oncol. 2009;48:549–555. doi: 10.1080/02841860802680419. [DOI] [PubMed] [Google Scholar]
- 6.Guillamondegui OM, Byers RM, et al. Aggressive surgery in treatment for parotid cancer: the role of adjunctive postoperative radiotherapy. Am J Roentgenol Radium Ther Nucl Med. 1975;123:49–54. doi: 10.2214/ajr.123.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Jackson GL, Luna MA, Byers RM. Results of surgery alone and surgery combined with postoperative radiotherapy in the treatment of cancer of the parotid gland. Am J Surg. 1983;146:497–500. doi: 10.1016/0002-9610(83)90239-8. [DOI] [PubMed] [Google Scholar]
- 8.Johns ME. Parotid cancer: a rational basis for treatment. Head Neck Surg. 1980;3:132–141. doi: 10.1002/hed.2890030207. [DOI] [PubMed] [Google Scholar]
- 9.Koul R, Dubey A, et al. Prognostic factors depicting disease-specific survival in parotid-gland tumors. Int J Radiat Oncol Biol Phys. 2007;68:714–718. doi: 10.1016/j.ijrobp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Gleave EN, Slevin NJ, McGurk M. Clinico-pathological and treatment-related factors influencing survival in parotid cancer. Br J Cancer. 1999;80:1296–1300. doi: 10.1038/sj.bjc.6990501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberson DW, Chu FW, Yarington CT. Parotid cancer: treatment and results. Ear Nose Throat J. 1993;72:787–793. [PubMed] [Google Scholar]
- 12.Toonkel LM, Guha S, Foster P, Dembrow V. Radiotherapy for parotid cancer. Ann Surg Oncol. 1994;1:468–472. doi: 10.1007/BF02303611. [DOI] [PubMed] [Google Scholar]
- 13.Tu G, Hu Y, Jiang P, Qin D. The superiority of combined therapy (surgery and postoperative irradiation) in parotid cancer. Arch Otolaryngol. 1982;108:710–713. doi: 10.1001/archotol.1982.00790590032010. [DOI] [PubMed] [Google Scholar]
- 14.Zbären P, Schüpbach J, et al. Carcinoma of the parotid gland. Am J Surg. 2003;186:57–62. doi: 10.1016/s0002-9610(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 15.Nagliati M, Bolner A, et al. Surgery and radiotherapy in the treatment of malignant parotid tumors: a retrospective multicenter study. Tumori. 2009;95:442–448. doi: 10.1177/030089160909500406. [DOI] [PubMed] [Google Scholar]
- 16.Jeannon JP, Calman F, et al. Management of advanced parotid cancer. A systematic review. Eur J Surg Oncol. 2009;35:908–915. doi: 10.1016/j.ejso.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Renehan A, Gleave EN, et al. Long-term follow-up of over 1000 patients with salivary gland tumours treated in a single centre. Br J Surg. 1996;83:1750–1754. doi: 10.1002/bjs.1800831228. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th edn. New York, US: Wiley-Liss; 2002. [Google Scholar]
- 19.Royal College of Pathologists. Datasets for Histopathology Reports on Head and Neck Carcinomas and Salivary Neoplasms. 2nd edn. London: RCPath; 2005. [Google Scholar]
- 20.Stafford ND, Wilde A. Parotid cancer. Surg Oncol. 1997;6:209–213. doi: 10.1016/s0960-7404(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 21.Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope. 2002;112:2141–2154. doi: 10.1097/00005537-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Webb AJ, Eveson JW. Pleomorphic adenomas of the major salivary glands: a study of the capsular form in relation to surgical management. Clin Otolaryngol Allied Sci. 2001;26:134–142. doi: 10.1046/j.1365-2273.2001.00440.x. [DOI] [PubMed] [Google Scholar]
- 23.Gleeson M. Scott-Brown's Otorhinolaryngology, Head and Neck Surgery. 7th edn. London: Hodder; 2008. [Google Scholar]
- 24.Tweedie DJ, Jacob A. Surgery of the parotid gland: evolution of techniques, nomenclature and a revised classification system. Clin Otolaryngol. 2009;34:303–308. doi: 10.1111/j.1749-4486.2009.01953.x. [DOI] [PubMed] [Google Scholar]
- 25.Hodgkinson DJ, Woods JE. The influence of facial-nerve sacrifice in surgery of malignant parotid tumors. J Surg Oncol. 1976;8:425–432. doi: 10.1002/jso.2930080509. [DOI] [PubMed] [Google Scholar]
- 26.Woods JE, Chong GC, Beahrs OH. Experience with 1,360 primary parotid tumors. Am J Surg. 1975;130:460–462. doi: 10.1016/0002-9610(75)90484-5. [DOI] [PubMed] [Google Scholar]
- 27.Valstar MH, van den Brekel MW, Smeele LE. Interpretation of treatment outcome in the clinically node-negative neck in primary parotid carcinoma: a systematic review of the literature. Head Neck. 2010;32:1402–1411. doi: 10.1002/hed.21316. [DOI] [PubMed] [Google Scholar]
- 28.Nutting CM, Rowbottom CG, et al. Optimisation of radiotherapy for carcinoma of the parotid gland: a comparison of conventional, three-dimensional conformal, and intensity-modulated techniques. Radiother Oncol. 2001;60:163–172. doi: 10.1016/s0167-8140(01)00339-5. [DOI] [PubMed] [Google Scholar]
- 29.Bragg CM, Conway J, Robinson MH. The role of intensity-modulated radiotherapy in the treatment of parotid tumors. Int J Radiat Oncol Biol Phys. 2002;52:729–738. doi: 10.1016/s0360-3016(01)02660-8. [DOI] [PubMed] [Google Scholar]
- 30.Keyes JW, Jr, Harkness BA, et al. Salivary gland tumors: pretherapy evaluation with PET. Radiology. 1994;192:99–102. doi: 10.1148/radiology.192.1.8208973. [DOI] [PubMed] [Google Scholar]