Abstract
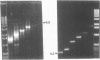
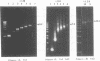
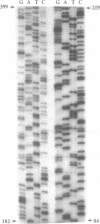
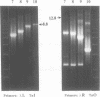
The 264 bp mini-transposon Tn5supF was constructed to sequence DNAs cloned in phage lambda without extensive shotgun subcloning or primer walking. Unique sequences near each transposon end serve as primer binding sites, and a supF gene is used to select transposition to lambda. We describe here PCR methods that facilitate Tn5supF-based sequencing. In a first pass, insertions are mapped relative to the ends of the cloned fragment using pairs of primers specific for vector DNA next to the cloning site and for a Tn5supF end. Most insertions not mapped in this step are near the center of the cloned fragment or in the vector arms, and are then mapped relative to the two innermost insertions by 'crossover' PCR. This involves amplification from primers on different DNA molecules, and generates hybrid DNA products whose lengths correspond to the distances between the two insertions. We routinely amplified more than 6 kb in direct PCR and 3 kb in crossover PCR; at the limit we amplified up to approximately 10 kb in direct PCR and approximately 6 kb in crossover PCR, but not reproducibly. Crossover PCR products were also obtained with insertions separated by only 200 bp, indicating that no rare sites are needed to switch templates. PCR products were purified by adsorption and then elution from glass slurry, and sequenced directly. Ladders of more than 400 bp were obtained from primer sites on each DNA strand; 2 kb was read from crossover PCR products, and showed that they were amplified with fidelity. In conclusion, direct and crossover PCR methods expedite transposon insertion mapping, and yield templates for accurate sequencing of both DNA strands.
Full text
PDF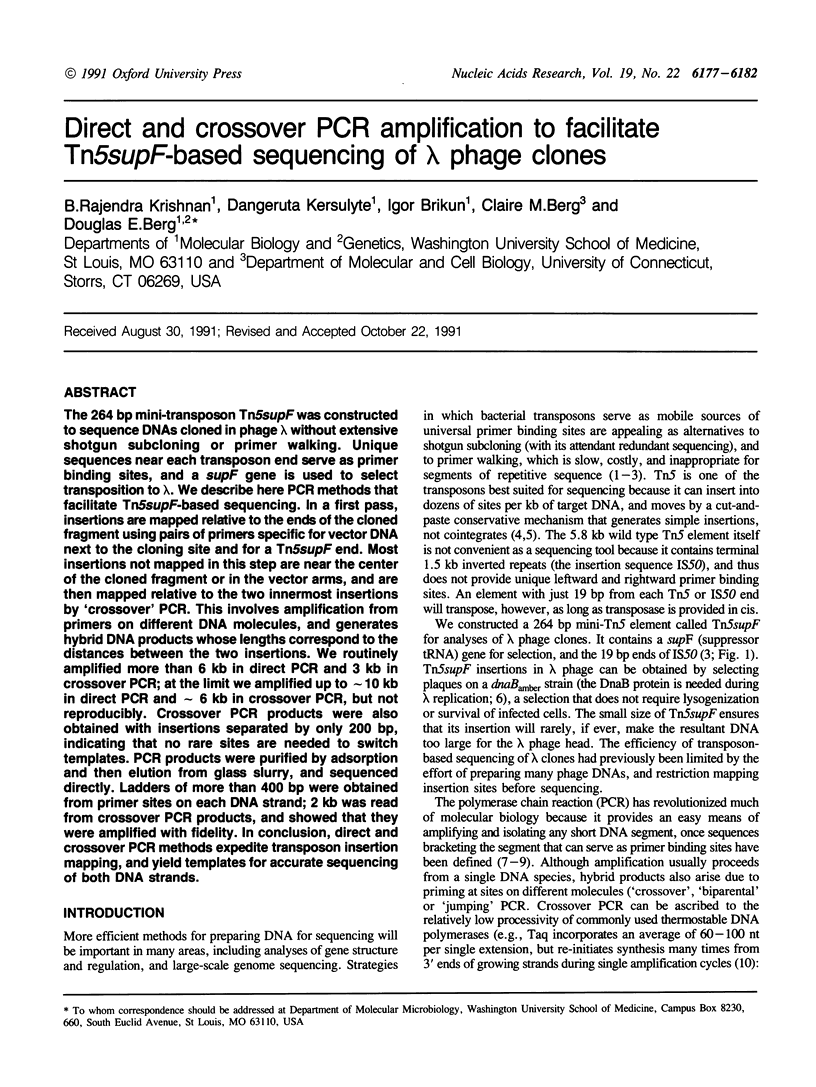




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brakenhoff R. H., Schoenmakers J. G., Lubsen N. H. Chimeric cDNA clones: a novel PCR artifact. Nucleic Acids Res. 1991 Apr 25;19(8):1949–1949. doi: 10.1093/nar/19.8.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley R. L., Jr, Smith L. M. Rapid DNA sequencing by horizontal ultrathin gel electrophoresis. Nucleic Acids Res. 1991 Aug 11;19(15):4121–4126. doi: 10.1093/nar/19.15.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty B. L., DeMartino J. A., Law M. F., Kawka D. W., Singer I. I., Mark G. E. Polymerase chain reaction facilitates the cloning, CDR-grafting, and rapid expression of a murine monoclonal antibody directed against the CD18 component of leukocyte integrins. Nucleic Acids Res. 1991 May 11;19(9):2471–2476. doi: 10.1093/nar/19.9.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Jansen R., Ledley F. D. Disruption of phase during PCR amplification and cloning of heterozygous target sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5153–5156. doi: 10.1093/nar/18.17.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman K., Fingar S. A., Shah J., Fyles J. Polymerase chain reaction-mediated gene synthesis: synthesis of a gene coding for isozyme c of horseradish peroxidase. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4084–4088. doi: 10.1073/pnas.88.10.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug J., Wolf M., Beato M. Creating chimeric molecules by PCR directed homologous DNA recombination. Nucleic Acids Res. 1991 May 25;19(10):2793–2793. doi: 10.1093/nar/19.10.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Blakesley R. W., Berg D. E. Linear amplification DNA sequencing directly from single phage plaques and bacterial colonies. Nucleic Acids Res. 1991 Mar 11;19(5):1153–1153. doi: 10.1093/nar/19.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulakauskas S., Wikström P. M., Berg D. E. Efficient introduction of cloned mutant alleles into the Escherichia coli chromosome. J Bacteriol. 1991 Apr;173(8):2633–2638. doi: 10.1128/jb.173.8.2633-2638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M., Seed B. Improved genetic selection for screening bacteriophage libraries by homologous recombination in vivo. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3166–3169. doi: 10.1073/pnas.87.8.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton A., Delbecchi L., Bourgaux P. DNA nicking favors PCR recombination. Nucleic Acids Res. 1991 May 11;19(9):2423–2426. doi: 10.1093/nar/19.9.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Kulakauskas S., Krishnan B. R., Hiemstra J., Berg D. E. Transposon Tn5supF-based reverse genetic method for mutational analysis of Escherichia coli with DNAs cloned in lambda phage. J Bacteriol. 1991 Jan;173(2):896–899. doi: 10.1128/jb.173.2.896-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Rychlik W., Spencer W. J., Rhoads R. E. Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res. 1990 Nov 11;18(21):6409–6412. doi: 10.1093/nar/18.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Taylor L. A., Rose R. E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988 Jan 11;16(1):358–358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolov A. A., Shabarova Z. A. Constructing DNA by polymerase recombination. Nucleic Acids Res. 1990 Jul 11;18(13):3983–3986. doi: 10.1093/nar/18.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]