Abstract
We have recently demonstrated that intra-articular (IA) administration of human recombinant lubricin, LUB:1, significantly inhibited cartilage degeneration and pain in the rat meniscal tear model of post-traumatic arthritis. In this report, we show that after a single IA injection to naïve rats and rats that underwent unilateral meniscal tear, [125I]LUB:1 had a tri-phasic disposition profile, with the alpha, beta, and gamma half-life estimates of 4.5 h, 1.5 days, and 2.1 weeks, respectively. We hypothesize that the terminal phase kinetics was related to [125I]LUB:1 binding to its ligands. [125I]LUB:1 was detected on articular cartilage surfaces as long as 28 days after single IA injection. Micro-autoradiography analysis suggested that [125I]LUB:1 tended to localize to damaged joint surfaces in rats with meniscal tear. After a single intravenous (IV) dose to rats, [125I]LUB:1 was eliminated rapidly from the systemic circulation, with a mean total body clearance of 154 mL/h/kg and a mean elimination half-life (t1/2) of 6.7 h. Overall, LUB:1 has met a desired disposition profile of a potential therapeutic intended for an IA administration: target tissue (knee) retention and fast elimination from the systemic circulation after a single IA or IV dose.
Key words: lubricin, osteoarthritis, pharmacokinetics
INTRODUCTION
The low coefficient of friction at articular cartilage surfaces is maintained in part by lubrication of the boundary, and lubrication deficiency is considered a risk factor for development of degenerative joint diseases (1). Lubricin, also referred to as proteoglycan 4 (PRG4), superficial zone protein (SZP), and megakaryocyte stimulating factor, is a mucinous glycoprotein with lubricating properties that prevents wear and adhesion at cartilage surfaces (2). It is synthesized by articular chondrocytes of the superficial zone and synoviocytes (3,4).
Dysregulation of lubricin has been associated with development and progression of degenerative joint disease in both animals and humans. Mice lacking lubricin develop progressive joint degeneration (2). Reduced expression of lubricin has been reported in several animal models of osteoarthritis (OA) and post-traumatic arthritis (PTA) (1,5,6). In humans, loss-of-function mutations in the PRG4 gene are associated with synovial hyperplasia and joint failure observed in individuals with camptodactyly–arthropathy–coxa vara–pericarditis syndrome, CACP (7). Similarly, lubricin levels are reduced in synovial fluid from patients after acute joint injury (8,9). In addition, osteoarthritis cartilage and fibrocartilaginous deposits in regions of exposed bone in human OA express lubricin (PGR4), suggesting a role of lubricin in regenerative processes (10).
We reported that binding of lubricin to the articular cartilage surfaces is dependent on protein secondary structure (11). Using a proteomic-based approach, we found various ligands that display significant binding to lubricin at cartilage surfaces, including type VI collagen, tenascin-C, aggrecan, fibronectin, COMP, vitronecting, and decoring (Flannery et al., (12)). Analysis of conserved/functional domains revealed collagen triple helical repeats, thrombospondin-like repeats, EGF-like, and fibronectin type II repeats to be the most abundant sequences, suggesting the potential for multivalent interactions at cartilage surfaces. Collectively, these data support the hypothesis that binding of lubricin to ligands at articular cartilage surfaces may be critical for lubricin function in the joint.
We recently developed a novel lubricin recombinant construct, LUB:1, which contains a truncated mucin-like domain (13). This lubricin construct maintains essential properties of cartilage binding and lubrication. We demonstrated that intra-articular (IA) administration of LUB:1 significantly inhibited cartilage degeneration in a rat model of PTA (13). Efficacy of native and recombinant human lubricin has also been demonstrated in other animal models of OA and PTA (14,15). These data suggest the potential use of recombinant lubricin as a biotherapeutic for the treatment of OA in humans.
In this report, we characterize the disposition of [125I]LUB:1 after intravenous (IV) and IA administration to naïve rats and in a rat model of PTA. Specifically, we sought to (1) characterize retention kinetics of radiolabeled LUB:1 in the rat knee joint following single IA administration, (2) determine LUB:1 tissue and fluid distribution following IV or IA administration, and (3) determine the concentration of LUB:1 in the knee that is needed to achieve the pharmacological effect in the rat model of PTA.
MATERIALS AND METHODS
Test Article, Iodination, and Dosing Solution Preparation
A recombinant lubricin construct, LUB:1, which represents a modified version of human lubricin (~250 kDa MW), was expressed in Chinese hamster ovary cells and purified, as described previously (13). LUB:1 was formulated in either phosphate-buffered saline (PBS) or in “Arg Buffer”(0.2 M arginine; 10 mM citrate), as specified in Table I.
Table I.
Study Design
| Protocol | Animals | Route, dose (injection volume) | Formulation | Sampling time points | |||
|---|---|---|---|---|---|---|---|
| Knee | Tissuesa | Urine | Blood, plasma, or serum | ||||
| Study 1 | Naïve female rats (n = 3 per time point) | IA, 20 μg (70 μL) per knee | PBS | 5 min–28 h | NC | NC | NC |
| Study 2 | Naïve female rats (n = 5 per time point) | IA, 20 μg (40 μL) per knee | PBS | 24–672 h | NC | NC | 10 min–672 h (serum) |
| Study 3 | Naïve male rats (n = 5) per time point | IA, 20 μg (40 μL) per knee | Arg Buffer | 6–504 h | 6 and 24 h | 24, 48, 72 h | 6–168 h (plasma) |
| Study 4 | Naïve and MT male rats (n = 5 per time point per group) | IA, 8.3 μg (40 μL) per knee | PBS | 24 and 168 h | NC | NC | 5 min–48 h (blood and plasma) |
| Study 5 | Naïve male rats (n = 5, serial sampling) | IV, 0.5 mg/kg (1 mL/kg) | Arg Buffer | NC | NC | 24, 48, 72 h | 2 min–72 h (plasma) |
Lewis rats were used for all studies
MT meniscal tear surgery, Arg Buffer 0.2 M arginine, 10 mM Citrate, PBS phosphate-buffered saline, NC not collected
aTissues were liver, kidney, and spleen
Iodination of the protein was performed using either the IODO-BEADS or the pre-coated IODO-GEN tubes, according to manufacturer’s instructions (Pierce, Rockford, IL), with 0.2–0.8 mg of LUB:1 and 1–2 mCi of 125-iodine (Perkin Elmer; Waltham, MA). A dosing solution was prepared by mixing an unlabeled test article, a trace amount of 125I-labeled test article, and a formulation buffer, with the range of specific activities of 23–480 μCi/mg for the five studies used in this report (Table I). Each dosing solution was characterized by gamma counting of trichloroacetic acid (TCA)-precipitable radioactivity (Model 1480 WIZARD™, Wallac Inc., Gaithersburg, MD or Model 2470 Perkin Elmer, Waltham, MA) and gel electrophoresis, as previously described (16). Free iodine was less than 10%, except for study 4, in which free iodine was 19%.
Animals and Meniscal Tear Surgery
Lewis rats (females for studies 1 and 2 and males for studies 3–5, Table I) were obtained from Taconic (Germantown, NY). For study 4, the rats underwent medial meniscal tear (MT) on the right knee to induce joint instability as previously described (17,18). IA-dosing was performed ~3 weeks post-surgery to allow the development of cartilaginous lesions in rats. Naive rats were used in the other studies (Table I). For study 5 (IV dosing), rats with two jugular vein catheters were used.
All animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations. Specifically, all in vivo procedures and aspects of this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Pfizer Inc (formerly Wyeth Inc.).
Study Design and Sample Collection
The study designs for the five different studies described in the “Results” are summarized in Table I. Rats were not fasted and were given water containing potassium iodide (0.1 mg/mL) for 3 days prior to dosing. All rats in study 5 and five rats in study 3 (designated for 168 h tissue harvest) were placed in metabolic cages (Ancare Corporation, North Bellmore, NY) prior to dosing.
For studies 1 and 2, rats (n = 3 or 5 per time point, respectively) received a single bilateral injection of [125I]LUB:1 with the dose/injection volumes specified in Table I. For studies 3 and 4, rats (n = 5 per time point per group) were given a single intra-articular (IA) injection of [125I] LUB:1 into the right knee (dose/volume specified in Table I). The knees were collected at the specified time points (Table I and Figs. 1 and 2) and the total counts for the right knee (IA injection site) were obtained by gamma counting. The limit of detection in the knee (defined as 3 × background counts per minute (cpm)) ranged from 0.05 to 1.1 ng eq/knee (depending on the specific activity of the dosing solution obtained). Since it was not technically feasible to homogenize the knees for TCA precipitation, TCA-soluble counts for the knee samples were not obtained. In some IA studies, whole blood samples were also obtained either by retro-orbital bleeding or by cardiac puncture (for terminal time points at which the knee collection was also performed) at the specified sampling time points (Table I), and processed for serum or plasma by centrifugation. The total and the TCA-soluble counts in 50–100 μL aliquots (in duplicates) were obtained and TCA-precipitable counts were calculated, as described previously (16). The limit of detection in the blood, serum, and plasma ranged from 0.53 to 23 ng eq/mL. For study 2, the right knees from three of five rats per time point (sampling time points specified in the text) were further dissected into tibial plateau, femoral condyle, meniscus, patella, and synovium and the counts of the dissected regions were obtained by gamma counting. For study 3, animals were whole body perfused with 10 mL heparinized (25 U/mL) PBS and additional tissues (liver, spleen, and kidney) were collected at 6 and 24 h. Total counts were obtained and radioactive equivalent concentrations for tissues were calculated as previously described (16). For study 4, the right knees were processed for micro-autoradiography analysis, as described below.
Fig. 1.
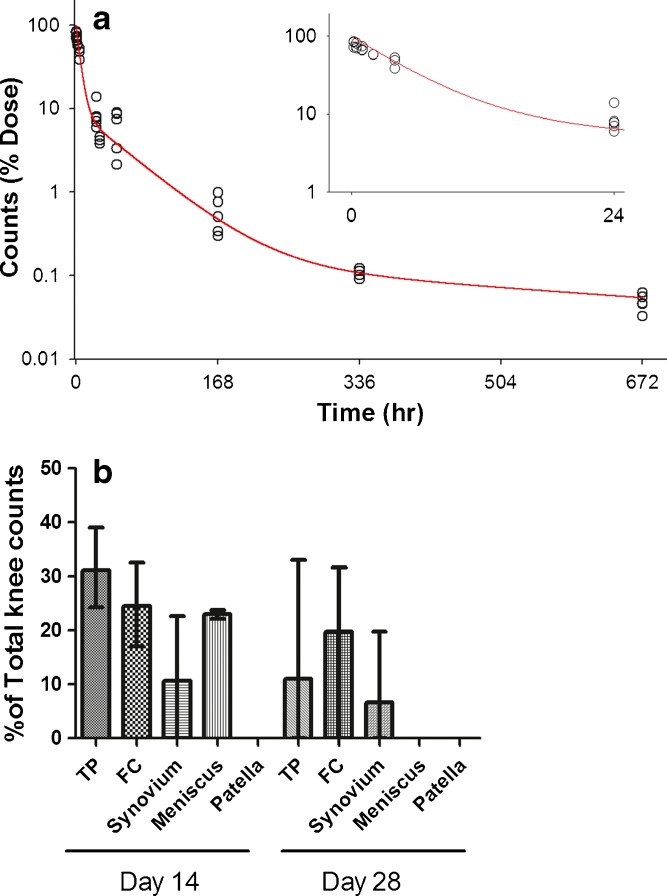
Total whole knee counts (as % dose) and knee joint tissue counts (as % total knee counts) after a single intra-articular (IA) dose of [125I]LUB:1 to naïve rats. Naïve rats were administered 20 μg/knee of [125I]LUB:1 and knees were collected at the time points described in the text and Table I (studies 1 and 2). (a) Individual animal total knee counts obtained by gamma counting were expressed as the percentage of total administered dose (open circles) and fitted simultaneously using the three-compartment model (solid line), as described in the text. Insert shows data up to 24 h post-dose. (b) For the 336 h (14 days) and 672 h (28 days) time points, joint tissues were further dissected into tibial plateau (TP), femoral condyle (FC), meniscus, patella, and synovium (n = 3 per time point) crude sections, gamma counting of these specific sections was conducted, the section counts were expressed as the percentage of total knee counts for each individual animal, and the mean and range values were plotted
Fig. 2.
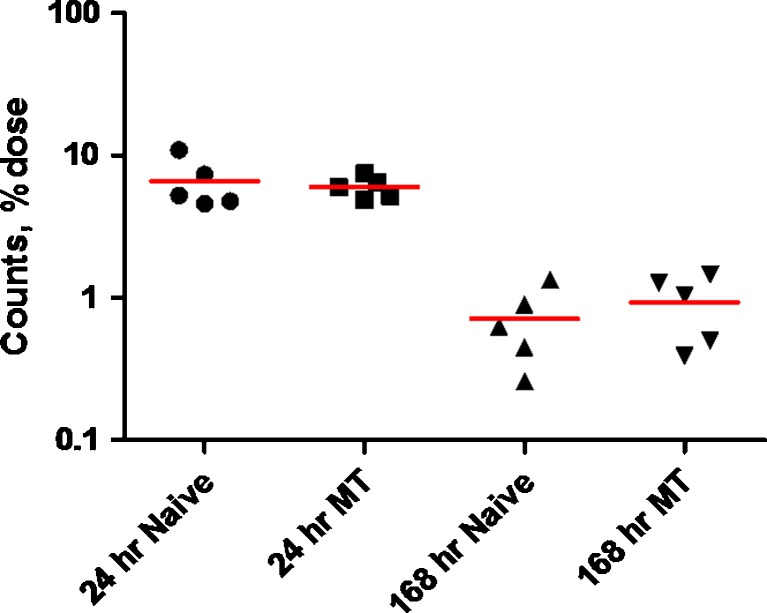
Total knee counts (as % dose) after a single IA dose of [125I]LUB:1 to naïve rats or rats that underwent MT surgery. Naïve or operated rats (n = 5 per time point per group) were given a single 8.3 μg/knee dose of [125I]LUB:1, knees were collected at 24 and 168 h time points, and individual animal (symbols) and mean (solid line) total knee counts, as the percentage of total administered dose was obtained as described in the text (study 4 in Table I, unilateral knee surgery). MT meniscal tear
For study 5, the rats (n = 5) were given a single 0.5 mg/kg IV bolus dose [125I]LUB:1 via a jugular vein catheter. Whole blood samples were obtained from contralateral jugular vein catheter using serial sampling design at pre-specified time points (Fig. 4 and Table I) and radioactive equivalent plasma concentrations were determined based on TCA-precipitable counts, as previously described (16).
Fig. 4.
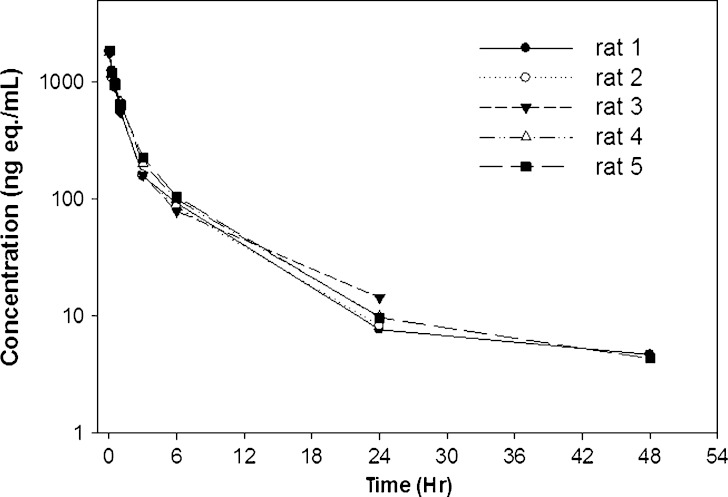
Individual animal radioactive equivalent concentrations of [125I]LUB:1 in plasma after a single 0.5 mg/kg IV dose to naïve rats. Lewis rats (n = 5) were administered a single IV dose of 0.5 mg/kg of [125I]LUB:1 into jugular vein (study 5 in Table I) and plasma samples were analyzed for radioactive equivalent concentrations by gamma counting, as described in the text
For studies 3 and 5, daily urine samples for rats placed in metabolic cages were collected over the 3-day period and analyzed by gamma counting to obtain cumulative total counts. Accumulated radioactivity excreted in urine was expressed as percent dose administered.
Micro-autoradiography
The knee samples were decalcified in 20% EDTA, embedded with paraffin, then sectioned at 5 μm, and placed on super frost plus slides. Slides were deparaffinized using xylene and then the following procedures were carried out in a dark room with safelight illumination only: slides were dipped into liquid emulsion, air dried, transferred into a light proof slide box wrapped with aluminum foil, then placed in a refrigerator for a 3-month exposure period. After 3 months, slides were placed in a slide holder and immersed into Eastman-Kodak D19 Developer, followed by a 30-s immersion in 1% acetic acid and a 10-min immersion in 30% sodium thiosulfate for fixation. Thereafter, slides were stained with eosin and placed into Mirax MIDI Histology Digital Scanning System to obtain digital images, which could be viewed at the enlargement range of ×1 to ×100 objective magnification.
Digital images of the knee joints were qualitatively reviewed to determine the radioactive signals induced by [125I]LUB:1. The intensity and tissue locations of radioactive signals were determined, and comparisons were performed to evaluate the difference between surgical-induced damage and intact joints, as well as between samples harvested at 24-h and 7-day time points.
Pharmacokinetic Calculations and Simulations
For studies 1 and 2, the knee count data (expressed as percentage of total dose) from all rats was simultaneously fitted into the three-compartment model using MATLAB software (2010b, The MathWorks, Natick, MA). The data were fitted to the analytical solution for a three-compartment model, C(t) = A×EXP(-α×t) + B×EXP(-β×t) + C×EXP(-γ×t), where C(t) counts as % dose at time t, and a weighted against the inverse of the observation squared.
For study 5 (IV dosing), pharmacokinetic (PK) parameters were calculated for each individual animal based on radioactive equivalent plasma concentrations by non-compartmental analysis using the WinNonlin software (ver. 5, Model 201; Pharsight, Inc., St. Louis, MO).
For estimation of an upper bound for the “Meff” described in the “Discussion”, the mass profile of LUB: 1 in the knee after 4 weeks of three per week IA injections of 20 μg per knee, was simulated using the nonparametric superposition module of WinNonlin software based on the mean data from the single dose IA studies (20 μg per knee injections, Fig. 1a).
RESULTS
Disposition After a Single Intra-articular Injection in Naïve Rats
The retention kinetics of [125I]LUB:1 in the knee of naïve Lewis rats after a single 20 μg/knee IA injection were assessed in two studies. In study 1 (pilot), the sampling time points were 5 min–28 h, while in study 2 sampling time points covered a long duration up to 28 days. The total radioactivity (cpm) in the knee was expressed as a percentage of the total administered dose, and data from both studies were analyzed simultaneously (Fig. 1a). There was a rapid decline in the knee counts during the first several days post-dose, such that there was approximately only 6% of total injected counts remaining in the knee at 48 h. A slower decline in knee radioactivity was observed at the later time points, with a measurable, albeit comparatively low amount of radioactivity remaining in the knees up to 28 days (672 h) post-dose (~0.05% of dose). The radioactivity–time profile was tested for conformity of fit using both the two-compartment and the three-compartment models. While no formal comparison of goodness of fit between the two models could be performed (because of limited dataset compared to the number of parameters for the three-compartment fitting), the apparent improvement in model fit due to adding a third compartment appeared to justify the added complexity. The three-compartment model was chosen as the final model (Fig. 1a) and the alpha, beta, and gamma half-life estimates were of 4.5 h, 1.5 days, and 2.1 weeks, respectively. After a single IA injection of [125I]LUB:1, total counts in serum or plasma were relatively low to undetectable during the 10 min–672 h sampling period (data not shown).
In study 2, joint tissues were also dissected into the tibial plateau (TP), femoral condyle (FC), meniscus, patella, and synovium at 14 days (336 h) and 28 days (672 h) and gamma counting of these specific sections was conducted after total knee counts were obtained (Fig. 1b). [125I]LUB:1 was detected in articular cartilage and synovial surfaces as long as 28 days after a single IA injection.
Study 3 was conducted to assess tissue distribution in highly perfused organs and excretion of radioactivity into urine after a single IA dose of [125I]LUB:1 of ~20 μg per knee. Similar to the observations in the first two studies, there was rapid elimination of total counts from the knee during the first 48 h, followed by a slow decay in counts in the terminal phase (data not shown). Radioactive equivalent concentrations and total radioactivity, expressed as % dose, were also analyzed for liver, spleen, and kidney at 6 and 24 h after a single IA dose of ~20 μg per knee (Table II). In all tissues, except for the injected knee, radioactive equivalent concentrations, and counts as % dose were relatively low (<1.5% of total administered dose). The mean cumulative counts in urine (expressed as a percentage of the injection dose) were ~77%, 92%, and 95% at 24, 48, and 72 h, respectively, indicating that most of injected dose was eliminated from the body in 2 to 3 days after a single IA injection.
Table II.
Radioactive Equivalent Concentrations of [125I]LUB:1 and Counts, as % Dose, in Liver, Spleen, Kidney, and Knee After a Single IA Dose of ~20 μg/Knee to Lewis Rats
| 6 h | 24 h | |||
|---|---|---|---|---|
| Mean concentration (ng eq/g) | Radioactivity (% dose) | Mean concentration (ng eq/g) | Radioactivity (% dose) | |
| Kidney | 98 | 1.29 | 7 | 0.09 |
| Knee | 1,320 | 27.7 | 132 | 2.37 |
| Liver | 22 | 1.17 | 2 | 0.13 |
| Spleen | 25 | 0.09 | 2 | 0.01 |
Naïve Lewis rats (n = 5 per time point) were administered a single IA dose of ~20 μg/knee of [125I]LUB:1 into the right knee (study 3 in Table I) and tissues were analyzed for total counts and the radioactive equivalent concentrations by gamma counting, as described in the text
Disposition After a Single IA Injection in Rat Meniscal Tear Model of PTA
To support the pharmacological assessment of LUB:1 in the rat MT model of PTA, study 4 with two treatment groups (naïve and MT Lewis rats) was conducted. The total knee counts were assessed at 24 h (1 day) and 168 h (7 days) following a single 8.3 μg/knee IA dose. At both time points, the mean total knee counts (as the percentage of total dose) were similar between the naïve and MT rats (Fig. 2), as well as similar to the profiles observed in earlier studies described above. Likewise, in accord with earlier studies, total and TCA-precipitable counts in circulation (whole blood, plasma, and/or serum) in study 4 were relatively low to undetectable (data not shown).
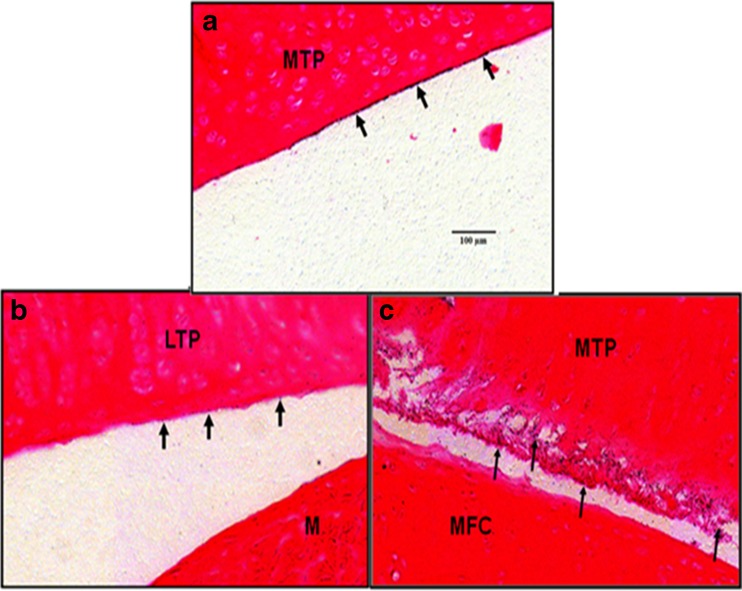
To further characterize the retention of [125I]LUB:1 in knee joints, the knees collected at 24 and 168 h (7 days) were analyzed by micro-autoradiography. Qualitative evaluation of digital images from intact or MT knee joints suggested that the radioactive signals were observed in all tissue surfaces within the articular joint for both naïve and operated animals (Fig. 3). These tissue surfaces include proximal tibial articular surface, distal femoral condyle articular surface, both proximal and distal surfaces of meniscus, joint capsule synovial tissue surface, and the surface of the cruciate ligament. However, the signals appeared significantly stronger on damaged articular surface (medial knee joint of the operated rats; Fig. 3c) compared to those on intact articular surfaces (lateral knee joint of operated rats; Fig. 3b) and on whole knee joint of naïve rats (Fig. 3a). The intensity and extent of the radioactive signal were at the highest levels at the 24-h time point while they were both significantly diminished at the 7-day time point. These results suggested that [125I]LUB:1 was retained in operated knee joints, with apparent localization to the target action site of damaged joint surfaces.
Fig. 3.
Micro-autoradiography analysis of knee joint sections after a single IA dose of [125I]LUB:1 to naïve rats or rats that underwent MT surgery. Naïve or MT rats were given a single 8.3 μg/knee dose of [125I]LUB:1, knees were collected at 24 and 168 h time points, and micro-autoradiography analysis followed by eosin staining of knee sections was conducted, as described in the text (study 4 in Table I, unilateral knee surgery). Representative digital images of the articular surface of the MTP in a naive animal a, LTP (without damage) of a MT animal b, and MTP (damaged) of the same MT animal c at 24 h time point. The intensity and extend of radioactive signals on the articular surfaces without damage a and b are significantly less than the damaged articular surface. Eosin staining; bar length in a equals 100 μm. Arrows point to the surfaces with radioactive signals. MT meniscal tear. MTP medial tibial plateau, LTP lateral tibial plateau, M meniscus, MFC medial femoral condyle
Disposition After Single IV Administration
In study 5, the pharmacokinetic parameters of [125I]LUB:1 were determined after a single 0.5 mg/kg IV dose to naïve Lewis rats (n = 5), based on TCA-precipitable radioactive equivalent plasma concentrations (Fig. 4 and Table III). [125I]LUB:1 was eliminated rapidly from the systemic circulation with a mean total body clearance (CL) of 154 ± 13 mL/h/kg and a short mean elimination half-life (t1/2) of 6.66 ± 1.72 h. The mean volume of distribution at steady-state (Vdss) was moderate (714 ± 120 mL/kg) and the mean exposure (AUC0-∞) was 3,271 ± 285 ng eq∙h/mL. In accord with a short half-life of [125I]LUB:1, about 87% of total administered dose were excreted into urine within 24 h post-drug administration. Mean cumulative counts in urine as % dose were 91% and 92%, respectively, at 48 and 72 h time points.
Table III.
Individual Animal and Mean Plasma PK Parameters of [125I]LUB:1 After a Single 0.5 mg/kg IV Dose to Lewis Rats
| Animal_ID | C 2min (ng eq/mL) | AUC0−∞ (ng eq∙h/mL) | CL (mL/h/kg) | Vdss (mL/kg) | t 1/2 (h) |
|---|---|---|---|---|---|
| Rat 1 | 1,823 | 3,231 | 155 | 865 | 8.79 |
| Rat 2 | 1,808 | 2,979 | 168 | 628 | 5.05 |
| Rat 3 | 1,752 | 3,049 | 164 | 784 | 6.42 |
| Rat 4 | 1,805 | 3,412 | 147 | 566 | 5.01 |
| Rat 5 | 1,846 | 3,683 | 136 | 726 | 8.03 |
| Mean | 1,807 | 3,271 | 154 | 714 | 6.66 |
| SD | 35 | 285 | 13.0 | 120 | 1.72 |
Naïve Lewis rats (n = 5) were administered a single IV dose of 0.5 mg/kg of [125I]LUB:1 into jugular vein (study 5 in Table I), plasma samples were analyzed for radioactive equivalent concentrations by gamma counting and individual animal PK parameters were determined, as described in the text
DISCUSSION
In this report, we characterized the disposition of [125I]LUB:1 (radiolabeled human recombinant lubricin) after a single IA or IV dose to naïve rats and in a rat model of PTA induced by meniscal tear surgery. After a single IA injection to rats, a rapid decline in knee radioactivity was observed during the first several days post-dose, such that approximately 6% of total injected counts remained in the knees at 48 h. At the later time points, the decline in total knee counts was relatively slow and [125I]LUB:1 was detected in whole knee samples, as well as in articular cartilage and synovial surfaces as long as 28 days after a single IA injection. The radioactivity–time profile in the knee appeared to be best fitted by a three-compartment model and the alpha, beta, and gamma half-life estimates were of 4.5 h, 1.5 days, and 2.1 weeks, respectively. The total knee radioactivity profile after a single IA injection appeared similar in both naïve rats and rats that underwent MT surgery at selected time points examined (24 and 168 h). However, micro-autoradiography analysis suggested that [125I]LUB:1 was retained in operated knee joints, with apparent localization to the target site of damaged joint surfaces.
After a single IV dose to rats, [125I]LUB:1 was also eliminated rapidly from the systemic circulation, with a mean total body clearance of 154 mL/h/kg and a short mean elimination half-life (t1/2) of 6.7 h. After a single IA injection of [125I]LUB:1, relatively low to undetectable amounts of radioactivity were observed in the circulation (whole blood, serum, or plasma), as well as in the spleen, liver, and kidney (<1.5% of total administered dose). The analysis of urine samples confirmed that >90% of each injected IA and IV dose was eliminated from the body in 2–3 days. Further investigations are needed to understand the LUB:1 clearance and distribution pathways, including additional tissue distribution data for the knee-draining lymph nodes. It should also be noted that for IA studies for which blood samples were collected, the total dose per rat, specific activity, and the resulting total μCi/rat were, in general, lower compared to those for an IV study. These experimental details may, in part, account for the low/undetectable amount of radioactivity observed in the circulation after IA administration. Overall, LUB:1 has appeared to meet the desired disposition profile of a potential therapeutic intended for an IA dosing: target tissue (knee) retention and fast elimination from the systemic circulation.
The reason for multi-phasic elimination profile after a single IA injection of LUB:1 remains to be understood. We hypothesize that the rapid drop in concentration during the first phase is related to the release of the protein/larger fragments to the lymphatic system and then into circulation, as well as radiolabeled degradation products/free iodine directly to the circulation. It should be noted that a non-binding macromolecule is believed to be cleared from the joint with the rate of synovial fluid turnover, i.e., with an apparent half-life of 2–16 h (19), which is in line with the apparent alpha half-life of LUB:1 of ~4.5 h.
We also hypothesize that the retention of LUB:1 in the terminal phase is related to ligand binding. While the ligands of lubricin remain to be established, recent data indicates that lubricin is likely to have a set of binding partners in the joint. Efficient and specific binding to the articular surface (the superficial zone) of cartilage explants was observed for extracted synovial and recombinant lubricin (11). Immunoprecipitation and mass spectrometry-based proteomic analysis identified a number of extracellular matrix components (type VI collagen, tenascin-C, aggrecan, fibronectin, COMP, vitronectin, and decorin) present at the surface of human cartilage which are able to bind to lubricin (Flannery et al., (12)). Several independent binding experiments also indicated that lubricin (SZP) binds to articular cartilage matrix components (fibronectin, hyaluronan, and collagen type I and II) (Schmid et al. and Elsaid et al., posters at the 48th and 53th Annual Meeting of the Orthopedic Research Society, respectively).
A derivative of hyaluronan (Synvisc), a potential binding partner of lubricin, is used in the clinic to treat pain associated with osteoarthritis in the knee joint and is given by either a single IA injection or by 3-weekly IA injections. The selective joint retention of hyaluronan is suggested both by preclinical and human clinical data. In rabbits, Synvisc was cleared from the joint much slower than it did from the systemic circulation, (half-life of ~8.8 and 1.5 days, respectively, versus ~30 min in circulation; Synvisc data sheet). In humans, the elimination kinetics of [131I]-labeled stabilized hyaluronan (non-animal stabilized hyaluronic acid) from the knee joint following a single IA dose, were described by three distinct phases, with alpha, beta, and gamma half-lives of 1.5 h, 1.5 days, and 4 weeks, respectively, and the hepatic radioactivity uptake was low (20). Thus, the disposition profile of stabilized hyaluronan is similar to the disposition profile reported here for radiolabeled recombinant lubricin.
It should be noted that since [125I]LUB:1 quantification in the knee was conducted by the counting of total knee radioactivity, it cannot be excluded that some or even major fraction of the counts are accounted for by lubricin degradation products or free iodine, in both early and late phases of the count–time profile in the knee. This is a common limitation of tissue distribution studies for protein therapeutics, as discussed by Vugmeyster et al. (16). However, several lines of evidence/reasoning suggest that considerable percentage of lubricin-associated radioactivity likely corresponds to biologically active lubricin at least during the later phases of the kinetic curve. First, smaller 125I-labeled degradation fragments, including “free” iodine, are more likely to cross semi-permeable synovial membrane and enter systemic circulation directly, (and be cleared from SF within minutes to hours). Second, in our study, radioactive signal tended to localize to damaged joint surfaces at 24 h and 7 days post a single injection. Third, LUB:1 had pharmacological activity in the rat MT model after weekly injections, albeit to a lesser extent than after 3 × per week injections (13). Fourth, when human synoviocyte lubricin was administered by IA injection of 10 μg twice a week (twofold lower dose that used in our studies) in a rat model of anterior cruciate ligament (ACL) injury (15), lubricin was detected by immunohistochemistry in rats receiving lubricin (with or without the concomitant injections of hyaluronic acid) but not in the saline-treated controls. Moreover, a significant reduction in cartilage degeneration score was observed in those lubricin-treated rats (with or without the concomitant injections of hyaluronic acid), but not in rats that were treated with hyaluronic acid alone or with saline (15). Finally, when a pain endpoint (assessed by weight bearing) was assessed in the MT model using 3 weekly 20 μg/knee doses, analgesic effects of LUB:1 were observed 13 days after the last dose (Rivera-Bermudez et al., poster at the 2012 World Congress on Osteoarthritis).
The rat meniscal tear model of PTA is a primary in vivo system for testing the ability of potential therapeutics to slow cartilage degradation and associated symptoms following acute injury. Further investigations are needed to establish a translation relation between this model and human OA. Specifically, the rat MT model may not accurately reflect the progression of human degenerative OA but rather reflects injury-induced OA (21), including differences in response to IA treatments. For example, IA hyaluronan was effective in a 40-month multicenter clinical trial (22), but not in a rat model of ACL injury (15).
Finally, the disposition profiles after a single IA injection and the dose–response data (including pharmacodynamic endpoints and minimum efficacious dose determination) in the MT rat model may be used to estimate a minimum mass of LUB:1 per knee (“Meff”) that is needed to achieve a significant pharmacological effect in this model. In our previously published study (13), only one dose level for IA dosing of LUB:1 to MT rats was used, albeit given by two different dosing regimens: (1) 4 weeks of three per week injections of 20 μg per knee and (2) 4 weeks of once per week injections of 20 μg per knee. LUB:1 administered by the first dosing regimen resulted in a significant pharmacological effect in all measured parameters, while LUB:1 given by the second dosing regimen ameliorated disease progression to a lesser extent. This limited pharmacology data, together with the pharmacokinetic simulations that are based on the assumption of linearity and the assumption that LUB:1 disposition profile in MT rats is similar to that in naïve rats, can be used to obtain a preliminary estimate (likely an upper bound) of Meff as 5.6 μg (approximately 1.6 × 10−2 nmole) per knee, using a model-independent approach (as described in the “Materials and Methods” section). However, for an accurate determination of Meff, additional dose–response information supported with the development of an appropriate mechanistic model that includes binding to a putative ligand is needed. Ultimately, an appropriate pharmacodynamic (PD) marker and a mechanistic PKPD model would need to be developed for an accurate projection of efficacious dosing regimen(s) in humans based on the disposition data presented in this report.
CONCLUSION
After a single IA injection of [125I]LUB:1 to naïve rats and in the rat model of PTA, a tri-phasic disposition profile was observed, with the alpha, beta, and gamma half-life estimates of 4.5 h, 1.5 days, and 2.1 weeks, respectively. We hypothesize that the terminal phase kinetics was related to [125I]LUB:1 binding to its ligands and micro-autoradiography analysis suggested that [125I]LUB:1 tended to localize to the target action site of damaged joint surfaces in this model.
Due to limitations in utilizing the 125I-labeling method to study disposition of protein therapeutics and biological differences between human OA and the rat MT model, the analyses presented in this study needs to be interpreted with caution. Additional dose–response studies, an appropriate pharmacodynamic marker, and a mechanistic PKPD model would need to be developed for an accurate projection of efficacious dosing regimen(s) in humans based on the disposition data presented in this report.
Acknowledgments
Authors thank David Defranco, Jennifer Spencer-Pierce, Cyndi Filliettaz, Adam Root, Tracey Blanchet, Vikram Patel, and Sonya Glasson for their assistance with these studies.
References
- 1.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52(6):1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 2.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17(1):110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 4.Su JL, Schumacher BL, Lindley KM, Soloveychik V, Burkhart W, Triantafillou JA, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20(3):149–157. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 5.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26(2):231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis res therapy. 2006;8(2):R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly–arthropathy–coxa vara–pericarditis syndrome. Nat Genet. 1999;23(3):319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 8.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis res therapy. 2010;12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Johnson LJ, Hsu HP, Spector M. Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res. 2007;25(7):873–883. doi: 10.1002/jor.20344. [DOI] [PubMed] [Google Scholar]
- 11.Jones AR, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res. 2007;25(3):283–292. doi: 10.1002/jor.20325. [DOI] [PubMed] [Google Scholar]
- 12.Flannery et al. 56th Annual Meeting of the Orthopedic Research Society. 2010: Paper 164.
- 13.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 14.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–2391. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39(1):164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vugmeyster Y, DeFranco D, Szklut P, Wang Q, Xu X. Biodistribution of [125I]-labeled therapeutic proteins: application in protein drug development beyond oncology. J Pharm Sci. 2010;99(2):1028–1045. doi: 10.1002/jps.21855. [DOI] [PubMed] [Google Scholar]
- 17.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthr Cartil. 2002;10(10):785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 18.Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr Cartil. 2005;13(7):623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Nemirovskiy O, Zheng YJ, Tung D, Korniski B, Settle S, Skepner A, et al. Pharmacokinetic/pharmacodynamic (PK/PD) differentiation of native and PEGylated recombinant human growth hormone (rhGH and PEG-rhGH) in the rat model of osteoarthritis. Xenobiotica foreign comp biol sys. 2010;40(8):586–592. doi: 10.3109/00498254.2010.488303. [DOI] [PubMed] [Google Scholar]
- 20.Lindqvist U, Tolmachev V, Kairemo K, Astrom G, Jonsson E, Lundqvist H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin Pharmacokinet. 2002;41(8):603–613. doi: 10.2165/00003088-200241080-00004. [DOI] [PubMed] [Google Scholar]
- 21.Findlay DM. If good things come from above, do bad things come from below? Arthritis res therapy. 2010;12(3):119. doi: 10.1186/ar3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro-Sarabia F, Coronel P, Collantes E, Navarro FJ, Rodriguez de la Serna A, Naranjo A, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Annals of the rheumatic diseases. 2011 Aug 17. [DOI] [PMC free article] [PubMed]