Abstract
Objectives
Bacterial vaginosis (BV) is common in lesbians, and treatment fails in up to 28%. Risks include sexual behaviours that transmit vaginal fluid. The authors measured efficacy of a behavioural intervention to reduce sexual transfer of vaginal fluid between female sex partners in reducing BV persistence.
Methods
Women aged 16–35 years with BV who reported sex with women (prior year) were eligible. Participants were randomised to intervention (motivational interviewing designed to reduce sharing of vaginal fluid on hands or sex toys post-treatment, by provision of condoms, gloves and water-based lubricant) or control (general STI education) arms. All were treated with vaginal metronidazole and underwent computer-assisted self-interview to ascertain sexual behaviours, with test-of-cure at 30 days.
Results
Of 129 women with BV, 108 (84%) were eligible; 89 (69%) agreed to enrol. 43 were randomised to control and 46 to intervention; 81 (91%) returned for test-of-cure. BV persisted in 12 (27.9%) of 43 women in intervention and 8 (21.1%) of 38 women in control arms (p1/40.6). Digital-vaginal sex was common post-treatment (50% intervention and 68% control); women randomised to the intervention were less likely to report receptive digital-vaginal sex without gloves than control (31% vs 61%; p1/40.01), without reported lower frequency of other sexual practices. Shared vaginal use of sex toys was infrequent.
Conclusions
Although the intervention effected a significant increase in glove use during digital-vaginal sex post-BV treatment, this was not associated with reduction in BV persistence. Shared use of vaginal sex toys was infrequent, suggesting that other mechanisms promote BV in lesbians.
INTRODUCTION
Bacterial vaginosis (BV) is the most prevalent vaginal infection in reproductive age women and has been consistently associated with adverse outcomes related to the upper genital tract and with increased risk of HIV acquisition.1, 2 Of 3739 women enrolled during 2001–2004 in a nationally representative sample of the US civilian non-institutionalised population, almost one in three (29.2%; 95% CI 27.2 to 31.3) had BV diagnosed using a Gram stain of vaginal fluid.3, 4 BV is characterised by the depletion of hydrogen peroxide-producing lactobacilli that characterise normal vaginal flora, with profound overgrowth of anaerobic bacteria.5 However, the aetiology of BV remains elusive. Several cross-sectional studies have reported a wide variety of risks for this common condition, including black race, hormonal contraception, douching, smoking, menses, chronic stress and sexual behaviours (including higher numbers of male sex partners, unprotected vaginal intercourse, anal intercourse and sex with other women).6–8 Fewer studies have followed heterosexual women prospectively for incident BV. These studies have reported that risks included black race, report of a regular male sex partner or a female partner during follow-up, higher Nugent score at initial treatment and recent vaginal lubricant use or anal sex.9–12 One study with 619 woman-years of follow-up found that BV acquisition was independently associated with black race, cigarette smoking, vaginal intercourse, receptive anal sex before vaginal intercourse, sex with an uncircumcised male partner, lack of precedent hydrogen peroxide-producing vaginal lactobacilli and detection of herpes simplex virus-2 serum antibodies at the visit prior to BV diagnosis.13
Women who have sex with women (WSW) have had a high prevalence of BV (25%–52%) as reported in several cross-sectional studies.3, 14–16 We have previously reported risks for both prevalent and incident BV in a group of WSW in the previous year. Risks for prevalent BV included higher lifetime number of female sex partners, shared use of a vaginally inserted sex toy and oral–anal sex with a female partner8; behavioural risks for incident BV included the report of a new female partner with BV and possibly the frequency of receptive oral sex.12 Previous studies also reported that BV-associated risks in lesbians include having a female sex partner with BV and sharing vaginally insertive sex toys, suggesting that sexual exchange of vaginal fluid may be an explanation.14 We hypothesised that interrupting sexual transfer of vaginal fluid between women with BV (index cases) and their sex partner(s) would reduce the rates of BV persistence. With the assistance of focus groups and expert behavioural science consultants, we developed an intervention to prevent sexual transfer of vaginal fluid between female sex partners. We then measured the efficacy of the intervention in reducing the rate of BV persistence 30 days after treatment for BV. In addition to measuring the contribution of previously recognised risk factors for BV, we used comprehensive computer-assisted self-interview (CASI) to assess behavioural risks. CASI has been shown to yield significantly higher rates of disclosure for same-sex behaviour and undesirable social behaviours when compared directly with self-administered questionnaires.17, 18 CASI also provides other benefits, including standardised delivery of survey content, eliminating variation in interviewer or day, and computer-controlled branching, automated consistency and range checking.
METHODS
Formative research and development of the intervention
When this study was implemented, no prior research on the development and validation of behavioural interventions to prevent the transmission of sexually transmitted infections in WSW was available. Thus, four focus groups (two for women aged 18–22 years and two for women aged 23–29 years) were held to inform the development of a behavioural intervention to reduce the sexual transfer of vaginal fluid between women. As we have previously reported, the focus group discussion guides emphasised sexual practices, penetrative versus non-penetrative sex, lubricant use, sex toy-cleaning practices, knowledge of BV and risk perception for BV/STD (sexually transmitted disease), STD prevention practices and preferences for a behavioural intervention, and STD-related information needs.19 Briefly, two major routes for sexual transfer of vaginal fluid were identified: insertive use of vaginal sex toys and digital sex (using hands or fingers). Regarding the possible means of reducing sexual transfer of vaginal fluid, most subjects had experience with using male condoms on sex toys and viewed this technique positively. Focus group participants uniformly rejected the concept of using topical antimicrobial solutions on hands prior to digital-vaginal sex; however, a high proportion of participants had used and viewed non-latex gloves favourably.
The health belief model (HBM) was selected as most applicable for designing and measuring compliance with the intervention, as its underlying premise is that the individual’s attitudes and beliefs are important determinants of health actions.20 The HBM has been successfully used to explain and predict sexual risk and protective behaviours associated with STD/HIV transmission. Behaviour is predicted from an assessment of the individual’s perceived threat and an estimate of the potential benefits of health-seeking action to reduce susceptibility or severity weighed against perceived barriers or costs inherent in undertaking an action. The intervention addressed the four factors that the HBM postulates to account for variation in health behaviour as follows. To address perceived susceptibility to BV, participants were educated about the relatively high prevalence of BV among women reporting sex with other women and the high degree of concordance of BV within monogamous female sexual partnerships. Perceived severity was addressed by educating participants on the symptoms of BV and its consequences. To address perceived benefits, we emphasised the benefits of treating and preventing BV, including the possibility of the reduced likelihood of BV transmission to female sex partners. Finally, perceived barriers to implementing the behavioural intervention were explored with participants, including ways to incorporate cleaning of sex toys or using male condoms on sex toys into participants’ sexual routines.
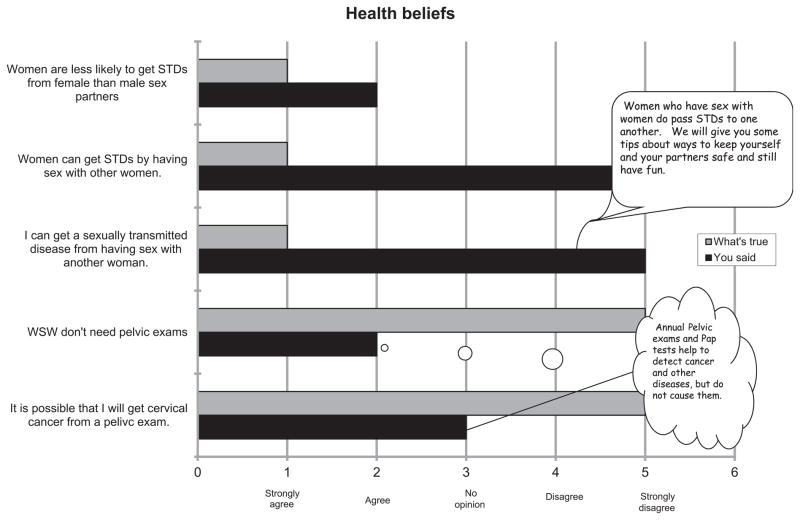
With these parameters in mind, study staff used a motivational interviewing model to deliver the client-centred interaction applicable to both the intervention and control arms of the randomised trial. An example of an interactive schematic for the delivery of the intervention is depicted in figure 1, which was shared with each participant as a visual aid to discuss the barriers that prevented the participant from adopting the intervention. In delivering the intervention, the study staff instructed participants how to employ behaviours aimed at reducing the likelihood of transferring vaginal fluid to female partners during sex and providing ‘safe sex’ kits, which included male condoms, nitrile gloves and water-based lubricant. Behaviours that were targeted included the use of gloves during digital-vaginal sex, the use of a condom if insertive sex toys were shared during the same sexual encounter and the use of the lubricant provided or another water-based lubricant if they used a lubricant. Participants randomised to the control group did not receive information on sexual behaviour modification or the safe sex kit, but instead received information on the need for adherence to routine Papanicolaou smear screening guidelines. The intervention was delivered by one of two study staff, both trained previously in the delivery of health-related behavioural counselling.
Figure 1.
Example of an interactive schematic for the delivery of the intervention, which used a motivational interviewing model to deliver the client-centred interaction applicable to both the intervention and control arms of the randomised trial. STD, sexually transmitted disease; WSW, women who have sex with women.
Implementation of the intervention in the randomised trial
The study population of the parent study, a yearlong prospective study of vaginal microbiology, was composed of women aged 16–30 years who reported having sex with at least one other woman in the previous year and who responded to recruitment through advertisements, media and community referral between October 2004 and December 2006, as previously described.21 At the time of enrolment into the parent study, participants completed CASI on demographics and medical, reproductive and sexual history. Participants underwent standardised examination including the collection of vaginal fluid for Gram stain, saline microscopy, pH measurement, potassium hydroxide evaluation and culture of Trichomonas vaginalis. BV was diagnosed if three of four clinical (Amsel) criteria (vaginal pH >4.5, clue cells on saline microscopy >20% of epithelial cells, amine odour on addition of potassium hydroxide and homogeneous vaginal discharge) were present,22 and Gram stain of vaginal fluid confirmed abnormal flora (Nugent score >3).4 Participants were tested at enrolment for Chlamydia trachomatis and Neisseria gonorrhoeae using the APTIMA-COMBO 2 assay (Gen-Probe, San Diego, California, USA) on urine and during follow-up visits if they reported interim risk behaviour (new sex partner, >1 partner) or genitourinary symptoms.
As part of the parent study, all participants were asked to return for three subsequent quarterly visits (total duration of follow-up, 1 year) or for evaluation at any time if genitourinary symptoms occurred. Women with BV diagnosed at enrolment or during prospective follow-up were eligible to enrol in the intervention. Other eligibility criteria included report of having sex with a woman in the prior 60 days. Women were excluded from enrolment into the intervention if they reported having a current sex partner (defined as sex planned during the next 30 days with that partner) already enrolled in the intervention study, reported prior hypersensitivity to metronidazole or to other nitroimidazole derivatives, were unwilling or unable to modify sexual behaviours targeted by the intervention or were unable to adequately comprehend the consent material because of language barriers or psychological difficulty. All women with BV enrolled in the trial were treated with vaginal metronidazole gel (37.5 mg every night for 5 days).
The randomisation included a simple randomisation scheme generated by a statistician. After enrolment, the study coordinator obtained the randomisation assignment from a sealed envelope that was generated in a sequence reflecting this scheme.
The primary study outcome was BV diagnosed 1 month after the first diagnosis and randomisation into the behavioural intervention, defined as persistent BV. Persistent BV was defined by Amsel criteria and confirmed by a Nugent score of vaginal fluid >6 at 1 month following treatment and randomisation into the trial. Secondary outcomes by randomisation arm included (1) detection of abnormal vaginal flora as defined by a Nugent score >3 at the 1-month post-treatment/randomisation visit and (2) time to first episode of BV (defined above) over the course of follow-up in the parent study, with participants censored after their first episode of BV. CASI was used at all follow-up visits to solicit reports of interim sexual behaviours.
Written informed consent was obtained from all participants. The study adhered to the standard guidelines for research involving human subjects and was approved annually by the University of Washington Human Subjects Review Committee.
Statistical analysis
For the calculation of the study’s power to detect a difference in the ability of the intervention to reduce the rate of BV persistence, we assumed that the probability of persistent BV at 4 weeks after antibiotic therapy with no intervention would be 20% and that the behavioural intervention would reduce BV persistence rates in index cases by an absolute difference of 15%. We determined that a sample of at least 45 women in each arm would yield a power of at least 80% to detect this difference. Detection of BV at the 1-month post-treatment visit was compared by randomisation arm using Fisher’s exact test (primary outcome). Cox regression analysis was used to generate HRs for the risk of BV over post-randomisation parent study participation by randomisation arm (secondary outcome), both unadjusted and adjusted for enrolment characteristics differing enough by arm to potentially influence results. All comparisons by arm were intent-to-treat, meaning that outcomes were compared by randomisation arm regardless of compliance with the intervention. All tests for statistical significance were two sided and a level of p<0.05 was considered statistically significant. Analyses were conducted using Stata 9.2.
RESULTS
The characteristics of 335 women enrolled in the overall study have been reported previously.8, 21 Briefly, participants’ median age was 27 years; 24% self-categorised as non-Caucasian, one-third reported a prior diagnosis of BV and 24% reported recent (past 90 days) sex with a male partner. At enrolment, two women (0.6%) had C trachomatis. None had N gonorrhoeae, trichomoniasis or clinically evident genital herpes.
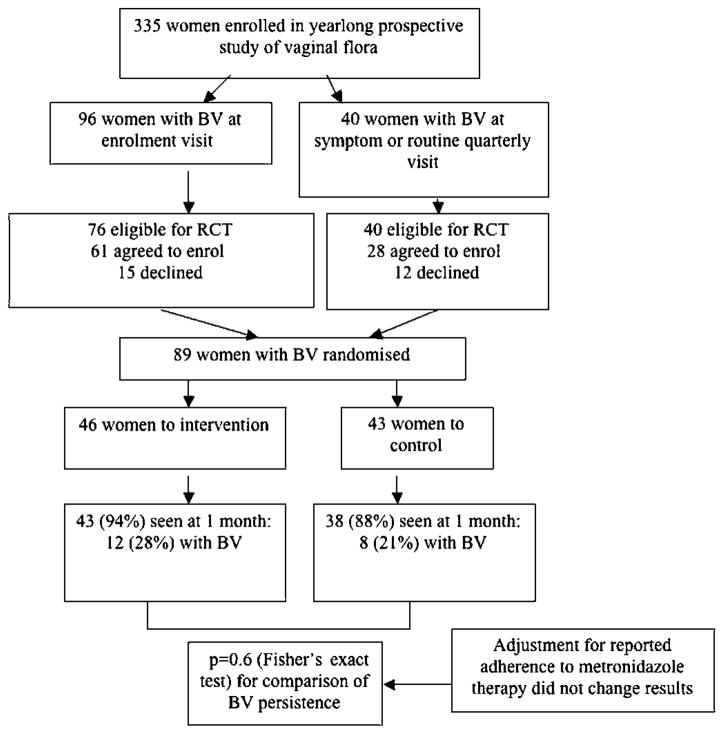
BV was diagnosed in 96 participants at their enrolment visit (28.7% of all enrolment visits) and in 40 additional participants at routinely scheduled quarterly visits or visits during which evaluation for genitourinary symptoms was sought (figure 2). Of all participants diagnosed with BV (N=131), 116 (88.5%) were eligible for the intervention trial; of these, 89 (76.7%) agreed to enrol and were randomised. These women did not differ significantly in the characteristics noted above from the participants enrolled in the study overall. As shown in table 1, demographic and recent sexual behaviour was similar by randomisation arm, although women randomised to the intervention group happened to include a somewhat lower proportion with a history of BV (33% vs 47%) and a higher proportion reporting recent genital-to-genital contact with female partner (58% vs 37%) and black race (12% vs 5%).
Figure 2.
CONSORT diagram of screening, enrolment and outcomes in the trial. BV, bacterial vaginosis; RCT, randomised controlled trial.
Table 1.
Characteristics of participants (N=89) enrolled in the randomised controlled trial, by study arm
| Intervention group N = 46 | Control group N = 43 | |
|---|---|---|
| Demographics and history of bacterial vaginosis (BV) | ||
| Mean age—years | 25.6 | 25.1 |
| Black race (self-defined)—no (%) | 5 (12) | 2 (5) |
| Current cigarette smoking | 15 (37) | 13 (37) |
| History of BV | 15 (33) | 20 (47) |
| Current sex partner with history of BV | 10 (23) | 10 (26) |
| Behavioural risk factors, prior 3 months | ||
| Douching | 2 (4) | 2 (5) |
| Sex with male partner | 11 (24) | 8 (19) |
| >1 female sex partner | 9 (20) | 13 (30) |
| Use of vaginal insertive sex toy with female partner | 20 (44) | 23 (53) |
| Shared vaginal insertive sex toys with female partner | 13 (28) | 13 (30) |
| Genital-to-genital contact with female partner | 26 (58) | 16 (37) |
| Receptive oral sex with female partner | 32 (71) | 32 (74) |
The overall rate of return for the 1-month assessment of primary outcome was 91.0% and did not differ between study arms. Table 2 displays the frequency of behaviours reported by participants at the 1-month follow-up visit, reflecting activity during the 30 days after BV treatment and randomisation. Most women reported sexual activity during that time frame (most commonly, receptive oral sex or digital-vaginal sex). Use of shared sex toys was infrequent in both groups, but nearly one in five women reported vaginal intercourse with a male partner; all women in the intervention group, interestingly, reported not using condoms with this behaviour during that time. There was no difference in the reported frequency of any behaviours by randomisation arm with the exception of digital-vaginal sex without using gloves, which was lower by nearly half among participants randomised to the intervention arm (31% vs 61%, p=0.01).
Table 2.
Behaviours reported by participants in the 30 days post-randomisation, by study arm (n (%))*
| Intervention group N = 46 | Control group N = 43 | p Value | |
|---|---|---|---|
| Behaviours not targeted by the intervention | |||
| Compliance with full course of metronidazole | 35 (85) | 32 (97) | 0.12 |
| Vaginal douching | 0 (0) | 0 (0) | – |
| Any sex | 35 (81) | 29 (76) | 0.6 |
| Sex with >1 female partner | 2 (5) | 6 (16) | 0.14 |
| Receptive oral sex | 23 (53) | 25 (66) | 0.4 |
| Genital-to-genital contact | 10 (23) | 8 (21) | >0.9 |
| Behaviours targeted by the intervention | |||
| Receptive digital-vaginal sex without gloves | 13 (31) | 23 (61) | 0.01 |
| Any receptive digital-vaginal sex | 24 (56) | 27 (71) | 0.17 |
| Receptive digital-anal sex without gloves | 2 (5) | 2 (5) | >0.9 |
| Any receptive digital-anal sex | 3 (7) | 7 (18) | 0.18 |
| Sharing sex toys without cleaning them | 1 (2) | 1 (3) | >0.9 |
| Any shared sex toy use | 5 (12) | 3 (8) | 0.7 |
| Vaginal intercourse with men without condom use | 8 (19) | 4 (11) | 0.4 |
| Any vaginal intercourse with male partner | 8 (19) | 6 (16) | 0.8 |
Includes participants who returned for 30-day follow-up (43 (94%) of those randomised to intervention and 38 (88%) to control arms).
Some percentages differ because information on some behaviours was not available for a few participants.
At the 1-month visit, the rates of persistent BV did not differ between the intervention and control arms (28% vs 21%, respectively; p=0.6). Although not statistically significantly different, compliance with metronidazole treatment by arm was different enough to prompt an assessment as to whether differential adherence could be contributing to this result. To do so, we compared BV rates by arm among the 67 women who confirmed at the 1-month visit that they had taken the full course of metronidazole treatment and again found no difference between arms (26% of 35 in the intervention arm had BVat 1 month vs 19% of 32 in the controls, p=0.6). BV rates did not differ by reported glove use; in the intervention arm, 31% of 13 reporting digital-vaginal sex without gloves had BV versus 28% of those not reporting this behaviour. In both randomisation arms combined, 25% of women experienced persistent BV whether or not they reported digital-vaginal sex without gloves.
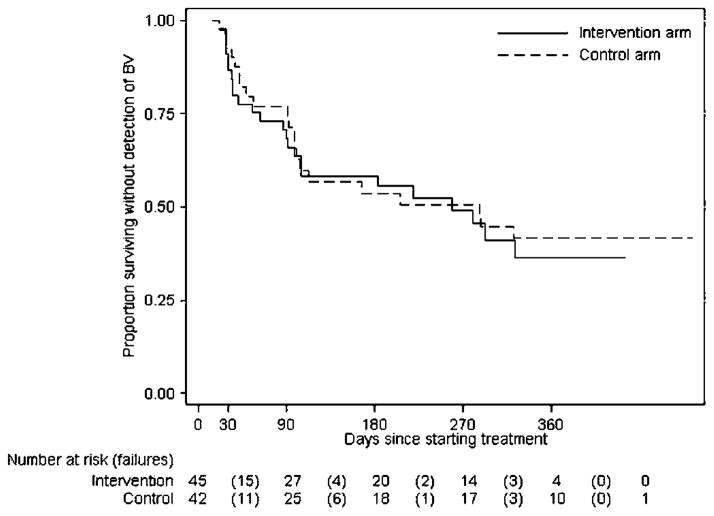
For the secondary objective of assessment of any BV following randomisation and treatment by study arm, 87 of 92 participants returned for at least one visit, of whom 45 had another episode of BV during follow-up. Of women originally randomised to the intervention arm, there were 24 episodes of BV during 261 person-months at risk (9.2% incidence of BV per month in those at risk). In women originally randomised to the control arm, there were 21 episodes of BV during 271 person-months at risk (7.7% incidence of BV per month in those at risk). Median number of days to the second BV diagnosis in all women was 280 days (259 in the intervention group and 287 in control). Figure 3 depicts the survival curves for remaining free of BV by study arm throughout study participation. Overall, the HR for participants in the intervention arm for the detection of subsequent BV was 1.12 (95% CI 0.62 to 2.01) relative to the control arm participants. Adjustment for black race, enrolment report of genital-to-genital contact and enrolment report of history of BV made little difference to the estimate of the effect of the intervention (adjusted HR=1.03 (0.54 to 1.97).
Figure 3.
Kaplan–Meier estimates of survival until the first detection of bacterial vaginosis (BV) by Amsel criteria after the initial BV-positive visit, by randomisation arm. A 30-day test-of-cure visit was scheduled for each woman and quarterly visits thereafter until the woman’s 1-year follow-up in the larger longitudinal study was complete. Twenty-eight of 89 women enrolled in the randomised controlled trial (RCT) had BV for the first time (and enrolled in the RCT) part way through the larger longitudinal study; thus their expected follow-up is <1 year. In the intervention arm, there were 24 failures during 261 person-months at risk (9.2% incidence of BV per month in those at risk). In the control arm, there were 21 failures during 271 person-months at risk (7.7% incidence of BV per month in those at risk).
DISCUSSION
In our effort to address the potential for the transfer of infected vaginal fluid to promote BV among female sex partners, we used focus group findings to design and deliver the first reported behavioural intervention to impact sexual behaviours that might result in such transmission in this group. Despite subsequently lower rates of unprotected digital-vaginal contact (significantly lower in the intervention group) and of shared sex toy use (low in both groups), we did not observe a reduction in the rates of persistent BV at 1 month following the intervention (primary study outcome) or in recurrent/persistent BV over the course of longer term follow-up (secondary outcome).
A number of possible explanations for the failure of the behavioural intervention to effect a reduction in BV persistence are plausible. First, our study design did not address a potentially causative role for oral sex in promoting BV and participants engaged in this behaviour with equal, and not uncommon, frequency in both the study arms. Receptive oral sex has been associated with a trend towards abnormal vaginal microbiota or frank BV, and several of the bacteria associated with BV have been identified in the oral cavity.23 Second, report of sharing sex toys—what might arguably be viewed as the most efficient means of transferring vaginal fluid between sex partners—was low in both arms after BV diagnosis/treatment; the intervention did emphasise avoiding sharing sex toys during the same sexual encounter and advised the participants to use a condom if they did do so. Third, although the intervention did result in behaviour change during the time between BV treatment and assessment of cure at 1 month, changes in sexual practices might need to be sustained over a longer period of time to favorably impact the vaginal microenvironment. Finally, it is noteworthy that participants had a very high rate of BV during follow-up (more than half experienced a recurrence), indicating a possible role for other aetiological factors such as a BV-associated biofilm (a highly adherent layer that may be relatively impenetrable to antibiotics),24 intrinsic characteristics of specific BV-associated bacteria21 or host immunity. We have previously reported that among women with BV in this cohort, who were treated with vaginal metronidazole, predictors of treatment failure included the detection of specific BV-associated bacteria at baseline, including the Clostridia-like bacteria BVAB1, BVAB2 and BVAB3, Peptoniphilus lacrimalis and Megasphaera phylotype 2, as well as failure to adhere to 5 days of vaginal metronida-zole.21 Importantly, report of no specific sexual practices with either male or female partners in the month after treatment predicted either persistent BV or abnormal flora. We have also shown in this cohort that the detection of specific BV in vaginal fluid weeks to months prior to the diagnosis of BV strongly predicts the development of BV, suggesting that changes in vaginal microbiota may precede the development of BV by a considerable time period. Thus, behaviours that promote vaginal colonisation by key BV-associated bacteria might predate the development of BV itself by some time, making it even more difficult to link these behaviours and incident BV. If some of these reasons are contributory, the type of intervention we employed might work better in preventing incident BV over the long term than facilitating its resolution in the short term.
In our previously reported study of BV acquisition in this cohort, only the report of a new female sex partner who provided a history of BV was associated with increased risk of BV acquisition in univariate analysis and was not significant in multivariate analysis.12 Given the absence of associations between recent sexual practices and BV acquisition, the significance of association between BV and the report of a new female partner with BV history remains unclear. Our inability to establish a role for sexual transmission of BV in this cohort may also relate to imprecise assessment of the time of sexual exposure to acquisition of a ‘precipitant’ BV and the possibility that multiple sexual practices may be involved and are often practiced concurrently, or, as is highly likely, that BV pathogenesis is multifactorial: while unprotected sex is a possible contributing factor, other factors are probably involved and probably contributed to the failure of randomised trials of short-term treatment of male sex partners of women with BV to improve cure or prevent BV recurrence.25 In this trial, we invited participants to let their female partners know about the study, but we did not systematically attempt to evaluate and treat partners for BV either empirically or based on the detection of BV.
Our study has limitations. First, while participants were selected on the basis of reporting sex with other women and the intervention was designed to affect sexual behaviours between female partners, 16% of them reported having sex with men in the month post-enrolment. Second, we relied on self-report of sexual behaviours, although we used CASI to minimise under- or over-reporting of these behaviours. Third, we intentionally did not target oral sex behaviours in our intervention, as we intended to study only the effect of reducing behaviours that were likely to transmit vaginal fluid. It is possible that an intervention that aimed more broadly at reducing the exchange of oral and vaginal microbiota could impact the likelihood of subsequently abnormal vaginal microbiota. Finally, it is possible that an intervention based on another theoretical model might have been more effective in promoting behaviour change in our participants.
Our findings raise several important areas for future research. First, the establishment of consensus definitions for incidence, persistence and recurrence of BV would assist in achieving progress towards describing the natural history of this condition. Whether risk factors that promote incidence are the same as those that promote persistence or recurrence is not clear. A recent National Institutes of Health-sponsored workshop has resulted in a proposal for defining these conditions.5 Second, the role of extragenital environments, particularly the oral cavity, in serving as reservoirs for BV-associated bacteria should be examined. A link between periodontal disease and BV in individual women has been observed,26 whether this is due to autoinoculation of both sites, concomitant exposure to an external source (such as sex partners’ oral or genital microbiota) or host genetic susceptibility27 is not clear. Among 3569 women enrolled in the Longitudinal Study of Vaginal Flora, a non-significant increased risk of periodontal disease was noted among women who reported performing oral sex on uncircumcised, relative to circumcised, male partners, suggesting the potential for partners’ genital microbiota to impact each others’ exposed microenvironments.
In summary, we demonstrated that although targeted sexual behaviours could be successfully modified among WSW with BV who were enrolled in a behavioural intervention aimed at reducing the risk of sexual transfer of vaginal fluid, this did not result in enhanced cure of BV. To date, the only proven interventions that prevent the development or recurrence of BV are circumcision of male partners28 and chronic suppressive antibiotic therapy.10 Because BV significantly affects women’s health, accounting for significant morbidity and conferring an increased risk of poor pregnancy outcome, HIV acquisition and upper genital tract disease, the development of effective approaches to prevent this infection should be a priority.
Key messages.
Bacterial vaginosis (BV) is common among women who have sex with women (WSW) and frequently occurs in both members of monogamous couples, suggesting a role for sexual transmission.
An intervention that successfully reduced the frequency of sexual practices likely to transmit vaginal fluid did not enhance the cure rates of BV in WSW.
The aetiology of high rates of BV in WSW may be multifactorial.
Acknowledgments
We are grateful to the study staff, including Susan Heideke, Nancy Dorn, Lauren Asaba, Dana Varon and Emily Hancock, to Kathy Agnew for performance of Gram stains on vaginal fluid, to Susan Rosenthal, PhD and Pamina Gorbach, DPH, for helpful discussions in developing the behavioural intervention, and to the women who enrolled.
Funding National Institute of Allergy and Infectious Diseases grants RO1 AI052228 (JMM).
Footnotes
Presented in part as a poster at the Annual Meeting of the Infectious Disease Society of Obstetrics and Gynecology, Seattle, WA, in August 2008.
Competing interests None.
Ethics approval This study was conducted with the approval of the University of Washington Human Subjects Division.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors JMM originated, designed and supervised the study, obtained funding, analysed the data and wrote the manuscript. KR coordinated day-to-day conduct of the research, developed study materials, assisted with data analysis and reviewed the manuscript for accuracy. KKT, who was the primary data manager and biostatistician, analysed the data and assisted in manuscript preparation.
References
- 1.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leitich H, Bodner-Adler B, Brunbauer M, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189:139–47. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 3.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 4.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex Transm Dis. 2010;37:732–44. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fethers KA, Fairley CK, Morton A, et al. Early sexual experiences and risk factors for bacterial vaginosis. J Infect Dis. 2009;200:1662–70. doi: 10.1086/648092. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Thomas KK, Agnew K, et al. Prevalence and risks for bacterial vaginosis in women who have sex with women. Sex Transm Dis. 2010;37:335–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 10.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0. 75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Thomas KK, Fiedler TL, et al. Risks for acquisition of bacterial vaginosis among women who report sex with women: a cohort study. PLoS One. 2010;5:e11139. doi: 10.1371/journal.pone.0011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherpes TL, Hillier SL, Meyn LA, et al. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Koutsky LA, Eschenbach DA, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 15.Fethers K, Marks C, Mindel A, et al. Sexually transmitted infections and risk behaviours in women who have sex with women. Sex Transm Infect. 2000;76:345–9. doi: 10.1136/sti.76.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey JV, Farquhar C, Owen C. Bacterial vaginosis in lesbians and bisexual women. Sex Transm Dis. 2004;31:691–4. doi: 10.1097/01.olq.0000143093.70899.68. [DOI] [PubMed] [Google Scholar]
- 17.Turner CF, Ku L, Rogers M, et al. Adolescent sexual behavior, drug use, and violence: increase reporting with computer survey technology. Science. 1998;280:867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 18.Kissinger P, Rice J, Farley T, et al. Application of computer-assisted interviews to sexual behavior research. Am J Epidemiology. 1999;149:950–4. doi: 10.1093/oxfordjournals.aje.a009739. [DOI] [PubMed] [Google Scholar]
- 19.Marrazzo JM, Coffey P, Bingham A. Sexual practices, risk perception and knowledge of sexually transmitted disease risk among lesbian and bisexual women. Perspect Sex Reprod Health. 2005;37:6–12. doi: 10.1363/psrh.37.006.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenstock I, Strecher VJ, Becker MH. The health belief model and HIV risk behavior change. In: DiClemente RJ, Peterson JL, editors. Preventing AIDS: Theories and Methods of Behavioral Interventions. New York, NY: Plenum Publishing Corporation; 1994. [Google Scholar]
- 21.Marrazzo JM, Thomas KK, Fiedler TL, et al. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med. 2008;149:20–8. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.Kumar PS, Griffen AL, Moeschberger ML, et al. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–55. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol. 2005;106:1013–23. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 25.Hamrick M, Chambliss ML. Bacterial vaginosis and treatment of sexual partners. Arch Fam Med. 2000;9:647–8. doi: 10.1001/archfami.9.7.647. [DOI] [PubMed] [Google Scholar]
- 26.Zabor EC, Klebanoff M, Yu K, et al. Association between periodontal disease, bacterial vaginosis, and sexual risk behaviours. J Clin Periodontol. 2010;37:888–93. doi: 10.1111/j.1600-051X.2010.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pretorius C, Jagatt A, Lamont RF. The relationship between periodontal disease, bacterial vaginosis, and preterm birth. J Perinat Med. 2007;35:93–9. doi: 10.1515/JPM.2007.039. [DOI] [PubMed] [Google Scholar]
- 28.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200:42.e1–7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]