Abstract

In this report we present a new chemical probe, 3-HTC, that can reversibly and ratiometrically measure the thioldisulfide equilibrium of biological systems. 3-HTC is composed of a coumarin that has a thiolate directly conjugated to its extended aromatic π system while formation of a disulfide attenuates this conjugation. The fluorescence and absorption properties of 3-HTC are therefore very sensitive to the redox state of its thiol. 3-HTC reacts reversibly with thiols and disulfides enabling its use to measure dynamic GSH/GSSH ratios in vitro as well as monitor the reversible redox status of whole cell lysates.
The thiol-disulfide interchange reaction is a key biochemical transformation that controls cellular processes such as gene regulation, cell division and differentiation.1 In addition, the disregulation of the thiol-disulfide steady state is a hallmark of oxidative stress and has been implicated in numerous diseases, such as atherosclerosis, obesity, inflammation and aging related complications.2 Hence, there is great interest in measuring the thiol-disulfide redox state of biological samples. Although several small molecule probes have been reported for thiol detection, they are not reversible and therefore do not provide a direct report on the thioldisulfide redox couple.3 Redox sensitive ratiometric green fluorescent proteins (roGFPs) that can monitor dynamic redox changes have been developed; however, these probes require genetic manipulation, which is frequently impossible and also has several additional complications.4 Currently, chemical probes that can measure the thiol-disulfide state of living cells do not exist, presenting a need for novel chemical tools that can measure biological thiol-disulfide dynamics.
In this report we present a new chemical probe, termed 3-hetaryl-7-thiol coumarin (3-HTC), that can reversibly and ratiometrically measure the thiol-disulfide equilibrium of biological systems. 3-HTC is composed of a coumarin that has a thiolate directly conjugated to its extended aromatic π system while formation of a disulfide attenuates this conjugation (Figure 1 and Scheme 1). The fluorescence and absorption properties of 3-HTC are therefore very sensitive to the redox state of its thiol and offer a convient method to measure and monitor redox changes, respctively.
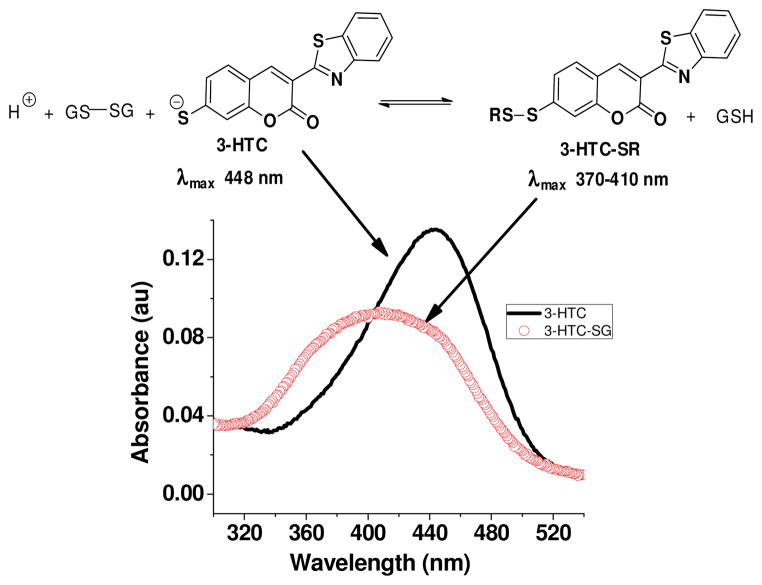
Figure 1.
3-HTC: A new molecule designed to image the thiol-disulfide redox couple in biological systems. 3-HTC can participate reversibly in thiol-disulfide interchange reactions to produce redox-dependent changes in its absorbance due to formation of the thiolate (448 nm) and disulfide species (370–410 nm). Ratiometric fluorescence imaging of the thiol-disulfide status is obtained by excitation of the thiolate (448 nm) and disulfide (370–410 nm) and monitoring emission at 490–500 nm (Fig 2).
Scheme 1.
Synthesis of 3-HTC
For example, in its reduced state, 3-HTC absorption and fluorescent excitation maxima are 448 nm but blue shifts to 370–410 nm in its oxidized form, due to a reduction in resonance forms available to the newly formed disulfide. 3-HTC can also participate in thiol-disulfide interchange reactions and its ratio of thiol-disulfide will therefore be dependent upon the cellular thiol-disulfide status. Importantly, the absorption of 3-HTC in the reduced and oxidized state are sufficiently different to allow for ratiometic imaging of the thiol-disulfide status within cells. For example, for systems with a low disulfide/thiol ratio, 3-HTC will exist predominantly as the free thiolate and therefore its ratio of excitation at 448/380 nm will be high. In contrast, in systems that have a high disulfide/thiol ratio, 3-HTC will exist predominantly in the disulfide form and its ratio of excitation at 448/380 nm will be low. Importantly, the disulfide exchange reaction is reversible, and thus, 3-HTC should be able to monitor the thiol/disulfide status in real time. Herein, we describe the synthesis, fluorescent ratiometic properties of 3-HTC thiolate and disulfides and the use of 3-HTC to measure GSH/GSSG ratios and the redox status of whole cell lysates.
3-HTC (1) was synthesized in 7 steps according to the methodology described in Scheme 1. The compound (6), the key intermediate in the synthesis, was generated from 4-hydroxy-2-methoxybenzaldehyde (2) in 4 steps. Briefly; compound (2) was converted to an O-arylthiocarbamate via reaction with DMTCC, and was then rearranged via a Newman-Kwart reaction at 185 °C generating the compound (4). Tandem deprotection of the methoxy and carbamate groups was then done with BBr3 and 5N KOH, generating (6), which, after purification, was immediately set for a Knoevenagel condensation with commercially available 2-cyanomethylbenzothiazole (7). The resulting iminocoumarin (8) was hydrolyzed with acid generating 3-HTC, which was air oxidized to the disulfide (9) and purified. 3-HTC was stored as a disulfide, and then reduced with aqueous TCEP before use.
To be a biochemically useful sensor, 3-HTC needs to efficiently participate in thiol/disulfide interchange reactions at physiological pH. The pKa of 3-HTC is a critical factor that determines its ability to reversibly detect thiols in aqueous environments. Cleavage and formation of a disulfide bond is mediated by the thiolate anion, which has increased nucleophilicity compared to the corresponding thiol. In addition, rates of disulfide interchange reactions are ideal when both the pKa of the thiol and the pH of the solution are equal.5 We therefore determined the pKa of 3-HTC via spectrometric titration. Figures S1 a–b demonstrate that 3-HTC has a pKa of 7.06 ± 0.05, and hence should be predominantly (~70%) ionized at pH 7.4. More importantly, the pKa of 3-HTC indicates that the compound is poised to efficiently interchange with disulfides. We anticipate that the kinetics of disulfide exchange with 3-HTC should be faster than with roGFPs, because the pKa’s of the cysteines in roGFPs have been estimated to be approximately 9.3 Interestingly, simple aromatic thiols such as thiophenol are more acidic than 3-HTC, and typically have pKa value of 6.6.5 The higher pKa of 3-HTC may be due to the electron-donating effect of the benzothiazolyl substituent on the coumarin moiety.
We synthesized the phenylmethane thiol disulfide of 3-HTC (Supplemental synthesis) to determine if the fluorescent properties of 3-HTC and its disulfide were sufficiently different to allow for ratiometric imaging of the thiol-disulfide status. Figures 2a and 2b demonstrate that the fluorescence emission of 3-HTC and its disulfide are dramatically different. This difference was identified by exciting 3-HTC and its disulfide at either 372 or 448 nm and measuring the emission. For example, 3-HTC free thiolate has a 448/372 nm emission ratio of 5, whereas for the disulfide this value is only 0.5. Thus 3-HTC should allow for ratiometric measurement of thiols and disulfide in biological systems.
Figure 2.
Fluorescence emission spectra of 3-HTC and 3-HTC benzyl disulfide. (a) Fluorescence emission spectra of 3-HTC (10 μM) in PBS (pH 7.4) after excitation at 372 and 448 nm. (b) Fluorescence emission spectra of 3-HTC benzyl thiol disulfide (compound 10) (20 μM) in PBS (pH 7.4) after excitation at 372 and 448 nm. Representative spectra for triplicate experiments.
3-HTC is designed to measure the thiol-disulfide ratio in cells by participating in disulfide exchange reaction with cellular thiols. We therefore investigated if 3-HTC could participate in a disulfide exchange reaction with glutathione disulfide (GSSG), and consequently measure the thiol-disulfide ratio of glutathione and its disulfide. 3-HTC was incubated with a 250-fold excess of GSSG for 10 min, and the fluorescence of the resulting solution was measured after excitation at either 448 nm or 372 nm (Fig S2 a–c and S3). Figure 3a demonstrates that the reaction of 3-HTC with GSSG can be monitored by measuring the emission of the reaction solution, after excitation at either 372 nm or 448 nm. 3-HTC has a 448/372 nm emission ratio of 2.4, and this changes to 0.4 in the presence of excess GSSG, due to formation of 3-HTC-SG.
Figure 3.
Ratiometric fluorescence response of 3-HTC and 3-HTC-SG. (a) Normalized fluorescence emission of 3-HTC and 3-HTC-SG at λex 370 and 448 nm and emission at 490 nm (averaged results for duplicate experimets). (b) 3-HTC (10 μM) in PBS (pH 7.4) was incubated with GSH/GSSG (0.001–1000; 1 mM total) for 10 min in a sealed vial before the emission at 500 nm was read after excitation at 370 nm and 458 nm (see supporting info pg S9 for replicate graphs, n=3).
In addition, we also determined if 3-HTC could measure different ratios of GSH to its disulfide, and potentially measure different oxidation environments within cells.6 3-HTC was mixed with varying ratios of GSH and its disulfide for 10 min and the resulting emission after excitation at either 372 nm or 458 nm was measured. Figure 3b (and Fig S4) demonstrates that the emission ratio of 3-HTC at 458 nm versus 372 nm changes dramatically in response to the GSH/GSSG ratio. Figure 3b (and Fig S7) also shows that the probe has a detection limit of 3 × 10−6 M. In addition, 3-HTC displays redox mid-point ratio of 0.5 but is able to respond most effectively to thiol-disulfide ratios of 0.1–10 with a maximum six-fold change in emission fluorescence between the thiol and disulfide.
Taken together, our data establishes that 3-HTC has the photophysical and chemical properties needed to act as a ratiometric, quantitative, reversible probe and should be capable of estimating the thiol-disulfide status by comparing the ratio of emissions following excitation at 370–380 nm and 448–458 nm. In order to detect changes within a redox environment a probe must have a redox mid-point that matches that of the environment under study. 3-HTC therefore provides a new avenue to study oxidizing environments such as blood plasma, ER, Golgi and the endosomal-lysosomal system.
Finally, we examined if 3-HTC could measure the redox balance in cell lysates. 3-HTC was incubated with air oxidized Jurkat cell lysates and the ratiometric fluorescence excitation at 500/380 nm was measured before and after reduction with GSH (used as an approximation to the total lysate thiol pool). Figure 4a–b (and Fig S5a–b) demonstrate that 3-HTC is predominantly in the disulfide form after addition to Jurkat cell lysates based on the high absorbance at 380 nm and low 500/380 nm excitation ratio. In contrast, Jurkat cell lysate disulfides reduced with 10 mM GSH are now predominantly in the free thiol state, as evidenced by the absorption shift to 500 nm and the high 500/380 nm excitation ratios. Furthermore, Figure 4a demonstrates that the redox dynamics of the lysate begins to change on standing at room temperature for 80 min. Our results confirm an oxidative thiol environment for whole cell lysates that have not been treated to prevent post-lytic oxidation (e.g with 10% TCA). Furthermore, our finding has implications for the activity of redox sensitive proteins in lysates prepared under conditions that do not halt thiol-disulfide interchange.7
Figure 4.
3-HTC measures the thiol-disulfide dynamics of cell lysates. (a) Jurkat lysate (0.5 mg/mL) or GSH (10 mM) reduced lysate in Tris lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Trition X) was incubated with 3-HTC (20 μM) and the absorbance spectra were taken. (b) Excitation ratio (500 nm/380 nm) of oxidized and reduced Jurkat lysates (0.5 mg/mL) were determined at 530 nm emission, following incubation with 3- HTC (20 μM). Experiments were carried out in duplicate.
In summary, we have produced a new small molecule, termed 3-HTC, that measures thiol-disulfide couples in biological systems. This probe provides the first synthetic molecular alternative to roGFPs for studying dynamic disulfide bond formations. 3-HTC is a fluorescent probe that exhibits λmax of 448 nm in its reduced thiolate form and a λmax 370–410 nm for the oxidized mixed disulfide. 3-HTC is a bona fide ratiometric, reversible, probe which is synthetically accessible, easy to handle and gives robust signals with both absorbance and fluorescence measurements and accumulates in cells within 30 min. 3-HTC can measure redox changes within cell lysates. We anticipate wide interest in the utility of 3-HTC to study the thiol-disulfide redox couple of relevant systems.
Supplementary Material
Acknowledgments
This work was funded in whole or in part with federal funds from the National Institute of Health RO1AI88023 to M.L.K and N.M, RO1HL096796-01 to N.M. and NSF-BES 0546962 Career Award to N.M.
Abbreviations
- 3-HTC
3-hetaryl-7-thiol coumarin
- 3-HTC-SG
3-hetaryl-7-thiol coumarin glutathione mixed disulfide
- GSH
glutathione
- GSSG
glutathione disulfide
- DMTCC
Dimethyl thiocarbamoyl chloride
- TCEP
tris(2-carboxyethyl)phosphine
Footnotes
Supporting Information Available. Detailed experimental procedures and compound characterization. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.(a) Antelmann H, Helmann JD. Antioxid Redox Signal. 2010;14:1049. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Matthais LJ, Yam PTW, Jiang XM, Vandegraaff N, Li P, Poumbourios P, Donoghue N, Hogg PJ. Nat Immunol. 2002;3:727. doi: 10.1038/ni815. [DOI] [PubMed] [Google Scholar]; (c) Paulsen CE, Carroll KS. ACS Chem Biol. 2009;5:47. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pan S, Berk BC. Circ Res. 2007;100:213. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 2.(a) Gupta D, Griendling KK, Taylor RW. In: Oxidative Stress and Cardiovascular Disease in Diabetes Mellitus Studies on Cardiovascular Disorders. Sauer H, Shah AM, Laurindo FRM, editors. Humana Press; New York, New York: 2010. p. 263. [Google Scholar]; (b) Salmon AB, Richardson A, Pérez VI. Free Radical Bio Med. 2010;48:642. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Bohndiek SE, Kettunen MI, Hu DE, Kennedy BW, Boren J, Gallagher FA, Brindle KM. J Am Chem Soc. 2011;133:11795. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lim CS, Masanta G, Kim HJ, Han JH, Kim HM, Cho BR. J Am Chem Soc. 2011;133:11132. doi: 10.1021/ja205081s. [DOI] [PubMed] [Google Scholar]; (c) Kim GJ, Lee K, Kwon H, Kim HJ. Org Lett. 2011;13:2799. doi: 10.1021/ol200967w. [DOI] [PubMed] [Google Scholar]; (d) Long L, Lin W, Chen B, Gao W, Yuan L. Chem Commun. 2011;47:893. doi: 10.1039/c0cc03806g. [DOI] [PubMed] [Google Scholar]
- 4.For an excellent review on fluorescent protein-based redox probes, see: Meyer AJ, Dick TP. Antioxid Redox Signal. 2010;13:621. doi: 10.1089/ars.2009.2948.
- 5.Houk J, Whitesides GM. J Am Chem Soc. 1987;109:6825. [Google Scholar]
- 6.Kemp M, Go YM, Jones DP. Free Radical Bio Med. 2008;44:921. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton DJ, Aye SM, Rady J, Xu F, Cross JV. PLoS ONE. 2010;5:e15012. doi: 10.1371/journal.pone.0015012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.