Abstract
Methamphetamine interacts with sigma receptors at physiologically relevant concentrations suggesting a potential site for pharmacologic intervention. In the present study, a previous sigma receptor ligand, CM156, was optimized for metabolic stability, and the lead analog was evaluated against the behavioral effects of methamphetamine. Radioligand binding studies demonstrated that the lead analog, AZ66, displayed high nanomolar affinity for both sigma-1 and sigma-2 receptors (2.4 ± 0.63 and 0.51 ± 0.15, respectively). In addition, AZ66 had preferential affinity for sigma receptors compared to seven other sites and a significantly longer half-life than its predecessor, CM156, in vitro and in vivo. Pretreatment of male, Swiss Webster mice with intraperitoneal (10–20 mg/kg) or oral (20–30 mg/kg) dosing of AZ66 significantly attenuated the acute locomotor stimulatory effects of methamphetamine. Additionally, AZ66 (10–20 mg/kg, i.p.) significantly reduced the expression and development of behavioral sensitization induced by repeated methamphetamine administration. Taken together, these data indicate that sigma receptors can be targeted to mitigate the acute and subchronic behavioral effects of methamphetamine and AZ66 represents a viable lead compound in the development of novel therapeutics against methamphetamine-induced behaviors.
Key words: locomotor activity, methamphetamine, pharmacokinetics, sensitization, sigma receptors
INTRODUCTION
Methamphetamine is currently one of the most abused illicit substances worldwide (1,2). It is a highly addictive psychostimulant whose acute effects include hyperthermia, hypertension, severe cardiac pathologies, and potential convulsions (2,3). Prolonged abuse of methamphetamine can result in significant neurological changes that lead to addiction, drug seeking behavior, and eventual drug dependence (1–4). Currently, there is a lack of medications designed to treat either the detrimental physiologic effects of methamphetamine or the addiction liability associated with its use. There have been a substantial amount of clinical trials conducted with drug candidates aimed at treating the addictive properties of methamphetamine; however, at this time, there are still no FDA-approved pharmacotherapies for methamphetamine dependence (5). Innovative strategies are essential for the development of pharmaceutical agents intended to treat the negative effects of methamphetamine abuse.
Sigma receptors represent a novel target for the development of new therapeutics intended to treat methamphetamine-induced effects. There are currently two known subtypes of sigma receptors, designated sigma-1 and sigma-2. Sigma-1 receptors are 223-amino acid proteins that are distinct from any other known mammalian protein (6). Sigma-1 receptors have been cloned and have a high level of homology among differing species (6). They can translocate between intracellular compartments where they are able to modulate signaling pathways and intracellular calcium signaling, interact with specific ion channels, and affect neurotransmitter synthesis, release, and reuptake (7–13). Much less is known about sigma-2 receptors, as these receptors have yet to be cloned and truly selective sigma-2 receptor ligands are currently lacking. Sigma-2 receptors are believed to be involved in cellular survival and modulate calcium signaling through sphingolipid products (14–17).
Methamphetamine, in addition to interacting with monoamine transporters, interacts with both subtypes of sigma receptors at physiologically relevant concentrations (18). Repeated administrations of methamphetamine have been shown to increase binding of the sigma receptor ligand [3H](+)-pentazocine in the frontal cortex, substantia nigra, and cerebellum, indicative of an increase in sigma receptors in these regions of the brain (19). Notably, sigma-1 receptor protein or mRNA was upregulated in the midbrain and the hippocampus of rats trained to self-administer methamphetamine (20). These studies provide evidence that sigma receptors may, in part, be involved in the neuroadaptive changes that occur with repeated methamphetamine exposures.
The methamphetamine-induced changes in sigma receptor expression levels appear to have functional and behavioral consequences. Two different sigma receptor antagonists, MS-377 ((R)-(+)-1-(4-chlorophenyl)-3-[4-(2-methoxyethyl)piperazin-1-yl]methyl-2-pyrrolidinone l-tartrate) and BMY 14802 (α-(4-flourophenyl)-4-(5-flouro-2-pyrimidinyl)-1-piperazine), have been shown to prevent augmented responses to repeated methamphetamine administrations in rodents, using a paradigm known as behavioral sensitization (21,22). However, both of these compounds are unable to attenuate acute methamphetamine-induced behaviors (21,22). Additionally, the selective sigma receptor antagonists BD1047 ([2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(diamino)ethylamine), BD1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine), and AC927 (1-(2-phenethyl)piperidine oxalate) have been shown to attenuate many acute effects of methamphetamine, including methamphetamine-induced increases in locomotor activity and hyperthermia in rodent models (23). These studies indicate that a selective sigma receptor ligand may attenuate some of the acute effects of methamphetamine administration in addition to preventing the neurological and behavioral changes associated with repeated methamphetamine exposures. It is yet to be determined as to whether a preferential sigma receptor ligand can actually reverse, in addition to prevent, the behavioral changes associated with methamphetamine-induced behavioral sensitization.
The purpose of the present study was to determine if the preferential sigma receptor ligand, AZ66 (3-(4-(4-cyclohexylpiperazin-1-yl)pentyl)-6-flourobenzo[d]thiazol-2(3H)-one), is able to attenuate the acute behavioral effects of methamphetamine and, in addition, prevent the development and block the expression of methamphetamine-induced behavioral sensitization. AZ66 is an optimized sigma receptor ligand derived from a previously studied selective sigma receptor antagonist, CM156 (3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione), that has been reported to attenuate many cocaine-induced behaviors (24). Further drug development of CM156 was halted due to an exceedingly short half-life (24), and AZ66 was synthesized to avoid the metabolism issues associated with CM156. Herein, we report that AZ66, a novel sigma receptor preferring ligand, is capable of being administered orally and attenuates many methamphetamine-induced behaviors, including the development and expression of behavioral sensitization.
METHODS
Synthesis of AZ66
The synthetic scheme for AZ66 is shown in Fig. 1. Commercially available 4-fluoroaniline (1), hydrochloride was refluxed in water with NH4SCN for 4 h to afford compound 2 after recrystallization from ethanol. A solution of bromine in chloroform was added to compound 2 at 0°C and subsequently refluxed for 2 h to afford compound 3. Compound 3 was stirred with KOH to afford compound 4. Compound 4 was treated with carbonyl-1,1′-diimidazole under reflux for 3 h to provide the fluorinated benzothiazolone 5. Benzothiazolone 5 was alkylated with 1,4-dibromopentane in dimethylformamide (DMF) at 60°C over 3 h to afford compound 6. Compound 6 was reacted with commercially available cyclohexyl piperazine to afford AZ66. AZ66 was subsequently converted to the hydrochloride salt for biological studies. Elemental analysis (C, H, N) of AZ66 was determined using a Perkin-Elmer CHN/SO Series II Analyzer. Obtained results were within 0.4% of the theoretical values.
Fig. 1.
Synthesis of AZ66. Reagents and conditions: a NH4SCN, H2O, reflux, 4 h; b Br2, CHCl3, 1 h at 0°C, reflux, 2 h; c KOH; d Gl. acetic acid; e carbonyl 1,1′ diimidazole, THF, reflux, 3 h; f 1,4-dibromopentane, K2CO3, DMF, 60°C, 3 h; g cyclohexyl piperazine, K2CO3, TBAI, ACN, reflux, 6 h
Drugs and Reagents
Methamphetamine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO, USA). Radioligands for binding studies were purchased from Perkin-Elmer (Boston, MA, USA). Other chemicals which were used for the radioligand binding studies were obtained from standard commercial sources (Sigma-Aldrich, St. Louis, MO, USA).
Animals
Male, Swiss Webster mice (21–30 g, Harlan, Indianapolis, IN, USA; Frederick, MD, USA) were used in the present experiments. The mice were housed in groups of four to six with a 12:12-h light/dark cycle and ad libitum food and water. Each cage was made from polysulfone and provided 542 cm2 of floor space (Tecniplast, Philadelphia, PA, USA), which was covered with corn cob bedding and packing material (ULINE, Waukegan, IL, USA). The mice were acclimated for 1 week before being used in experiments, and they were randomly assigned to their treatment groups. Two different shipments of mice were used for each experimental group, regardless of the sample size. Male, Sprague Dawley rats were used for the pharmacokinetic studies. All procedures were performed as approved by the Institutional Animal Care and Use Committees at the West Virginia University Health Sciences Center and the University of Mississippi.
Radioligand Binding Studies
The radioligand binding assays were preformed in rat brain homogenates using methods previously described in detail (25,26). Briefly, sigma-1 receptors were labeled with 5 nM [3H](+)-pentazocine, and sigma-2 receptors were labeled with 3 nM [3H]di-o-tolylguanidine in the presence of 300 nM (+)-pentazocine to block sigma-1 receptors. Nonspecific binding was determined in the presence of 10 μM haloperidol. All of the assays were terminated with the addition of ice-cold buffer and rapid vacuum filtration over glass fiber filters. Separate synthetic batches of AZ66 were used in this study, and their Ki values were averaged together to obtain a mean binding affinity.
In Vitro Metabolism Studies
In order to confirm AZ66 had an increased metabolic stability compared to its parent compound, CM156, in vitro metabolism studies were conducted. AZ66 was evaluated in liver microsomes prepared from rat using standard procedures. AZ66 (5 μM) was incubated at 37°C in 50 mM potassium phosphate buffer (pH 7.4), 3 mM MgCl2, and 1 mM EDTA (pH 7.4), in the presence and absence of cofactor, NADPH-generating system [1 mM NADP (pH 7.4), 5 mM glucose 6-phosphate (pH 7.4), and 1 U/ml glucose-6-phosphate dehydrogenase]. Reactions were initiated by adding the cofactor mix and terminated at designated time points (0, 15, 30, and 60 min) with the addition of stop reagent (adding equal volume of ice-cold acetonitrile). The samples were centrifuged 10,000 rpm for 10 min at 4°C. The supernatants were collected and analyzed using ultra performance liquid chromatography (UPLC)/mass spectrometry/mass spectrometry.
The UPLC system consisted of a Waters Acquity UPLC (Milford, MA, USA) equipped with a binary solvent manager, vacuum degasser, and an autosampler. Chromatographic separations were performed on an Atlantis dC18 column (2.1 × 50 mm). The mobile phase consisted of 10 mM ammonium acetate containing 0.1% acetic acid and methanol (25:75, v/v) and pumped at a flow rate of 0.18 ml/min. The injection volume was 10 μl. A Micromass Quattro Micro™ system (Waters Corp., Manchester, UK) equipped with electrospray ionization source was used for the mass spectrophotometric detection. The electrospray ionization source was operated in positive ionization mode. The acquisitions were performed using multiple reaction monitoring. The mass transitions chosen for quantitation were m/z 405 → m/z 181 for AZ66 and m/z 448 → m/z 285 for aripiprazole (internal standard). The mass spectrophotometer parameters were optimized for maximum analyte detection: capillary voltage 4.88 kV, cone voltage 44 V, and argon was used as the collision gas at 3.5 × 10−3 Pirani. Collision energies were set at 30 and 21 eV for AZ66 and aripiprazole, respectively.
In Vivo Metabolism Studies
To further evaluate the metabolic stability of AZ66, pharmacokinetic studies were performed in vivo. Briefly, blood samples from male, Sprague Dawley rats (n = 5) outfitted with indwelling catheters in the jugular vein were collected. An initial blood volume of 0.1 ml was withdrawn to clear the line. A fresh syringe was used to withdraw a 0.15-ml blood sample. This and all subsequent blood samples were placed in heparinized microfuge tubes at 0°C. The rats were administered 20 mg/kg AZ66 orally (p.o.). Timed blood samples were then collected for up to 36 h. For each blood sample, plasma was separated by centrifugation at 10,000 rpm for 10 min at 4°C. AZ66 was quantified using ultra performance liquid chromatography/mass spectrometry/mass spectrometry as described above.
Locomotor Activity
Locomotor activity was measured as an index of the stimulant effects of methamphetamine using an automated activity monitoring system (San Diego Instruments, San Diego, CA, USA). The mice were given 30–60 min to acclimate to the testing room before being habituated to the testing chambers for an additional 30 min. Each testing chamber consisted of a 16 × 16 photobeam array to detect lateral movements and a separate 16 photobeam array to detect rearing by the animals. Ambulatory, fine, and rearing movements were quantified to give an overall locomotor activity score.
Mice (n = 24) were divided randomly into four treatment groups for the dose–response study. They received a pretreatment of saline 15 min prior to a dose of methamphetamine (0.1–5.0 mg/kg, i.p.). The mice were then returned to the testing chambers, and their activity was quantified for 30 min.
For the acute locomotor studies, mice (n = 65) were randomly assigned into a treatment group and pretreated with either saline or AZ66 (0–20 mg/kg, i.p.), followed 15 min later by either saline or a stimulant dose of methamphetamine (1 mg/kg, i.p.). The mice were then returned to the testing chambers, and their activity was quantified for the next 30 min. The evaluation of AZ66 in combination with saline or methamphetamine allowed the determination of the effects of AZ66 alone, as well as its ability to mitigate the stimulant actions of methamphetamine.
For the oral administration studies, mice (n = 42) were pretreated by oral gavage (p.o.) with either distilled water (0.1 ml/10 g) or AZ66 (20–30 mg/kg), and locomotor activity was recorded for 30 min after receiving an i.p. injection of either a stimulant dose of methamphetamine (1 mg/kg) or saline (0.1 ml/10 g). Pretreatment time was 120 min, based on the pharmacokinetic parameters (Tmax) of AZ66.
Behavioral Sensitization
The mice were acclimated to the treatment room for 60 min and then individually to a testing chamber for 30 min on each day. Locomotor activity was monitored on days 1, 2, 3, 4, 5, 6, 7, and 15 for 30 min immediately after the treatments by using an automated activity monitoring device (San Diego Instruments, San Diego, CA, USA).
For the dose–response study, mice (n = 12) were randomly assigned to one of four treatment groups. On days 1–7, mice received saline pretreatment followed 15 min later by either saline or methamphetamine (0.1, 0.5, or 1 mg/kg, i.p.). After an 8-day drug-free period, which allowed the drugs and their metabolites to be washed out, mice were administered either saline or methamphetamine (0.1, 0.5, and 1 mg/kg, i.p.).
For the development of sensitization studies, mice (n = 36) were randomly assigned to one of six treatment groups as shown in Table I and injected i.p. once a day for seven consecutive days. The two injections making up each treatment were separated by a 15-min pretreatment period; the dose of methamphetamine used was 1 mg/kg. Treatments on days 1 to 7 were followed by an 8-day drug-free period, and then all of the mice were challenged on day 15 with methamphetamine (1 mg/kg i.p.).
Table I.
Treatment Schedule for Sensitization Experiments
| Development of sensitization | Expression of sensitization | ||||||
|---|---|---|---|---|---|---|---|
| Group | Days 1–7 | Days 8–14 | Day 15 | Group | Days 1–7 | Days 8–14 | Day 15 |
| 1 | Sal + Sal | NT | Sal + Meth | 1 | Sal + Sal | NT | Sal + Meth |
| 2 | Sal + Meth | NT | Sal + Meth | 2 | Sal + Sal | NT | AZ(10 mg/kg) + Meth |
| 3 | AZ (10 mg/kg) + Meth | NT | Sal + Meth | 3 | Sal + Sal | NT | AZ (20 mg/kg) + Meth |
| 4 | AZ (20 mg/kg) + Meth | NT | Sal + Meth | 4 | Sal + Meth | NT | Sal + Meth |
| 5 | AZ (10 mg/kg) + Sal | NT | Sal + Meth | 6 | Sal + Meth | NT | AZ (10 mg/kg) + Meth |
| 6 | AZ (20 mg/kg) + Sal | NT | Sal + Meth | 7 | Sal + Meth | NT | AZ (20 mg/kg) + Meth |
n = 6–10/group. All compounds were administered intraperitoneally
NT no treatment, Meth methamphetamine (1 mg/kg), Sal saline, AZ AZ66
For the expression of sensitization studies, mice (n = 42) were randomly assigned to one of six treatment groups as shown in Table I and injected once a day (i.p.) for seven consecutive days with either saline or methamphetamine (1 mg/kg, i.p.). After an 8-day drug-free period, pretreatment with AZ66 (10–20 mg/kg, i.p.) or saline was administered 15 min prior to methamphetamine (1 mg/kg, i.p.).
Statistical Analyses
The data from the radioligand binding assays were analyzed using GraphPad Prism 4.0 (San Diego, CA, USA) to calculate IC50 values. Apparent Ki values were then calculated using the Cheng–Prusoff equation and Kd values determined in separate saturation assays (27). The data from the acute locomotor activity measurements, development, and expression of sensitization studies were analyzed using GraphPad Prism 4.0 (San Diego, CA, USA) using an analysis of variance (ANOVA). Significant ANOVAs were followed by post hoc Dunnett’s tests for comparisons to control or Bonferroni’s tests for other pairwise comparisons. p < 0.05 was considered statistically significant for all behavioral tests.
RESULTS
Competition Binding Assays
The average binding affinities of AZ66 for sigma-1 and sigma-2 receptors, in addition to seven other sites, are listed in Table II. AZ66 bound to both sigma-1 and sigma-2 receptors with nanomolar affinities (batch 1 had a sigma-1 affinity of 1.20 ± 0.15 nM and a sigma-2 affinity of 0.31 ± 0.09 nM; batch 2 had a sigma-1 affinity of 3.50 ± 0.13 nM and a sigma-2 affinity of 0.66 ± 0.15 nM). In addition, AZ66 had a >200-fold preference for sigma receptors compared to the other sites tested. However, AZ66 displayed a moderate to low affinity for the serotonin transporter, 5-HT2 receptor, dopamine (D2) receptor, and dopamine transporter.
Table II.
Binding Affinities of AZ66 for σ Receptors, Monoamine Transporters, and Non-sigma Sites
| Radioligand | Nonspecific binding | Tissue | K i | |
|---|---|---|---|---|
| Sigma receptors | ||||
| σ 1 | 5 nM [3H](+)-pentazocine | 10 μM haloperidol | Rat brain | 2.4 ± 0.63 |
| σ 2 | 3 nM [3H]di-o-tolylguanidine | 10 μM haloperidol | Rat brain | 0.51 ± 0.15 |
| Monoamine transporters | ||||
| Dopamine | 0.5 nM [3H]WIN 35,428 | 50 μM cocaine | Rat striatum | 872 ± 122 |
| Serotonin | 0.2 nM [3H]paroxetine | 1.5 μM imipramine | Rat brainstem | 612 ± 44 |
| Norepinephrine | 0.5 nM [3H]nisoxetine | 4 μM desipramine | Rat cerebral cortex | >10,000 |
| Non-sigma sites | ||||
| Opioid | 1 nM [3H]naloxone | 1 μM naloxone | Rat brain | >10,000 |
| 5-HT2 | 2 nM [3H]ketanserin | 1 μM mianserin | Rat brain | 535 ± 51 |
| Dopamine (D2) | 5 nM [3H](−)-sulpiride | 1 μM haloperidol | Rat brain | 1183 ± 272 |
| NMDA/PCP | 5 nM [3H]TCP | 10 μM cyclazocine | Rat brain | >10,000 |
Affinities (K i in nanomolars) were determined in rat brain. The values in this table represent the mean ± SEM from replicate assays
NMDA N-methyl-d-aspartic acid, PCP phencyclidine, TCP tenocyclidine
In Vitro Metabolism Studies
The in vitro half-life of AZ66 in rat liver microsomes was 115 ± 5 min, significantly higher than its predecessor CM156 (5 min).
In Vivo Metabolism Studies
The pharmacokinetic parameters associated with oral dosing of AZ66 (20 mg/kg) were area under the curve (158.22 ± 2.79 μg h/ml); half-life, T1/2 (8.79 ± 0.10 h); maximum concentration, Cmax (0.30 ± 0.02 μg/ml); mean residence time (8.82 ± 0.24 h); volume of distribution, Vd (78.06 ± 0.85 l/kg); apparent oral clearance at steady state, Clss/F (6.15 ± 0.11 l/h/kg); and time to reach maximum concentration, Tmax (2.00 ± 0.22 h). The oral bioavailability was 58.17%.
Locomotor Activity
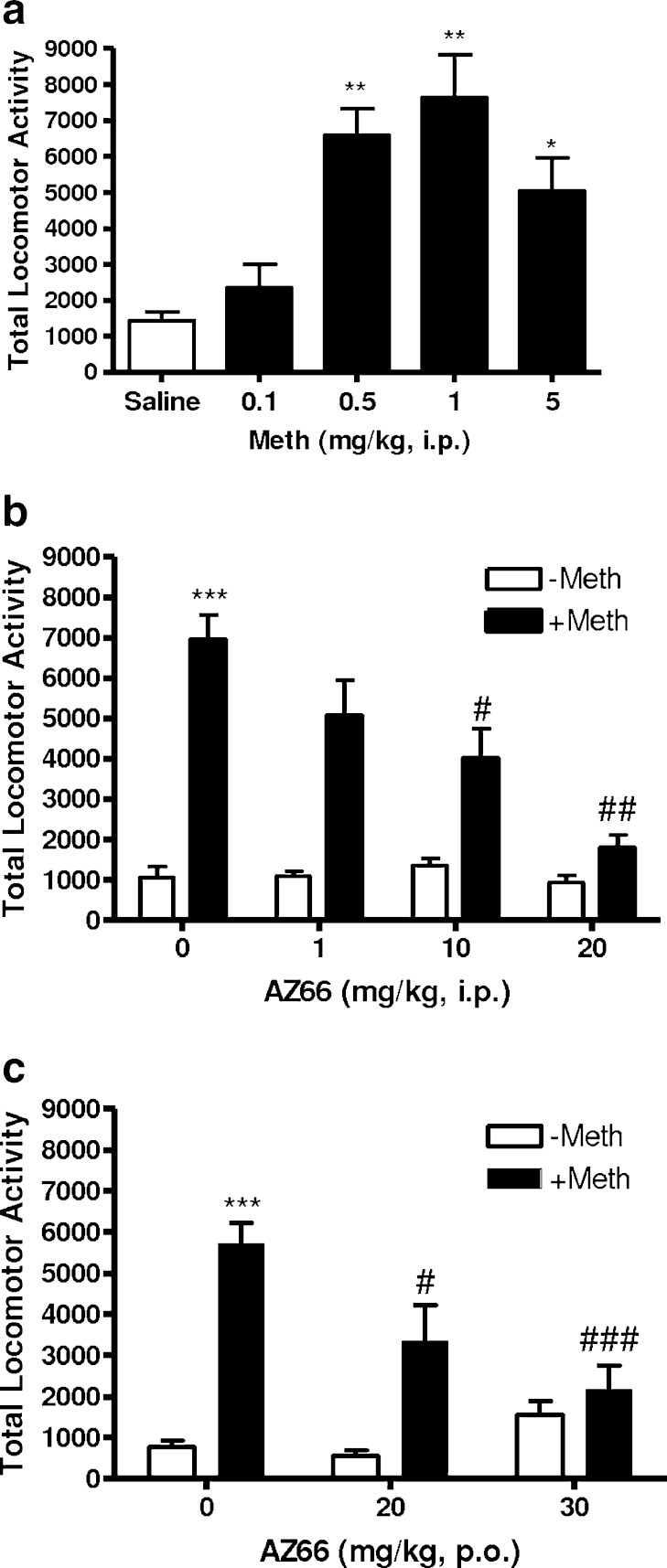
Methamphetamine produced an inverted U-shaped dose–response for locomotor activity (Fig. 2a); peak locomotor stimulant effects were observed at 1 mg/kg, which was thereafter administered as the challenge dose of methamphetamine for further studies. One-way ANOVA confirmed that the differences between methamphetamine doses were statistically significant (F(4, 29) = 10.92, p < 0.0001). Post hoc analysis using Dunnett’s multiple comparison tests revealed that three doses of methamphetamine (0.5, 1.0, and 5.0 mg/kg, i.p.) were significantly different from the saline control (Fig. 2a).
Fig. 2.
a Dose-dependent effects of methamphetamine on basal locomotor activity. Male, Swiss Webster mice (n = 6 per group) were injected with saline or methamphetamine (Meth, 0.1, 0.5, 1, and 5 mg/kg, i.p.), and their activity was quantified for 30 min. Methamphetamine caused an inverted U-shaped dose–response on locomotor activity. Data are reported as mean ± SEM. *p < 0.05, **p < 0.01 vs. saline, post hoc Dunnett’s test. b Effects of AZ66 on locomotor activity following methamphetamine treatment. Male, Swiss Webster mice (n = 6–10 per group) were pretreated with saline or AZ66 (1, 10, and 20 mg/kg, i.p.) 15 min prior to saline (0 mg/kg AZ66, white bars) or methamphetamine (1 mg/kg, i.p., black bars) administration. AZ66 significantly attenuated the locomotor stimulant effects of methamphetamine. Data are reported as mean ± SEM. ***p < 0.001 vs. saline; #p < 0.05, ##p < 0.01 vs. Meth, post hoc Bonferroni’s test. c Effects of orally administered AZ66 on methamphetamine-induced increases in locomotor activity. Mice (n = 6–10 per group) pretreated with oral administration of distilled water (0 mg/kg AZ66) were challenged (i.p.) with either a stimulant dose of methamphetamine (1 mg/kg, i.p., black bars) or saline (white bars); all other animals pretreated with oral dosing with AZ66 (20 or 30 mg/kg) were challenged with methamphetamine (1 mg/kg, i.p., black bars) or saline (white bars). Animals received distilled water or AZ66 120 min prior to methamphetamine administration. Methamphetamine-induced increases in locomotor activity were significantly attenuated by pretreatment with 20 and 30 mg/kg, p.o. AZ66. Data are reported as mean ± SEM. ***p < 0.001 vs. saline; #p < 0.05, ###p < 0.001 vs. Meth, post hoc Bonferroni’s test
Pretreatment with AZ66 prior to a stimulant dose of methamphetamine significantly attenuated methamphetamine-induced hyperactivity (Fig. 2b). One-way ANOVA confirmed a significant difference between treatment groups (F(4, 46) = 12.81, p < 0.0001). Post hoc analysis using Bonferroni’s multiple comparison tests revealed a significant attenuation of methamphetamine-induced hyperactivity at two doses of AZ66 (10 and 20 mg/kg, i.p.). In the absence of methamphetamine, AZ66 had no significant effect on locomotor activity (Fig. 2b).
To further evaluate the drug-like characteristics of AZ66, oral administration studies were conducted (Fig. 2c). One-way ANOVA confirmed that there was a significant difference between all treatment groups (F(5, 41) = 19.01, p < 0.0001). Post hoc analysis using Bonferroni’s multiple comparison tests revealed a significant attenuation of methamphetamine-induced hyperactivity at both doses of AZ66 (20 and 30 mg/kg, p.o.). Similar to the i.p. studies, AZ66 displayed no significant effects in the absence of methamphetamine compared to saline-treated mice (Fig. 2c).
Behavioral Sensitization
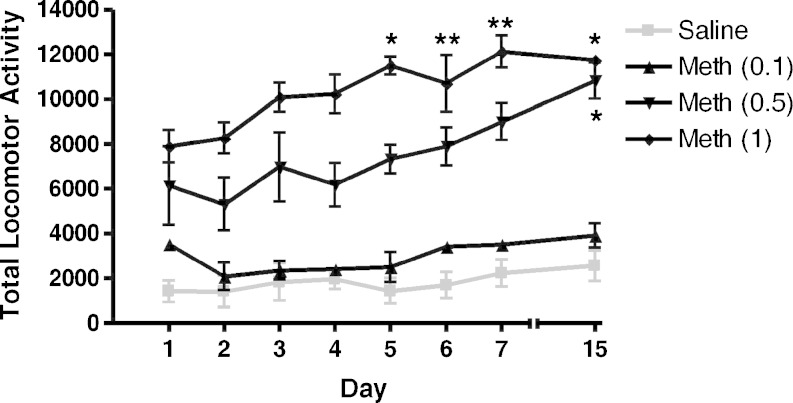
When a dose–response experiment was conducted to determine the optimal dose of methamphetamine needed to produce behavioral sensitization (Fig. 3), methamphetamine dose-dependently increased locomotor activity on days 1–7, on and remained significantly higher on the challenge day (day 15) when compared to day 1. ANOVA confirmed a significant difference between the experimental groups on all of the treatment days (F(3, 31) = 162, p < 0.0001). Repeated administration of methamphetamine on days 1–7 resulted in behavioral sensitization, which was measured as an enhanced response to methamphetamine on day 15 (0.5 mg/kg, q = 2.94, p < 0.05; 1.0 mg/kg, q = 3.15, p < 0.05; post hoc Dunnett’s test). Based on the larger increases in total locomotor activity, 1 mg/kg of methamphetamine was used as the challenge dose in subsequent behavioral sensitization studies.
Fig. 3.
Dose-dependent effects of methamphetamine on behavioral sensitization. Male, Swiss Webster mice (n = 12 per group) were injected with saline or methamphetamine (Meth; 0.1, 0.5, and 1 mg/kg, i.p.) on days 1–7. Following an 8-day drug-free period, the animals were challenged with either saline or methamphetamine (0.1, 0.5, and 1 mg/kg, i.p.) on day 15. Repeated administration of methamphetamine (0.5 and 1 mg/kg) on days 1–7 resulted in behavioral sensitization on day 15. Data are reported as mean ± SEM. *p < 0.05, **p < 0.01 vs. Meth (day 1), post hoc Dunnett’s tests
Development of Behavioral Sensitization
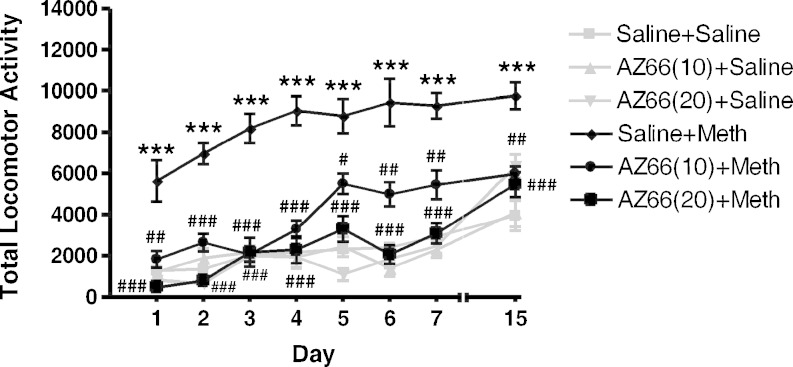
ANOVA demonstrated a significant difference between the treatment groups on all treatment days: day 1 (F(5, 35) = 16.89, p < 0.0001), day 2 (F(5, 35) = 43.95, p < 0.0001), day 3 (F(5, 35) = 25.98, p < 0.0001), day 4 (F(5, 35) = 27.30, p < 0.0001), day 5 (F(5, 35) = 27.55, p < 0.0001), day 6 (F(5, 35) = 25.21, p < 0.0001), day 7 (F(5, 35) = 29.56, p < 0.0001), and day 15 (F(5, 35) = 13.00, p < 0.0001). Post hoc Dunnett’s test confirmed that methamphetamine-treated mice displayed a significantly higher locomotor activity in comparison to the saline-treated controls for days 1–7. Additionally, with the exception of the methamphetamine-treated group that demonstrated an elevated locomotor response on days 1–7, none of the other groups had significantly different locomotor activity as compared to the saline control group for any of the treatment days. Pretreatment with AZ66 (10 and 20 mg/kg, i.p.) before methamphetamine administration on days 1–7 significantly attenuated methamphetamine-induced increases in locomotor activity compared to saline + methamphetamine-treated mice. On the challenge day (day 15), when each of the groups were administered methamphetamine, mice that received methamphetamine on days 1–7 exhibited a significantly higher locomotor activity than mice that received saline on days 1–7 (t = 7.22, p < 0.001), indicative of behavioral sensitization (Fig. 4). Mice that received AZ66 as a pretreatment to methamphetamine on days 1–7 demonstrated a significantly lower locomotor count compared to mice who received saline prior to methamphetamine on days 1–7: AZ66 10 mg/kg (t = 4.39, p < 0.01) and AZ66 20 mg/kg (t = 5.03, p < 0.001) (Fig. 4).
Fig. 4.
Effects of AZ66 on the development of behavioral sensitization to methamphetamine. Male, Swiss Webster mice were injected (i.p.) with either saline or AZ66 (10 or 20 mg/kg) followed 15 min later with either saline or methamphetamine (Meth; 1 mg/kg) once a day for 7 days. Following an 8-day drug-free period (day 15), all of the mice were injected (i.p.) with saline, followed 15 min later with methamphetamine (1 mg/kg). Pretreatment with AZ66 (10 or 20 mg/kg) on days 1–7 significantly blocked the development of sensitization. Data are reported as mean ± SEM. ***p < 0.001 vs. saline; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Meth, post hoc Bonferroni’s tests
Expression of Behavioral Sensitization
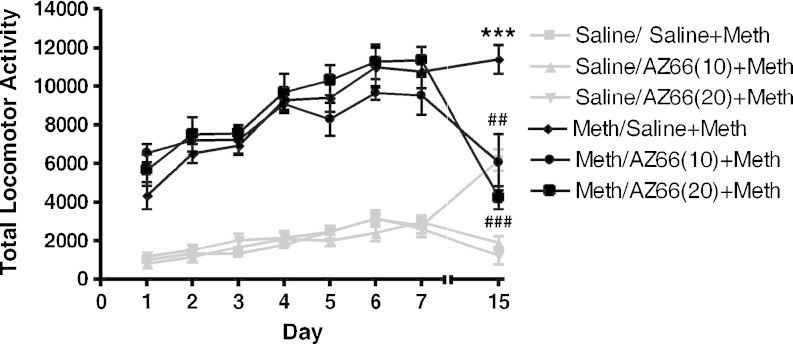
ANOVA confirmed a significant difference between the methamphetamine-treated mice and saline-treated mice on days 1–7: day 1 (F(5, 41) = 23.67, p < 0.0001), day 2 (F(5, 41) = 43.07, p < 0.0001), day 3 (F(5, 41) = 60.16, p < 0.0001), day 4 (F(5, 41) = 54.48, p < 0.0001), day 5 (F(5, 41) = 43.45, p < 0.0001), day 6 (F(5, 41) = 36.70, p < 0.0001), and day 7 (F(5, 41) = 40.05, p < 0.0001). On the challenge day (day 15), there was still a significant difference between the treatment groups (F(5, 41) = 25.34, p < 0.0001), and pretreatment with AZ66 significantly attenuated hyperactivity in mice that were sensitized (methamphetamine treatments on days 1–7). Post hoc Bonferroni’s tests confirmed that pretreatment with both doses of AZ66 prior to methamphetamine administration on day 15 caused a significant attenuation of methamphetamine-induced hyperactivity in mice receiving saline on days 1–7 (10 mg/kg, t = 4.03, p < 0.01; 20 mg/kg, t = 4.67, p < 0.001) and mice receiving methamphetamine on days 1–7 (10 mg/kg, t = 5.03, p < 0.001; 20 mg/kg, t = 6.75, p < 0.001). AZ66 pretreatment also attenuated acute increases in mice only receiving methamphetamine on day 15 (saline treatments on days 1–7) (10 mg/kg, t = 6.15, p < 0.001; 20 mg/kg, t = 7.12, p < 0.001). Both doses of AZ66 were shown to have no significant effects in the absence of methamphetamine compared to saline-treated mice (Fig. 5).
Fig. 5.
Effects of AZ66 on the expression of behavioral sensitization to methamphetamine. Male, Swiss Webster mice were injected (i.p.) with saline or methamphetamine (Meth; 1 mg/kg) once a day for 7 days (days 1–7). Following an 8-day drug-free period (day 15), mice were injected (i.p.) with saline or AZ66 (10 or 20 mg/kg), followed 15 min later with methamphetamine (1 mg/kg, Meth). Pretreatment with AZ66 (10 or 20 mg/kg) on day 15 significantly blocked the expression of sensitization. Data are represented as mean ± SEM. ***p < 0.001 day 15 vs. day 1 and ##p < 0.01, ###p < 0.001 day 15 vs. methamphetamine sensitized group (Meth/Saline + Meth), post hoc Bonferroni’s tests
DISCUSSION
Previously studied sigma receptor ligands attenuate many of the behavioral effects of methamphetamine administration in rodent models (18,21,22,26,28). Earlier studies have focused on the acute locomotor stimulant effects of methamphetamine in addition to the prevention of methamphetamine-induced behavioral sensitization. This study is the first to our knowledge showing that an optimized, orally bioavailable sigma receptor ligand attenuates the expression of methamphetamine-induced behavioral sensitization, in addition to mitigating the acute locomotor stimulant effects of methamphetamine and preventing the development of methamphetamine-induced behavioral sensitization.
Radioligand binding studies demonstrated that AZ66 displays high nanomolar affinities for both sigma-1 and sigma-2 receptors and a >200-fold preference for sigma receptors compared to any other site tested. These values are significant in that AZ66 represents an orally active, metabolically optimized ligand for further drug development that retains sigma receptor affinity and selectivity. More importantly, in addition to retaining its affinity and selectivity as compared to its parent compound CM156, it appears to have enhanced pharmacologic activity.
In this study, both i.p. injection and p.o. dosing of AZ66 attenuated methamphetamine-induced increases in acute locomotor activity. Additionally, both doses of AZ66 attenuated methamphetamine-induced locomotor activity while producing no effects on their own, a characteristic that is indicative of antagonist-like properties. This is consistent with past reports of sigma receptor antagonists attenuating these behaviors (18,23,26). Data from the present study support a role for sigma receptors in these effects and also show that a sigma receptor preferring compound administered orally can mitigate methamphetamine-induced hyperactivity. These protective effects are likely to involve both subtypes of sigma receptors; however, being that AZ66 has mixed affinity for both subtypes, the determination as to which subtype is responsible for the actions presented here cannot conclusively be made. Nevertheless, antisense oligonucleotide studies have shown that sigma-1 receptors play a role in the acute locomotor stimulant effects of methamphetamine (18). Knockdown of sigma-1 receptors mitigate the locomotor stimulant effects of methamphetamine, and similar results are obtained through the use of sigma receptor antagonists (18). However, sigma-1 receptor knockout mice still respond to methamphetamine suggesting a compensatory role for sigma-2 receptors (29), which is consistent with the reported ability of sigma-2 receptor agonists to produce motor activating effects (30). It is thus likely that AZ66 acts as a sigma receptor antagonist at both subtypes to block the acute hyperactivity induced by methamphetamine.
AZ66 also prevents the development of methamphetamine-induced behavioral sensitization. These results demonstrate the ability of AZ66 to block the neurological changes associated with repeated administration of psychostimulants such as methamphetamine. Repeated treatment with methamphetamine causes long-lasting neuroadaptations within regions of the brain associated with reward (31). Behavioral sensitization is a quantifiable measure of these neuroadaptations, and in this study, AZ66 attenuated the development of methamphetamine-induced behavioral sensitization. These results suggest that sigma receptors play a role in the establishment of sensitization and AZ66 may provide protective effects by blocking the evolution of neuroadaptations necessary for the development of behavioral sensitization. Neurons located in the ventral tegmental area (VTA) are believed to be responsible for the development of sensitization, and sigma receptors are widely distributed in the VTA (32). A number of other sigma receptor ligands have previously been shown to attenuate the development of sensitization to psychostimulants (21,22,24,33,34); however, the mechanism by which these events occur remains largely unknown.
Sigma receptors are involved in several aspects of neuroplasticity, including neurite outgrowth, and sigma receptor ligands modulate these processes (35–37). The neurologic changes associated with augmented locomotor responses to repeated administrations of methamphetamine may occur through neuroplasticity mediated in part by sigma receptors. Sigma receptors modulate dopaminergic neurotransmission in a variety of ways through protein kinase C, calcium/calmodulin, and calcium signaling (38,39). Another potential mechanism by which sigma receptors may modulate neuroplasticity is through the phosphorylation of CREB (40). CREB phosphorylation has been shown to be altered in discrete regions of the brain following methamphetamine-induced behavioral sensitization (41); however, the exact way in which this relates to behavioral consequences has yet to be determined. Other studies demonstrate close interactions between sigma receptors and the dopaminergic system in regions of the brain responsible for motor function, as well as sites likely responsible for the development of methamphetamine-induced behavioral sensitization (21,32,42–45).
In addition to blocking the development of behavioral sensitization, AZ66 significantly reversed the expression of methamphetamine-induced sensitization. Expression of sensitization is a clinically relevant behavioral paradigm that measures neuroadaptations that have already occurred after repeated methamphetamine exposures (46). AZ66 blocked the expression of sensitization when administered on the challenge day (day 15), further implicating a role for sigma receptors in sensitization. While neurons located in the VTA are important for the development of sensitization, the nucleus accumbens (NAc) is an essential region responsible for the expression of sensitization (47). Sigma receptor ligands have been shown to modulate dopamine uptake in the NAc (48), and sigma receptors may play a role in the reinforcing effects of drugs of abuse by modulating dopamine levels in the NAc (49). The ability of AZ66 to reverse the effects are most likely due to sigma receptor modulation of both dopamine transporters and dopamine levels in motor and limbic areas of the brain, specifically the NAc. Taken together, sigma receptors appear to have a modulatory role in both the development and expression of methamphetamine-induced sensitization.
The majority of clinical research centered on drug addiction and dependence focuses on the removal of physical dependence and withdrawal syndromes, neglecting the core symptoms of addiction and molecular etiology of the disease. Sigma receptors represent a novel strategy to understanding the molecular basis of drug addiction and may represent a plausible site for pharmacological intervention.
CONCLUSION
Data from radioligand binding studies and metabolism studies demonstrate that AZ66 is a sigma receptor preferring ligand with a favorable pharmacological profile. Moreover, AZ66 provides protection against acute and adaptive behavioral effects of methamphetamine while having no effects on its own. Although additional studies need to be conducted in order to further characterize AZ66 as a potential medication, the data presented herein support a role for sigma receptors in the acute and subchronic behavioral effects of methamphetamine.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (DA013978 and DA023205).
References
- 1.Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- 2.Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan KC, Lin YF, Yu FC, Lin CS, Chu P. Clinical manifestations and prognostic features of acute methamphetamine intoxication. J Formos Med Assoc. 1998;97:528–533. [PubMed] [Google Scholar]
- 4.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (σ(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Maurice T, Su TP. Ca2+ signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- 10.Booth RG, Baldessarini RJ. (+)-6,7-Benzomorphan sigma ligands stimulate dopamine synthesis in rat corpus striatum tissue. Brain Res. 1991;557:349–352. doi: 10.1016/0006-8993(91)90159-S. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron R, Debonnel G, De Montigny C. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- 12.Gronier B, Debonnel G. Involvement of sigma receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. Eur J Pharmacol. 1999;368:183–196. doi: 10.1016/S0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 13.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/S0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 14.Vilner BJ, de Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci. 1995;15:117–134. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharmacol Exp Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- 16.Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–322. [PubMed] [Google Scholar]
- 17.Crawford KW, Coop A, Bowen WD. σ(2) Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur J Pharmacol. 2002;443:207–209. doi: 10.1016/S0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Itzhak Y. Repeated methamphetamine-treatment alters brain sigma receptors. Eur J Pharmacol. 1993;230:243–244. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujike H, Okumura K, Zushi Y, Akiyama K, Otsuki S. Persistent supersensitivity of sigma receptors develops during repeated methamphetamine treatment. Eur J Pharmacol. 1992;211:323–328. doi: 10.1016/0014-2999(92)90388-K. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett. 2000;289:21–24. doi: 10.1016/S0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- 23.Seminerio MJ, Kaushal N, Shaikh J, Huber JD, Coop A, Matsumoto RR. Sigma (σ) receptor ligand, AC927 (N-phenethylpiperidine oxalate), attenuates methamphetamine-induced hyperthermia and serotonin damage in mice. Pharmacol Biochem Behav. 2011;98:12–20. doi: 10.1016/j.pbb.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, Matsumoto RR. A novel substituted piperazine, CM156, attenuates the stimulant and toxic effects of cocaine in mice. J Pharmacol Exp Ther. 2010;333:491–500. doi: 10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (σ) receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 28.Rodvelt KR, Oelrichs CE, Blount LR, Fan KH, Lever SZ, Lever JR, Miller DK. The sigma receptor agonist SA4503 both attenuates and enhances the effects of methamphetamine. Drug Alcohol Depend. 2011;116:203–210. doi: 10.1016/j.drugalcdep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker JM, Bowen WD, Patrick SL, Williams WE, Mascarella SW, Bai X, Carroll FI. A comparison of (−)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of sigma 2 receptors in motor function. Eur J Pharmacol. 1993;231:61–68. doi: 10.1016/0014-2999(93)90684-A. [DOI] [PubMed] [Google Scholar]
- 31.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/S0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 32.Graybiel AM, Besson MJ, Weber E. Neuroleptic-sensitive binding sites in the nigrostriatal system: evidence for differential distribution of sigma sites in the substantia nigra, pars compacta of the cat. J Neurosci. 1989;9:326–338. doi: 10.1523/JNEUROSCI.09-01-00326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ujike H, Kuroda S, Otsuki S. σ Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- 34.Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]
- 35.Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry. 2004;37(Suppl 3):S208–S213. doi: 10.1055/s-2004-832679. [DOI] [PubMed] [Google Scholar]
- 38.Derbez AE, Mody RM, Werling LL. σ(2)-Receptor regulation of dopamine transporter via activation of protein kinase C. J Pharmacol Exp Ther. 2002;301:306–314. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- 39.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-U. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Alkayed NJ, Hurn PD, Kirsch JR. Cyclic adenosine monophosphate response element-binding protein phosphorylation and neuroprotection by 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) Anesth Analg. 2009;108:964–970. doi: 10.1213/ane.0b013e318192442c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDaid J, Graham MP, Napier TC. Methamphetamine-induced sensitization differentially alters pCREB and ΔFosB throughout the limbic circuit of the mammalian brain. Mol Pharmacol. 2006;70:2064–2074. doi: 10.1124/mol.106.023051. [DOI] [PubMed] [Google Scholar]
- 42.Largent BL, Gundlach AL, Snyder SH. Psychotomimetic opiate receptors labeled and visualized with (+)-[3H]3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Proc Natl Acad Sci U S A. 1984;81:4983–4987. doi: 10.1073/pnas.81.15.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi S, Horikomi K, Kato T. MS-377, a novel selective sigma(1) receptor ligand, reverses phencyclidine-induced release of dopamine and serotonin in rat brain. Eur J Pharmacol. 2001;427:211–219. doi: 10.1016/S0014-2999(01)01254-7. [DOI] [PubMed] [Google Scholar]
- 44.Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tam SW. Naloxone-inaccessible sigma receptor in rat central nervous system. Proc Natl Acad Sci U S A. 1983;80:6703–6707. doi: 10.1073/pnas.80.21.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32:148–154. [PubMed] [Google Scholar]
- 47.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Thompson TL, Bridges S, Miller C. Modulation of dopamine uptake in rat nucleus accumbens: effect of specific dopamine receptor antagonists and sigma ligands. Neurosci Lett. 2001;312:169–172. doi: 10.1016/S0304-3940(01)02209-1. [DOI] [PubMed] [Google Scholar]
- 49.Ault DT, Werling LL. Phencyclidine and dizocilpine modulate dopamine release from rat nucleus accumbens via sigma receptors. Eur J Pharmacol. 1999;386:145–153. doi: 10.1016/S0014-2999(99)00769-4. [DOI] [PubMed] [Google Scholar]