Abstract
The prevalence of bacterial vaginosis among lesbians is high. We assessed whether unique Lactobacillus strains were shared by female sex partners. Cultures of vaginal and rectal specimens for detection of Lactobacillus organisms were performed for women who reported having had sex with women. Lactobacilli were identified on the basis of DNA homology and were typed and fingerprinted by repetitive element sequence– based polymerase chain reaction (rep-PCR). Of 237 women, Lactobacillus crispatus was detected in 98% and Lactobacillus gasseri in 21%. Detection of L. gasseri was associated with recent receptive digital-vaginal sex (P = .04) and increased bacterial vaginosis risk (odds ratio, 4.2; 95% confidence interval, 1.4– 13.4). Of 31 couples monogamous for >3 months, rep-PCR fingerprints were identical in both members in 23 (77%).
The flora of the healthy vagina is dominated byH2O2-producing Lactobacillus species, predominantly Lactobacillus crispatus and Lactobacillus jensenii [1]. Bacterial vaginosis (BV) is characterized by overgrowth of commensal anaerobic flora relative to lactobacilli. BV among heterosexual women is associated with a new male sex partner and unprotected intercourse [2, 3] and with vaginal intercourse immediately after anal intercourse [4]. The prevalence of BV among lesbians is high relative to that among heterosexual women [5, 6], and BV is frequently found in both members of lesbian couples [5]. Sexual practices involving digital-vaginal or digital-anal contact might transmit vaginal fluid and may promote abnormal vaginal flora [7]. Criswell et al. [8] “transmitted” BV from one woman to another by transferring vaginal secretions.
We hypothesized that sexual behaviors that could transfer vaginal fluid might also transmit lactobacilli between female sex partners. Repetitive element sequence– based PCR (rep-PCR), a DNA fingerprinting technique, has been successful in distinguishing a probiotic Lactobacillus crispatus strain from other L. crispatus strains and from other endogenous Lactobacillus species [9]. Rep-PCR uses repetitive sequences throughout the genome for direct amplification of genomic DNA, generating fingerprint patterns unique to bacterial species or strains [10]. We defined species-specific distribution of genital Lactobacillus isolates recovered from women who reported having had sex with other women and used rep-PCR fingerprinting to assess whether unique Lactobacillus strains were shared by female sex partners.
Subjects, materials, and methods
Women who reported having had sex with another woman during the preceding year were eligible and invited to refer female partners for enrollment. Medical histories were obtained by means of a standardized questionnaire. Vaginal specimens were obtained for Gram stain and for culture by swabbing the lateral vaginal wall; swabs for culture were placed in Port-a-Cul anaerobic transport tubes (Becton Dickinson), transported to the University of Washington Anaerobe Laboratory, and set up within 24 h. Rectal specimens were collected for culture by rotating a swab that was inserted 2 cm into the rectum; swabs were placed in Port-a-Cul transport tubes. Quantitative culture of lactobacilli was performed as previously described [11]. Gram stains were read using the Nugent scale [12].
Lactobacilli were identified to the genus level by means of Gram stain findings and colony morphologic characteristics [13]. After 48 h of anaerobic incubation, plates were exposed to ambient air. Lactobacillus isolates were preserved in reconstituted litmus milk (Becton Dickinson Microbiology Systems), stored at −70°C, and transported to the Magee-Womens Research Institute for DNA fingerprinting studies.
Each vial of litmus milk was thawed at room temperature and used to inoculate Columbia 5% sheep blood agar plates (PML Microbiologicals). Plates were incubated at 36°C in 5%–6%CO2 for at least 24 h. Each strain was passed at least 1 more time to ensure purity and vigor before procedures and restocking.
Strain typing of Lactobacillus was performed using rep-PCR fingerprinting as previously described [9]. If >2 isolates from the same participant had similar DNA rep-PCR DNA finger-prints, only 1 was subjected to species-level identification. Species-level identification of isolated lactobacilli was determined by means of hybridization of DNA from the isolates to DNA of American Type Culture Collection Lactobacillus strains [14].
Statistical analysis was performed using SPSS (SPSS). Tests for statistical significance were 2-tailed, with an α level of <.05. Couples were defined as monogamous if they reported having had no other partners during the 3-month period before enrollment. To characterize each couple by use of a composite measure, we averaged the ages of both partners and the number of sex partners reported outside the relationship during the 9-month interval before the monogamous period. To estimate recent sexual practices in the relationship, we used the most recent report and highest number of most recent sex acts if the values obtained from each partner did not agree. For each couple, we selected a pair of participants to serve as control subjects for rep-PCR analysis. Control subjects were participants who reported having had no sex in the past 60 days and were matched to case couples on the basis of the case couples’ mean age and approximate date of visit. Members of each case couple were classified as having shared Lactobacillus isolates if 2 strains with identical rep-PCR patterns were isolated from the vagina and/or rectum of each woman. Study procedures were approved by the University of Washington Human Subjects Research Review Committee, and all participants provided written informed consent.
Results
Participants were enrolled in a cross-sectional study described previously [5]. In brief, the 392 participants were predominantly white (88%), the median age was 28 years, and the median numbers of lifetime male and female sex partners reported were 7 and 6, respectively. Most (58%) reported having had only 1 female partner during the past 6 months. Almost all reported having received and performed oral-vaginal and digital-vaginal sex with female partners during the past year, and many reported having engaged in oral-anal and digital-anal sex during the past year (34% and 63%, respectively). Overall, 36.8% had abnormal vaginal flora (25.3% had BV, and 11.5% had intermediate flora as defined by the Nugent score).
Of 356 women who had rectal and vaginal specimens cultured, 262 (72%) had lactobacilli recovered from either site. Of these 262 women, 237 (91%) had viable lactobacilli for species-level identification. These 237 participants did not differ from the entire group of 392 participants with respect to factors such as age, race, and sexual history, but they were less likely to have BV. This difference was expected—the 237 women were selected because they had lactobacilli recovered from genital specimens.
The distribution of Lactobacillus species by anatomic site is depicted in table 1. L. crispatus was most common, followed by Lactobacillus gasseri and L. jensenii. For women colonized with L. crispatus, rectal colonization alone was uncommon; most women had either vaginal colonization only or both vaginal and rectal colonization. The overall prevalence of rectal colonization did not differ between L. crispatus and L. gasseri. However, relative to L. crispatus, the rectum was more commonly the sole site of L. gasseri colonization (P < .001). Relatively few women with L. jensenii had this organism recovered from the rectum. Of women who had any vaginal lactobacilli detected, 57 (24%) had >1 species type. L. jensenii was rarely detected as the single Lactobacillus species in either the vagina or the rectum; this occurred in only 4 of 26 women with L. jensenii colonization.
Table 1.
Distribution of Lactobacillus strains among 237 women for whom cultures of vaginal and rectal specimens were performed.
| Women colonized, by anatomic site(s) |
||||
|---|---|---|---|---|
| DNA homology group | Overall | Vagina only | Rectum only | Vagina and rectum |
| L. crispatus | 232 (97.9) | 117/232 (50.4) | 12/232 (5.1) | 103/232 (44.4) |
| L. gasseri | 50 (21.1) | 24/50 (48.0) | 13/50 (26.0) | 13/50 (26.0) |
| L. jensenii | 26 (11.0) | 18/26 (69.2) | 3/26 (11.6) | 5/26 (19.2) |
| L. iners | 2 (0.8) | 2/2 (100) | 0/2 (0) | 0/2 |
NOTE. Data are number or proportion of women (%). A total of 39 women (17%) were colonized with >1 Lactobacillus species.
Relative to women without L. gasseri, women with L. gasseri reported a shorter median interval since they last had receptive digital-vaginal sex (3 days vs. 21 days; P = .03) and were more likely to have abnormal vaginal flora, defined as a Nugent score of >4 (37.2% vs. 13.1%; OR, 3.9; 95% CI, 1.9–8.3; P = .001) or BV (14.0% vs. 3.7%; OR, 4.3; 95% CI, 1.4 –13.4; P = .02).
Both members from 72 monogamous partnerships were enrolled. In 40 couples (56%), lactobacilli were detected in cultures of vaginal or rectal specimens for both women. In 16 couples (22%), lactobacilli were detected by cultures of vaginal or rectal specimens for only 1 partner. No lactobacilli were isolated by culture for either partner in 15 couples (21%). Culture was not performed for 1 woman in the remaining couple. Of the 40 couples with both partners colonized by lactobacilli, 31 had strains available for rep-PCR. Isolates were not available for the remaining 9 couples because they were enrolled before initiation of the protocol for saving lactobacilli isolates. When both partners in a couple were colonized by lactobacilli, L. crispatus colonization of both was the most common outcome (74%). Nonetheless, of 31 couples with strains available for rep-PCR, identical strains were recovered from both women in 23 (74%). Of these 23 couples, partners in 18 shared the same L. crispatus strain, partners in 5 shared the same L. gasseri strain, and partners in 1 shared the same L. jensenii strain. Women in one of these couples shared an L. crispatus strain and an L. gasseri strain.
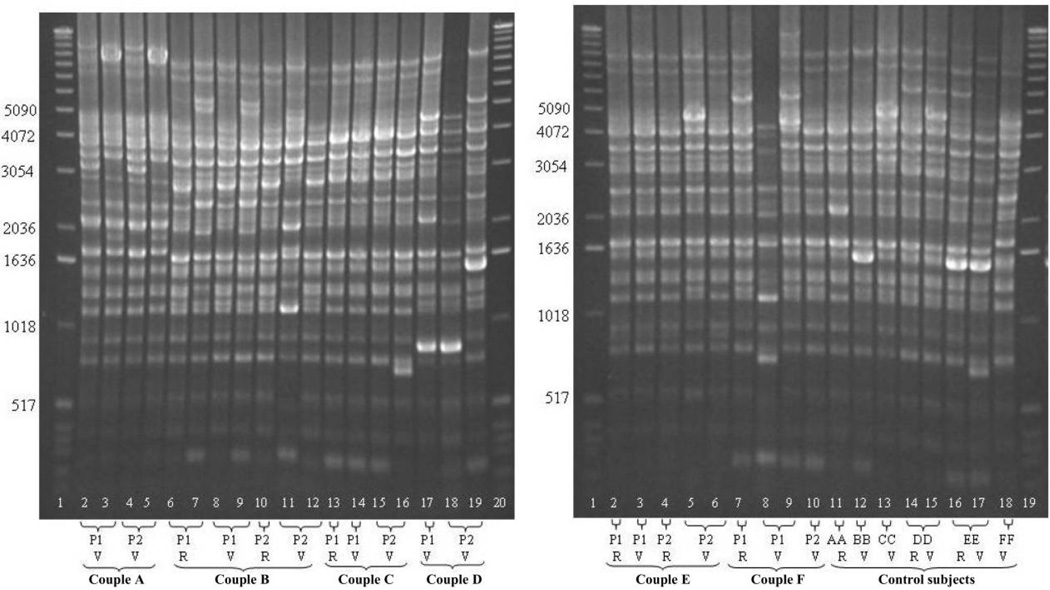
The gels in figure 1 depict rep-PCR findings for 18 women: 6 case couples (A through F) and 6 matched control women (AA through FF). Unique patterns are seen for each couple, and no similarity was seen for control subjects. Both partners of couple A were vaginally colonized by 2 different L. crispatus strains. Two different strains both vaginally and rectally colonized partner 1 of couple B. One strain colonized both the rectum and vagina of partner 1 of couple C and was only isolated from the vagina of partner 2. The same strain was detected from the vagina of both partners of couple D. Vaginal and rectal colonization by the same strain was found in both partners of couple E. Partners of couple F did not have a strain in common. However, partner 1 was colonized rectally and vaginally by the same strain. Rep-PCR findings were available for 46 other women in the study. Overall, at least half of the women (51% [60 of 118]) had >1 strain with a distinct rep-PCR pattern.
Figure 1.
Representative gels of repetitive element sequence– based PCR DNA fingerprints of Lactobacillus crispatus isolates from 18 subjects, of whom 12 were from 6 monogamous couples (A through F) and 6 were from matched control women who were unpartnered at the time of evaluation (AA through FF). “P” denotes sex partner, with members of each couple designated as “P1” or “P2.” Isolates obtained from the vagina are designated as “V,” and those obtained from the rectum are designated as “R.” A 1-kb DNA ladder (Invitrogen; lanes A1, A20, B1, and B19) served as a size standard on all gels.
Couples who shared identical Lactobacillus strains reported having had fewer female partners in the prior year (P = .03) and a longer median relationship duration (16 vs. 9.8 months; P = .6). The likelihood of sharing identical lactobacilli was not related to the mean age of the couple, the median number of lifetime male sex partners, or the practice, frequency, or timing of other types of sexual behaviors, including oral or anal sexual practices; one exception was reported use of shared vaginal sex toys, for which there was a trend toward an association with sharing identical lactobacilli strains (OR, 1.6; 95% CI, 0.94 –2.7; P = .2).
Discussion
The distribution of genital Lactobacillus species among lesbian and bisexual women in this study was characterized by a low prevalence of L. jensenii and high prevalence of L. gasseri relative to prevalences among heterosexual women [14]. Vaginal colonization with L. gasseri was associated with specific sexual practices that could have facilitated its introduction from a rectal reservoir into the vagina (e.g., via digital-vaginal sex) and with a significantly increased risk of abnormal vaginal flora. The rectum may provide a receptive environment for L gasseri, and sexual behaviors that allow for exchange of rectal flora may facilitate vaginal colonization with this species. Women with L. gasseri colonization of the vagina and/or rectum were more likely to have BV.
Nearly three quarters of the female partners we studied shared strains of lactobacilli with unique rep-PCR patterns, and the likelihood of sharing strains was directly related to having had fewer female sex partners in the prior year, with an association suggested for increasing duration of partnership and for sexual practices that could efficiently transfer vaginal fluid. Strains of lactobacilli having identical DNA fingerprinting patterns were commonly shared between the vagina and rectum. Among control “partners” matched for age and date of enrollment into the study, no similarities in rep-PCR patterns were seen. These data suggest that sexual behavior affects species-specific genital colonization and that women share vaginal Lactobacillus species with their female partners.
Data for heterosexual women indicate that the rectum may serve as a reservoir for vaginal lactobacilli that support normal vaginal flora. Antonio et al. [14] reported that only 9% of 147 women colonized by L. crispatus or L. jensenii vaginally and/or rectally had BV, compared with 44% of 27 women colonized by other H2O2-producing lactobacilli. Although the likelihood of rectal colonization increased with increasing colony counts from vaginal cultures, rectal colonization occurred in the absence of high-density vaginal colonization in one-third of women. Co-colonization of the vagina and rectum by these species was associated with the lowest prevalence of BV. Of note, the prevalence of L. gasseri among women in this study was 13%.
Our study has limitations. Subjects were self-referred and may not be representative of women who have sex with women. Most subjects were white, and our sample size may have been too small for subgroup analysis to detect statistical significance for some associations. Lactobacillus iners may have been underrepresented in our subjects, as we did not include methods specifically developed to cultivate this fastidious organism [15].
Our findings suggest areas for future study. First, a potential role of L. gasseri in BV pathogenesis could be explored. Vaginal colonization with L. gasseri might simply correlate with sexual practices that not only introduce this species into the vagina but may independently increase BV risk, including receptive anal intercourse or receptive oral-anal sex. Few studies of BV have assessed anal sexual behaviors as an exposure, but a prospective study identified report of anal intercourse immediately following vaginal intercourse as a significant risk [4]. Alternatively, vaginal conditions that promote colonization with L. gasseri may favor establishment of BV. Second, our observation that female sex partners share identical strains of lactobacilli has implications for vaginal application of probiotics to restore vaginal flora. Vaginal colonization with human-derived L. crispatus has been successful [9], and therapeutic applications are under study.
Acknowledgments
Financial support: National Institutes of Health (grants R29-AI41153-04 and RO1-AI52228-01 to J.M.M.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 44th Annual Meeting of the Infectious Diseases Society of America, 14 October 2004, Boston, MA.
References
- 1.Hillier SL. Normal vaginal flora. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sexually transmitted diseases. 4th ed. New York, NY: McGraw-Hill; 2008. pp. 289–307. [Google Scholar]
- 2.Sanchez S, Garcia P, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191:1898–1906. doi: 10.1016/j.ajog.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 3.Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005;32:654–658. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 4.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 5.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–1313. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 6.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw CS, Morton AN, Garland SM, Morris MB, Moss LM, Fairley CK. Higher-risk behavioral practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol. 2005;106:105–114. doi: 10.1097/01.AOG.0000163247.78533.7b. [DOI] [PubMed] [Google Scholar]
- 8.Criswell BSLC, Gardner HL, Dukes CD. Haemophilus vaginalis: vaginitis by inoculation from culture. Obstet Gynecol. 1969;33:195–199. [PubMed] [Google Scholar]
- 9.Antonio MA, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol. 2003;41:1881–1887. doi: 10.1128/JCM.41.5.1881-1887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16 Suppl 4:S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 12.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdeman L, Cato EP, Moore WEC. Anaerobe laboratory manual. 4th ed. Blacksburg, VA: Anaerobe Laboratory, Virginia Polytechnic Institute and State University; 1977. pp. 143–148. [Google Scholar]
- 14.Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis. 2005;192:394–398. doi: 10.1086/430926. [DOI] [PubMed] [Google Scholar]
- 15.Falsen E, Pascual C, Sjoden B, Ohlen M, Collins MD. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol. 1999;49:217–221. doi: 10.1099/00207713-49-1-217. [DOI] [PubMed] [Google Scholar]