Abstract
Hemophilia A is an X-linked bleeding disorder caused by the deficiency of factor VIII (FVIII). Exogenous FVIII is administered therapeutically, and due to a short half-life, frequent infusions are often required. Fifteen to thirty-five percent of severe hemophilia A patients develop inhibitory antibodies toward FVIII that complicate clinical management of the disease. Previously, we used phosphatidylinositol (PI) containing lipidic nanoparticles to improve the therapeutic efficacy of recombinant FVIII by reducing immunogenicity and prolonging the circulating half-life. The objective of this study is to investigate further improvements in the FVIII–PI formulation resulting from the addition of polyethylene glycol (PEG) to the particle. PEGylation was achieved by passive transfer of PEG conjugated lipid into the FVIII–PI complex. PEGylated FVIII–PI (FVIII–PI/PEG) was generated with high association efficiency. Reduced activity in vitro and improved retention of activity in the presence of antibodies suggested strong shielding of FVIII by the particle; thus, in vivo studies were conducted in hemophilia A mice. Following intravenous administration, the apparent terminal half-life was improved versus both free FVIII and FVIII–PI, but exposure determined by area under the curve was reduced. The formation of inhibitory antibodies after subcutaneous immunization with FVIII–PI/PEG was lower than free FVIII but resulted in a significant increase in inhibitors following intravenous administration. Passive transfer of PEG onto the FVIII–PI complex does not provide any therapeutic benefit.
Key words: factor VIII, hemophilia A, immunogenicity, inhibitor development, PEGylation
INTRODUCTION
Factor VIII (FVIII) is an essential cofactor in the intrinsic pathway of the blood coagulation cascade (1). Deficiency or dysfunction of FVIII causes hemophilia A, a severe X-linked bleeding disorder (2). Infusion of recombinant FVIII or plasma-derived FVIII as a replacement is the first line of therapy for hemophilia A patients (3). Between 15% and 35% of severe hemophilia A patients will develop inhibitory responses to FVIII, presenting a major challenge in the clinical management of the disease (4). Based on systematic epitope mapping experiments, the anti-FVIII antibodies have been found to mainly target amino acid regions 484–508 of the A2 domain (5,6), 1811–1818 of the A3, and 2181–2312 of the C2 domains(7–9). Inhibition of FVIII activity results from antibodies against the A2 and A3 domains blocking interaction with factor IXa (7,10) while antibodies against the C2 domain interfere with binding to platelet membranes (9,11). Shielding or modification of these epitopes could potentially reduce the immunogenicity of the protein (12–14).
The half-life of FVIII in humans is only 10–12 h (15). This short half-life is often due to poor in vivo stability resulting from a combination of binding to plasma proteins and antibodies, proteolytic inactivation by thrombin, factor Xa and activated protein C, and non-proteolytic degradation mechanisms (1,16,17). In addition, low-density lipoprotein receptor-related protein (LRP), a member of low-density lipoprotein receptor (LDLR) family of endocytic receptors, has been shown to contribute to FVIII catabolism (18,19). As a result, prophylactic FVIII replacement therapy can require up to two to four infusions per week to maintain hemostatic efficacy (20,21). As the frequency of infusions increases, so does the risk for inhibitor formation (22,23). Less frequent infusions have also been linked to increased patient compliance (24). A FVIII molecule or formulation that shows prolonged biological half-life and reduced immunogenicity would represent a major advancement in the treatment of hemophilia A.
Previously we reported that phosphatidylserine (PS)-containing liposomes could reduce the immunogenicity of FVIII but failed to provide sufficient systemic exposure following i.v. administration, believed to result from rapid uptake by the reticuloendothelial system (RES) (25). Grafting liposomes with polyethylene glycol (PEG) has been shown to prolong liposome circulation in vivo (26–28). The presence of PEG attracts water to the liposome surface and provides a barrier against opsonins and cells of the RES (29). In addition, PEG neutralizes the surface charge of liposomes, decreasing the interaction between charged phospholipid head groups and opsonizing proteins (26). PEGylation of FVIII–PS liposomes resulted in a further reduction of immune response compared to the un-PEGylated formulation but provided only a modest improvement in the pharmacokinetic profile (30). Replacement of PS with phosphatidylinositol (PI) in FVIII containing lipidic particles provided a significant improvement in the half-life and avoided the rapid RES clearance observed with the previous formulations (31). Both PS and PI interact with the amino acid 2303–2332 lipid binding C2 domain of FVIII, but PI also associates with the A2 domain and FVIII is believed to penetrate deeper into the lipid particle (32–34). The topology of the FVIII–PI complex is more beneficial in reducing FVIII exposure to plasma components such as proteases and IgGs, as well as protecting the LRP binding sites within the A2 and C2 domains (18,19). The FVIII–PI complex also reduced immunogenicity in hemophilia A mice, possibly due to the shielding of immunogenic epitopes.
In the present study, we incorporated PEG conjugated lipids into the FVIII–PI complex and investigated the use of PEGylated FVIII–PI (FVIII–PI/PEG) to further improve therapeutic efficacy of FVIII. In vitro characterization of the complex was performed. The in vivo pharmacokinetics and immunogenicity of PEGylated FVIII–PI complex were evaluated in hemophilia A mice.
MATERIALS AND METHODS
Materials
Albumin-free recombinant full-length FVIII (Baxter Healthcare, Glendale, CA, USA) was used for the studies. Dimyristoylphosphatidylcholine (DMPC), soybean PI, and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (DMPE-PEG2000) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol was purchased either from Avanti Polar Lipids or from Sigma-Aldrich (St. Louis, MO, USA). IgG-free bovine serum albumin and diethanolamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal antibodies ESH4 was purchased from American Diagnostica Inc. (Greenwich, CT, USA). Monoclonal antibody N77210M was purchased from Biodesign International (Saco, ME, USA). Normal coagulation control plasma, FVIII-deficient plasma, and activated partial thromboplastin time (aPTT) reagents were purchased from Trinity Biotech (County Wicklow, Ireland). The Coamatic FVIII kit from DiaPharma Group (West Chester, OH, USA) was used to determine FVIII activity in plasma samples. Endosafe Endochrom-K® kit was purchased from Charles River Laboratories Inc. (Charles River, MA, USA).
PEG-Coated FVIII–PI Complex
A thin lipid film composed of 50:50:5 molar ratio of DMPC/PI/cholesterol was rehydrated with Tris buffer (25 mM Tris and 150 or 300 mM NaCl, pH = 7.0) (31). In order to produce small unilamellar vesicles, multilamellar vesicles were extruded through double polycarbonate membranes of 80 nm pore size (GE Osmonics Labstore, Minnetonka, MN, USA) using a high-pressure extruder (Northern Lipids Inc., Burnaby, BC, Canada) and then sterile-filtered through a 0.22-μm MillexTM-GP filter unit (Millipore Corporation, Bedford, MA, USA). Recovery of the lipid preparation was determined by inorganic phosphorous assay (35). A Nicomp Model CW 380 particle size analyzer (Particle Sizing Systems, Santa Barbara, CA, USA) was used to determine the size of lipid vesicles. To form the FVIII–lipid complex, FVIII was incubated with vesicles at 37°C at a molar ratio of 1:10,000 with gentle swirling for 30 min for all experiments. Endotoxin levels were tested in all formulations using the Endosafe Endochrom-K® kit (Charles River, MA, USA) and found to be below the detection limit.
To associate the particle with PEG, the FVIII–PI complex was added to a dry film of DMPE-PEG2000 followed by incubation at 25°C for 45 min to facilitate the transfer of PEGylated lipid to lipidic particles. As micelle formation would lead to inefficient incorporation of PEG in liposomes, it is necessary to ensure that the volume of FVIII–PI complex added to the PEG film did not result in DMPE-PEG2000 concentrations exceeding the critical micelle concentration, which was determined to be ∼100 μM. The final formulation contained 5 mol% PEG.
In Vitro Characterization of the FVIII–PI/PEG Complex
The activity of the FVIII–PI/PEG complex was determined with both the one-stage aPTT assay and by two-stage chromogenic assay. aPTT readings were taken on a Coag-A-Mate XM coagulometer (Organon Teknika Corporation, Durham, NC, USA), and the chromogenic readings were determined according to manufacturer’s instructions (Coamatic FVIII kit). FVIII samples were also spiked with PI/PEG liposomes prepared in the absence of FVIII to determine if the particles themselves had any interfering effects on the assay.
To estimate the amount of FVIII associated with PEGylated liposomes, free protein was separated from bound protein using a discontinuous dextran density gradient (36). Protein association efficiency of FVIII–PI/PEG complex was determined from the activity before and after separation using the aPTT assay. PEGylated FVIII–PI obtained after separation was also lyophilized and reconstituted in d-chloroform for NMR analysis to confirm PEG incorporation into lipidic particles.
Activity of FVIII and FVIII–PI/PEG in the Presence of Monoclonal Antibodies
To determine the effect of antibodies on the activity of FVIII, 20 ng/mL of FVIII or PEGylated FVIII–PI was incubated with 5 μg/mL of monoclonal antibodies ESH4 and N77210M, targeted to the C2 and A2 domains, respectively, at 37°C for 2 h. Following the incubation, residual FVIII activity was measured with the Coamatic FVIII kit. FVIII and FVIII–PI/PEG free of monoclonal antibodies were treated as controls and normalized as 100% FVIII activity.
Animals for In Vivo Studies
Breeding pairs of hemophilia A mice (C57BL/6J) with a targeted deletion in exon 16 of the FVIII gene were provided by Dr. HH Kazazian and Dr. R Sarkar from University of Pennsylvania, Philadelphia, PA, USA (37). A colony was established, and animals between 8 and 12 weeks old were used for the in vivo studies. All animal experiments were approved and performed according to the guidelines of Institutional Animal Care and Use Committee of the University at Buffalo.
Pharmacokinetics Studies
FVIII–PI/PEG (400 IU/kg) was administered to a group of male hemophilia A mice as a single i.v. bolus injection via the penile vein. For FVIII–PI/PEG, dosing was specified by the activity of FVIII added to the particles, and plasma concentrations were determined by comparison to a standard curve of the same formulation. Blood samples were collected from at least three mice per time point by cardiac puncture using syringes containing acid citrate dextrose (ACD—85 mM sodium citrate, 110 mM d-glucose, and 71 mM citric acid) buffer (10:1 v/v blood/ACD) at 0.08, 0.5, 1, 2, 4, 8, 16, 24, 30, 36, 42, and 48 h post-injection. Plasma was separated immediately by centrifugation at 5,000×g at 4°C for 5 min and stored at −80°C until analysis. The activity of FVIII in all plasma samples was measured by chromogenic assay. Non-compartmental analysis was performed using Phoenix WinNonlin v6.2 (Pharsight Corporation, Mountain View, CA, USA), and basic pharmacokinetic parameters including half-life (t1/2), clearance (CL), volume of distribution (V), mean residence time (MRT), and area under the curve (AUC) were generated. Comparisons were made to the pharmacokinetics of free FVIII and FVIII–PI previously obtained under identical experimental conditions (31).
Immunogenicity Studies
The relative immunogenicity of FVIII–PI/PEG was determined in hemophilia A mice. Four weekly injections of 10 IU were administered subcutaneously to female mice (n = 8) or intravenously via the penile vein to male mice (n = 6). At the end of sixth week, blood samples were collected by cardiac puncture into ACD buffer at a 10:1 (v/v) blood/ACD ratio. Plasma was collected as before and stored immediately at −80°C. Inhibitory antibody titers were measured by the Nijmegen modification of the Bethesda assay (38,39). Clotting times determined from various dilutions (1:2 to 1:1,024) of normal coagulation control plasma were used to create a calibration curve. Mouse plasma samples were serially diluted (1:8 to 1:16,000) in human FVIII-deficient plasma. Each diluted plasma sample (100 μL) was mixed with an equal volume of normal human plasma and incubated at 37°C for 2 h. The remaining FVIII activity was determined at the end of the incubation using the aPTT assay. A plot of residual activity of test samples at various dilutions versus log of the dilution was created, and a linear regression was performed on the linear part of the curve. Inhibitory titers, expressed in Bethesda units, are defined as the dilution at which 50% of FVIII activity is inhibited. Inhibitory titer data for free FVIII and FVIII–PI/PEG has been previously reported (31).
Statistical Analysis
Statistical differences (p < 0.05) were tested using Student’s independent two-sample t test or one-way ANOVA with Bonferroni’s multiple comparison test. For pharmacokinetics studies, differences in systemic exposure between the two treatments were compared using the Bailer–Satterthwaite method (40).
RESULTS
In Vitro Characterization of PEGylated FVIII–PI
The activity measured after association was significantly reduced as determined by both aPTT and chromogenic assays, 53.4 ± 2.4% and 74.6 ± 3.2%, respectively (Table I). This reduction in FVIII specific activity is probably due to the steric effect of PEGylation, which may decrease the accessibility of FVIII for required interactions. FVIII is inactive in its native form. Upon activation to FVIIIa, it must associate with factor IXa on a phospholipid surface to form the tenase complex that converts factor X to factor Xa (41,42). The presence of PEG on the liposome surface may limit the ability of FVIII to exit the complex and engage in required molecular interactions. Our PEGylation approach does not involve cross-linking or covalent attachment of PEG to FVIII that might reduce the intrinsic activity of FVIII. The reduction may instead be due to the temporary shielding of the FVIII surface. Upon exposure to the plasma components, FVIII would be slowly released from the PEGylated lipidic particles and become available for protein interactions critical to its biological function. Full retention of FVIII activity was observed after spiking the FVIII samples with PEGylated PI particles suggesting that the presence of the particles did not have any impact on FVIII activity and did not interfere with the assay measurement (Table I).
Table I.
In Vitro FVIII Activity of PEGylated FVIII–PI
| Formulation | FVIII activity recovered (%) | |
|---|---|---|
| aPTT assay | Chromogenic assay | |
| FVIII–PI/PEG | 53.4 ± 2.4* | 74.6 ± 3.2* |
| FVIII spiked with PI/PEG particles | 100.1 ± 9.6 | 91.2 ± 2.6 |
FVIII activity was measured following complexation with PI/PEG and after spiking of the particles by aPTT and chromogenic assays. For both assays, FVIII activity was determined from a calibration curve constructed of free FVIII. Values are mean ± standard deviation; n = 3
*p < 0.05 compared to free FVIII by one-way ANOVA with Bonferroni’s multiple comparison test
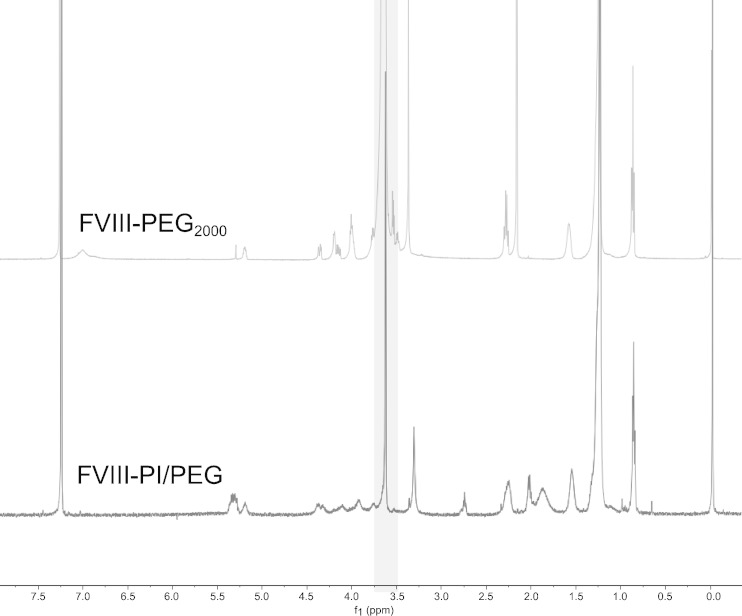
To ensure that PEG was successfully incorporated into the FVIII–PI particles, FVIII–PI/PEG was separated by centrifugation on a discontinuous dextran density gradient. Association efficiency was determined by comparing the activity recovered in the less dense upper layers to that of the total activity by aPTT. The association efficiency of FVIII–PI and FVIII–PI/PEG was 72.1 ± 9.1% and 82.1 ± 7.8%, respectively (mean ± SD). This change was not significantly different (p > 0.05, Student’s independent t test). The separated FVIII–PI/PEG was also analyzed using NMR spectroscopy to confirm the incorporation of PEG into the lipidic particles (Fig. 1). Peaks associated with the PEG component in FVIII–PI/PEG were detected at 3.63 ppm and are consistent with the DMPE-PEG2000 control. Our data indicated that PEG conjugated lipid was successfully transferred into the FVIII–PI complex without inhibiting the association of FVIII with PI particles.
Fig. 1.
NMR spectra of DMPE-PEG2000 (50 μmol total lipids) and PEGylated FVIII–PI complex in d-chloroform (upper spectrum DMPE-PEG2000 control, lower spectrum FVIII–PI/PEG). Separated FVIII–PI/PEG was lyophilization and reconstitution in d-chloroform
Activity of FVIII and FVIII–PI/PEG in the Presence of Monoclonal Antibodies
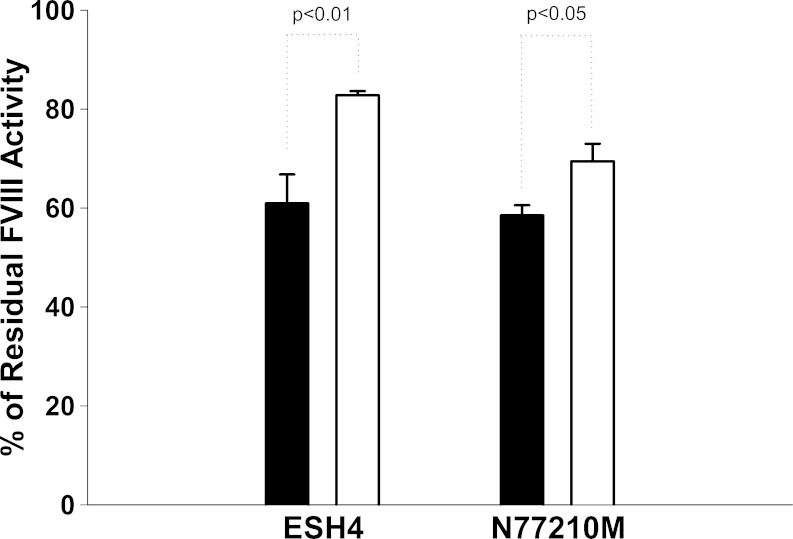
To show that the PEGylated FVIII–PI complex is more resistant to inhibitory antibodies binding compared to free FVIII, we performed in vitro studies with monoclonal antibodies ESH4 and N77210M, directed against the C2 and A2 domains, respectively. The activity of FVIII and FVIII–PI/PEG in the absence of monoclonal antibodies was measured using the chromogenic assay and normalized to 100%. Residual FVIII activity after incubation with the antibodies was then determined (Fig. 2). Our results showed that both free FVIII and FVIII–PI/PEG were inhibited by the monoclonal antibodies. However, PEGylated FVIII–PI retained 36% more activity than free FVIII in the presence of ESH4 and 19% more activity in the presence of N77210M. This suggests that PI/PEG may offer protection of immunogenic epitopes in the presence of antibodies. Translation of this effect to the in vivo setting could provide significant benefits in achieving efficacy in patients who have developed high antibody titers.
Fig. 2.
Percentage of residual FVIII activity in the presence of monoclonal anti-FVIII antibodies (solid bars FVIII, empty bars PEGylated FVIII–PI). FVIII samples (20 ng/mL) were incubated with 5 μg/mL monoclonal anti-FVIII antibodies ESH4 and N77210M targeting the C2 and A2 domains of FVIII, respectively, and the residual FVIII activity was determined by two-stage chromogenic assay. Each vertical bar presented is the mean of triplicate experiments. Error bars represent standard error of the mean. Data were analyzed by Student’s independent t test
Pharmacokinetics Studies
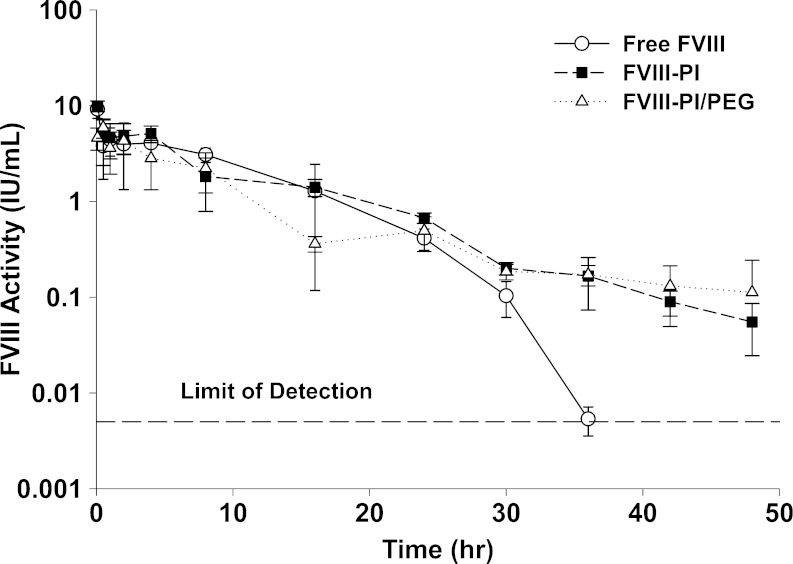
In order to evaluate the effect of PEGylation on the in vivo disposition of FVIII–PI/PEG, pharmacokinetic studies were conducted in hemophilia A mice (Fig. 3). It is interesting to note that only about half of the dose injected (∼5 IU/mL) was recovered in the plasma samples at 5 min post-injection. As the standard curve used to determine FVIII–PI/PEG activity already accounts for apparent activity loss from encapsulation, this low initial recovery represents a further loss of activity upon injection. FVIII–PI/PEG is expected to display limited distribution into extravascular tissues, so the source of this initial discrepancy is not readily apparent. The dispositions of all formulations appear similar from 0.5 to 30 h, but beginning at 36 h, they start to diverge. The plasma activity of the protein could be monitored only up to 36 h following administration of free FVIII, but for FVIII–PI- and FVIII–PI/PEG-treated mice, activity of the protein could be detected reproducibly even after 48 h post-injection. The apparent terminal t1/2 of FVIII–PI/PEG was increased ∼6-fold and ∼2 compared to free FVIII and FVIII–PI, respectively (Table II). Despite the terminal half-life increase, there was a reduction in AUC0–t largely due to the lower recovery at 5 min. The decrease in AUC0–t for FVIII–PI/PEG was not significantly different compared to free FVIII (p > 0.05, one-way ANOVA with Bonferroni’s multiple comparison test) but was significantly lower than that observed for FVIII–PI (p < 0.05, one-way ANOVA with Bonferroni’s multiple comparison test). Data for MRT0–t and AUC0–t are not extrapolated to infinity as predictions at time points beyond 48 h are difficult to validate and the contribution of this area to total AUC constitutes less than 5%.
Fig. 3.
Influence of PI/PEG on pharmacokinetics of FVIII in hemophilia A mice (open circles FVIII, close square FVIII–PI, open triangles FVIII–PI/PEG). The animals were given 400 IU/kg per animal via the penile vein, and plasma concentrations of FVIII were followed for 48 h. Error bars represent standard deviations. Free FVIII and FVIII–PI data have been previously published (31)
Table II.
Summary of Pharmacokinetic Parameters Generated with Phoenix WinNonlin v6.2 Following i.v. Administration of 400 IU/kg Free FVIII, FVIII–PI, and FVIII–PI/PEG
| Formulation | Pharmacokinetic parameters | ||||
|---|---|---|---|---|---|
| t 1/2 (h) | AUC0–t (IU h/mL) | CL (mL/h) | V (mL) | MRT0–t (h) | |
| FVIII | 2.62 | 57.7 ± 5.9 | 0.173 | 0.653 | 8.06 |
| FVIII–PI | 6.79 | 60.4 ± 3.9* | 0.164 | 1.61 | 9.33 |
| FVIII–PI/PEG | 15.6 | 44.4 ± 4.6* | 0.213 | 4.79 | 9.15 |
AUC values are presented with standard errors as determined by application of the Bailer–Satterthwaite method
*p < 0.05 by one-way ANOVA with Bonferroni’s multiple comparison test
Immunogenicity Studies
In vivo immunogenicity of free FVIII and PEGylated FVIII–PI was evaluated in a mouse model. Hemophilia A mice mount an IgG-based immune response similar to that of patients with hemophilia A (43). Immunizations were administered subcutaneously, and it has been shown that inhibitory titers to FVIII generated via this route are comparable to those following i.v. administration (31,44). The results obtained from immunogenicity studies are summarized in Table III. When administered subcutaneously, compared to free FVIII, inhibitory titers were significantly reduced by FVIII–PI (p < 0.001) and FVIII–PI/PEG (p < 0.01), but no statistical difference could be established between these two lipidic formulations (Table III). The precise mechanism responsible for the lower immune response observed following s.c. injection with the FVIII–PI/PEG complex has not yet been evaluated but is believed to be similar to the epitope protection offered by FVIII–PI.
Table III.
Mean Inhibitory Anti-FVIII Antibody Titers Following Repeated Administration of FVIII, FVIII–PI, or PEGylated FVIII–PI in Hemophilia a Mice via s.c. or i.v. Routes
| Formulation | Inhibitory titers via subcutaneous route | Inhibitory titers via intravenous route | ||
|---|---|---|---|---|
| n | Mean ± SEM | n | Mean ± SEM | |
| Free FVIII | 15 | 690 ± 78 | 8 | 675 ± 71 |
| FVIII–PI | 10 | 183 ± 57* | 8 | 385 ± 237*** |
| FVIII–PI/PEG | 8 | 298 ± 59** | 6 | 1,352 ± 229**** |
Results are expressed in Bethesda units. Data were analyzed by one-way ANOVA with Bonferroni’s multiple comparison test. Free FVIII and FVIII–PI data have been previously published (31)
*p<0.001 compared to free FVIII s.c.; **p<0.01 compared to free FVIII s.c.; ***p<0/05 compared to free FVIII i.v.; ****p<0.001 compared to both free FVIII and FVIII‐PI i.v.
A surprising observation was the increased immunogenic response for FVIII–PI/PEG when administered intravenously, and the results suggest that the beneficial effects of PEGylation were completely abolished. While PI–FVIII still resulted in a reduction over free FVIII (p < 0.05), FVIII–PI/PEG caused an increase in titers that were significant compared to both free FVIII and FVIII–PI (p < 0.001).
DISCUSSION
The overall goal of this research is to reduce the frequency of administration of FVIII by increasing the circulation time of the protein and reducing the intensity of immune response to FVIII in vivo. Previously we reported that FVIII associated with PI containing lipidic particles has potential to be a useful therapeutic approach for hemophilia A (31). A large surface area of FVIII was involved in the association with PI containing lipidic particles suggesting that the protein was buried inside the particles. This not only masks the immunogenic epitopes on FVIII but it could also reduce FVIII exposure to plasma components, protecting FVIII from proteolysis and binding of opsonizing proteins. Administration of FVIII–PI resulted in reduced immunogenicity and prolonged half-life of FVIII in a murine model for hemophilia A (31). Pisal et al. showed that this FVIII–PI continued to prolong the circulation time even in the absence of von Willebrand factor (vWF) (45). We investigated whether attachment of PEG to the complex would yield further in vivo pharmacokinetic and immunogenic improvements.
PEGylation is the covalent coupling of PEG chains to a therapeutic agent (46). Due to the non-toxic and biocompatible nature of PEG, PEGylation became a popular method for the delivery of biopharmaceuticals to improve biological efficacy during the 1970s (47,48). The hydrophilic nature of PEG polymers allow binding of water molecules to form a water layer over the surface of PEGylated drugs. This increases the hydrodynamic radius of biopharmaceuticals and interferes with renal clearance. In addition, PEGylation provides a steric barrier against proteolysis, binding of opsonizing proteins and interaction with clearance receptors or cellular surface like those of the RES, thereby prolonging the circulation half-life of biopharmaceuticals. PEGylation can often interfere with critical macromolecular interactions and lead to a substantial reduction in activity and efficacy, though it should be noted that Mei and coworkers recently generated a series of FVIII constructs covalently linked to PEG that retained full activity (49). PEGylation of lipid particles containing therapeutic proteins such as FVIII is an alternative option that imparts the protective beneficial effects of PEG on the particle without the risk of altering critical functions by covalently attaching it to the protein. Within the particle itself, FVIII is encapsulated in the lumen or intercalated in the lipid bilayer. The protein is released from the particle in vivo where it can participate in necessary intermolecular interactions such as association with vWF. Attachment of PEG to PI particles prior to loading of FVIII can drastically reduce the association efficiency, so PEGylation of FVIII–PI complex was achieved by passive transfer of 5 mol% DMPE-PEG2000 after complexation of FVIII with PI particles (30).
It is appropriate to mention here that our approach of complexing FVIII with PEGylated liposomes results in a configuration that is distinctively different from PEGylated liposomal FVIII reported by Baru et al. (50). Their approach utilized synthetic PEGylated liposomes consisting of 97% POPC and 3% 1,2‐distearoyl‐sn‐glycero‐3‐phosphoethanolamine‐n‐[methoxy(polyethylene glycol)‐2000] (DSPE‐PEG2000). FVIII was noncovalently attached to the outer surface of the PEGylated liposome by exploiting the interactions of specific protein sequences. While this approach allowed FVIII to be available for clotting at all times, it also exposed FVIII to plasma components such as proteases and IgGs. Moreover, as the protein was attached to the surface of PEG noncovalently, disassociation of FVIII immediately following administration was possible where it could be taken up by the hepatic clearance receptor LRP, providing little to no improvement of the pharmacokinetic profile.
In vitro we observed that addition of PEG allowed for the maintenance of high association efficiency as well as reductions in activity and protection from antibodies suggestive of substantial shielding of FVIII within the PI/PEG complex. Thus, we moved to test FVIII–PI/PEG in vivo. FVIII clearance from circulation is dependent on the specific binding to multiple catabolic receptors that mediate the endocytosis of FVIII (51). Electrostatic interactions between positive residues on FVIII and negative residues on LRP result in the FVIII-LRP binding (52). Several high-affinity binding sites for LRP interaction have been identified including sites in the A2 (Arg484–Phe509) and A3 (Glu1811–Lys1818) domains and a low-affinity binding site in the C2 domain (18,19,53). LRP-mediated endocytosis of FVIII is facilitated by heparan sulfate proteoglycans, a component of the extracellular matrix (54). The asialoglycoprotein receptor is another possible clearance mechanism that recognizes terminal β-d-galactose or N-acetyl-d-galactosamine residues and has been shown to interact with the B domain of full-length FVIII (55).
Shielding of FVIII from interactions with clearance receptors can prolong the circulation of FVIII, but the ability to disassociate from the particle as needed in vivo must also be maintained. Following administration of the FVIII–PI/PEG complex, lower recovery at the initial 5-min time point and decreases in AUC invoke the argument that the protein may not be able to effectively exit the particle in vivo. With FVIII–PI, the benefits of prolonged circulation only became evident at later time points (31). It follows that this region is where the additional protection of the particle from clearance mechanisms and opsonizing proteins would also become evident. It is possible that the uptake of PI containing particles by phagocytic cells of the RES is further reduced by the incorporation of PEGylated lipids. Inclusion of PEG has been shown to enhance the stability of liposomes in vivo by increasing the hydrophilicity of the liposome surface and minimizing nonspecific interaction with RES (56). A prolongation of half-life in the terminal phase for the FVIII–PI/PEG particle is evident, but benefits from this increase are offset by the overall reduction of exposure at earlier time points and the larger quantity of protein required to establish equivalent concentrations.
Despite a reduction in immunogenicity following s.c. administration, the inhibitory titers significantly increased when FVIII–PI/PEG was given intravenously, the clinical route of administration for FVIII. The reason behind the increased immunogenic response following i.v. administration is not clear, but we speculate that the free fraction of FVIII may remain associated on the exterior of the PEG particle via electrostatic interactions and immunologic epitopes of this exposed FVIII are presented in a highly repetitive manner. This could promote compliment activation. Additionally, PEG may shield PI and thus negate the immunoregulatory effects of PI. Recently, we showed that PI can downregulate the expression of co-stimulatory signals in antigen presenting cells (APCs), suppress T cell activation, and increase the secretion of regulatory cytokines such as TGF-beta (57).
Further, after intravenous administration, liposomes are in direct contact with the spleen, promoting exposure to B cells. This is opposed to the subcutaneous space which is primarily filtered to the spleen via lymphatic circulation, and the large size of the liposomes may limit distribution via this system. It is surprising given the fact that PEG itself is generally believed to be immunologically inert, but Ishida and coworkers have reported the development of an IgM-based immune response to liposomes incorporating PEG2000-DSPE (58). Development of this response is T cell-independent, B cell-mediated, and the spleen plays a critical role (59,60). Recognition of PEGylated liposomes by IgM could lead to compliment activation and further recruitment of APCs. If the development of an IgM-based response to the PEGylated liposomes is indeed the cause for this elevated immunogenicity, it is likely when extended to multiple dosing what little pharmacokinetic improvements we have observed will be lost due to the accelerated blood clearance phenomenon associated with this response (58). In these studies, we used PEG with a molecular weight of 2000. It is possible that the barrier provided by the PEG on the outer surface of the PI particle proved to be too substantial of a barrier for FVIII release, and use of a lower molecular weight PEG may help facilitate the release of FVIII. However, given the severity of the immune response and associated concerns, inclusion of PEG in this liposomal formulation is not recommended.
CONCLUSION
PEG was successfully added to our established FVIII–lipid formulation. In vitro experiments suggested increases in association efficiency and protection from antibodies that could provide additional benefits in vivo. Circulating half-life was improved as expected, but overall exposure as determined by AUC was decreased. Despite improvements in the subcutaneous immunogenicity, intravenous immunogenicity was significantly amplified. This response may result in an accelerated clearance for FVIII–PI/PEG in a multiple dosing situation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 HL-70227) to SVB. We are grateful to Dr. ZP Bernstein of Western New York Hemophilia Foundation, for providing albumin-free recombinant factor VIII (Advate). The authors thank Dr. K Huang from the Department of Chemistry at University at Buffalo for performing the NMR study.
Abbreviations
- aPTT
Activated partial thromboplastin time
- DMPC
Dimyristoylphosphatidylcholine
- DMPE-PEG2000
1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000]
- DSPE-PEG2000
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-n-[methoxy (polyethylene glycol)-2000]
- FVIII
Factor VIII
- LDLR
Low-density lipoprotein receptor
- LRP
Low-density lipoprotein receptor-related protein
- PEG
Polyethylene glycol
- PI
Phosphatidylinositol
- PS
Phosphatidylserine
- RES
Reticuloendothelial system
- rFVIII
Recombinant factor VIII
- i.v.
Intravenous
- s.c.
Subcutaneous
- vWf
von Willebrand factor
- APCs
Antigen presenting cells
References
- 1.Foster PA, Zimmerman TS. Factor VIII structure and function. Blood Rev. 1989;3(3):180–91. doi: 10.1016/0268-960X(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 2.Mannucci PM, Tuddenham EG. The hemophilias—from royal genes to gene therapy. N. Engl. J. Med. 2001;344(23):1773–9. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 3.Klinge J, Ananyeva NM, Hauser CA, Saenko EL. Hemophilia A—from basic science to clinical practice. Semin. Thromb. Hemost. 2002;28(3):309–22. doi: 10.1055/s-2002-32667. [DOI] [PubMed] [Google Scholar]
- 4.Brettler DB. Inhibitors in congenital haemophilia. Baillieres Clin Haematol. 1996;9(2):319–29. doi: 10.1016/S0950-3536(96)80066-7. [DOI] [PubMed] [Google Scholar]
- 5.Healey JF, Lubin IM, Nakai H, Saenko EL, Hoyer LW, Scandella D, et al. Residues 484–508 contain a major determinant of the inhibitory epitope in the A2 domain of human factor VIII. J. Biol. Chem. 1995;270(24):14505–9. doi: 10.1074/jbc.270.24.14505. [DOI] [PubMed] [Google Scholar]
- 6.Lubin IM, Healey JF, Barrow RT, Scandella D, Lollar P. Analysis of the human factor VIII A2 inhibitor epitope by alanine scanning mutagenesis. J. Biol. Chem. 1997;272(48):30191–5. doi: 10.1074/jbc.272.48.30191. [DOI] [PubMed] [Google Scholar]
- 7.Zhong D, Saenko EL, Shima M, Felch M, Scandella D. Some human inhibitor antibodies interfere with factor VIII binding to factor IX. Blood. 1998;92(1):136–42. [PubMed] [Google Scholar]
- 8.Healey JF, Barrow RT, Tamim HM, Lubin IM, Shima M, Scandella D, et al. Residues Glu2181-Val2243 contain a major determinant of the inhibitory epitope in the C2 domain of human factor VIII. Blood. 1998;92(10):3701–9. [PubMed] [Google Scholar]
- 9.Scandella D, Gilbert GE, Shima M, Nakai H, Eagleson C, Felch M, et al. Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood. 1995;86(5):1811–9. [PubMed] [Google Scholar]
- 10.Fay PJ, Scandella D. Human inhibitor antibodies specific for the factor VIII A2 domain disrupt the interaction between the subunit and factor IXa. J. Biol. Chem. 1999;274(42):29826–30. doi: 10.1074/jbc.274.42.29826. [DOI] [PubMed] [Google Scholar]
- 11.Fijnvandraat K, Celie PH, Turenhout EA, ten Cate JW, van Mourik JA, Mertens K, et al. A human alloantibody interferes with binding of factor IXa to the factor VIII light chain. Blood. 1998;91(7):2347–52. [PubMed] [Google Scholar]
- 12.Reding MT, Okita DK, Diethelm-Okita BM, Anderson TA, Conti-Fine BM. Human CD4+ T-cell epitope repertoire on the C2 domain of coagulation factor VIII. J. Thromb. Haemost. 2003;1(8):1777–84. doi: 10.1046/j.1538-7836.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 13.Reding MT, Okita DK, Diethelm-Okita BM, Anderson TA, Conti-Fine BM. Epitope repertoire of human CD4(+) T cells on the A3 domain of coagulation factor VIII. J. Thromb. Haemost. 2004;2(8):1385–94. doi: 10.1111/j.1538-7836.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu GL, Okita DK, Conti-Fine BM. T cell recognition of the A2 domain of coagulation factor VIII in hemophilia patients and healthy subjects. J. Thromb. Haemost. 2004;2(11):1908–17. doi: 10.1111/j.1538-7836.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- 15.Morfini M. Pharmacokinetics of factor VIII and factor IX. Haemophilia. 2003;9(Suppl 1):94–9. doi: 10.1046/j.1365-2516.9.s1.8.x. [DOI] [PubMed] [Google Scholar]
- 16.Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25(2):505–12. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 17.Fay PJ, Anderson MT, Chavin SI, Marder VJ. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim. Biophys. Acta. 1986;871(3):268–78. doi: 10.1016/0167-4838(86)90208-6. [DOI] [PubMed] [Google Scholar]
- 18.Lenting PJ, Neels JG, van den Berg BM, Clijsters PP, Meijerman DW, Pannekoek H, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J. Biol. Chem. 1999;274(34):23734–9. doi: 10.1074/jbc.274.34.23734. [DOI] [PubMed] [Google Scholar]
- 19.Saenko EL, Yakhyaev AV, Mikhailenko I, Strickland DK, Sarafanov AG. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J. Biol. Chem. 1999;274(53):37685–92. doi: 10.1074/jbc.274.53.37685. [DOI] [PubMed] [Google Scholar]
- 20.Ljung R. Prophylactic therapy in haemophilia. Blood Rev. 2009;23(6):267–74. doi: 10.1016/j.blre.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson M, Berntorp E, Bjorkman S, Lindvall K. Pharmacokinetic dosing in prophylactic treatment of hemophilia A. Eur. J. Haematol. 1993;51(4):247–52. doi: 10.1111/j.1600-0609.1993.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharathkumar A, Lillicrap D, Blanchette VS, Kern M, Leggo J, Stain AM, et al. Intensive exposure to factor VIII is a risk factor for inhibitor development in mild hemophilia A. J. Thromb. Haemost. 2003;1(6):1228–36. doi: 10.1046/j.1538-7836.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 23.Koestenberger M, Raith W, Muntean W. High titre inhibitor after continuous factor VIII administration for surgery in a young infant. Haemophilia. 2000;6(2):120. doi: 10.1046/j.1365-2516.2000.00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001;23(8):1296–310. doi: 10.1016/S0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 25.Ramani K, Miclea RD, Purohit VS, Mager DE, Straubinger RM, Balu-Iyer SV. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J. Pharm. Sci. 2008;97(4):1386–98. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002;240(1–2):95–102. doi: 10.1016/S0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 27.Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int. J. Pharm. 2003;253(1–2):121–32. doi: 10.1016/S0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 28.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 29.Zeisig R, Shimada K, Hirota S, Arndt D. Effect of sterical stabilization on macrophage uptake in vitro and on thickness of the fixed aqueous layer of liposomes made from alkylphosphocholines. Biochim. Biophys. Acta. 1996;1285(2):237–45. doi: 10.1016/S0005-2736(96)00167-8. [DOI] [PubMed] [Google Scholar]
- 30.Ramani K, Purohit V, Miclea R, Gaitonde P, Straubinger RM, Balu-Iyer SV. Passive transfer of polyethylene glycol to liposomal-recombinant human FVIII enhances its efficacy in a murine model for hemophilia A. J. Pharm. Sci. 2008;97(9):3753–64. doi: 10.1002/jps.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng A, Straubinger RM, Balu-Iyer SV. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. AAPS J. 2010;12(3):473–81. doi: 10.1208/s12248-010-9207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99(4):1215–23. doi: 10.1182/blood.V99.4.1215. [DOI] [PubMed] [Google Scholar]
- 33.Pratt KP, Shen BW, Takeshima K, Davie EW, Fujikawa K, Stoddard BL. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999;402(6760):439–42. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]
- 34.Purohit VS, Ramani K, Kashi RS, Durrani MJ, Kreiger TJ, Balasubramanian SV. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochim. Biophys. Acta. 2003;1617(1–2):31–8. doi: 10.1016/j.bbamem.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett GR. Phosphorus assay in column chromatography. J. Biol. Chem. 1959;234(3):466–8. [PubMed] [Google Scholar]
- 36.Heath TD, Macher BA, Papahadjopoulos D. Covalent attachment of immunoglobulins to liposomes via glycosphingolipids. Biochim. Biophys. Acta. 1981;640(1):66–81. doi: 10.1016/0005-2736(81)90532-0. [DOI] [PubMed] [Google Scholar]
- 37.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat. Genet. 1995;10(1):119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 38.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb. Haemost. 1995;73(2):247–51. [PubMed] [Google Scholar]
- 39.Purohit VS, Ramani K, Sarkar R, Kazazian HH, Jr, Balasubramanian SV. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J. Biol. Chem. 2005;280(18):17593–600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nedelman JR, Gibiansky E, Lau DT. Applying Bailer’s method for AUC confidence intervals to sparse sampling. Pharm. Res. 1995;12(1):124–8. doi: 10.1023/A:1016255124336. [DOI] [PubMed] [Google Scholar]
- 41.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 42.Lollar P, Fay PJ, Fass DN. Factor VIII and factor VIIIa. Methods Enzymol. 1993;222:128–43. doi: 10.1016/0076-6879(93)22010-D. [DOI] [PubMed] [Google Scholar]
- 43.Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb. Haemost. 2000;84(5):826–32. [PubMed] [Google Scholar]
- 44.Peng A, Gaitonde P, Kosloski MP, Miclea RD, Varma P, Balu-Iyer SV. Effect of route of administration of human recombinant factor VIII on its immunogenicity in hemophilia A mice. J. Pharm. Sci. 2009;98(12):4480–4. doi: 10.1002/jps.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb. Haemost. 2010;104(5):1073–5. doi: 10.1160/TH10-06-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J. Pharm. Sci. 2010;99(6):2557–75. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977;252(11):3578–81. [PubMed] [Google Scholar]
- 48.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977;252(11):3582–6. [PubMed] [Google Scholar]
- 49.Mei B, Pan C, Jiang H, Tjandra H, Strauss J, Chen Y, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270–9. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 50.Baru M, Carmel-Goren L, Barenholz Y, Dayan I, Ostropolets S, Slepoy I, et al. Factor VIII efficient and specific non-covalent binding to PEGylated liposomes enables prolongation of its circulation time and haemostatic efficacy. Thromb. Haemost. 2005;93(6):1061–8. doi: 10.1160/TH04-08-0485. [DOI] [PubMed] [Google Scholar]
- 51.Ananyeva NM, Kouiavskaia DV, Shima M, Saenko EL. Catabolism of the coagulation factor VIII: can we prolong lifetime of f VIII in circulation? Trends Cardiovasc Med. 2001;11(6):251–7. doi: 10.1016/S1050-1738(01)00124-4. [DOI] [PubMed] [Google Scholar]
- 52.Bieri S, Djordjevic JT, Daly NL, Smith R, Kroon PA. Disulfide bridges of a cysteine-rich repeat of the LDL receptor ligand-binding domain. Biochemistry. 1995;34(40):13059–65. doi: 10.1021/bi00040a017. [DOI] [PubMed] [Google Scholar]
- 53.Bovenschen N, Boertjes RC, van Stempvoort G, Voorberg J, Lenting PJ, Meijer AB, et al. Low density lipoprotein receptor-related protein and factor IXa share structural requirements for binding to the A3 domain of coagulation factor VIII. J. Biol. Chem. 2003;278(11):9370–7. doi: 10.1074/jbc.M212053200. [DOI] [PubMed] [Google Scholar]
- 54.Sarafanov AG, Ananyeva NM, Shima M, Saenko EL. Cell surface heparan sulfate proteoglycans participate in factor VIII catabolism mediated by low density lipoprotein receptor-related protein. J. Biol. Chem. 2001;276(15):11970–9. doi: 10.1074/jbc.M008046200. [DOI] [PubMed] [Google Scholar]
- 55.Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJ, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J. Thromb. Haemost. 2005;3(6):1257–65. doi: 10.1111/j.1538-7836.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 56.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–7. doi: 10.1016/0014-5793(90)81016-H. [DOI] [PubMed] [Google Scholar]
- 57.Gaitonde P, Peng A, Straubinger RM, Bankert RB, Balu-Iyer SV. Downregulation of CD40 signal and induction of TGF-beta by phosphatidylinositol mediates reduction in immunogenicity against recombinant human factor VIII. J Pharm Sci. 2011; doi:10.1002/jps.22746. [DOI] [PMC free article] [PubMed]
- 58.Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115(3):251–8. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115(3):243–50. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, et al. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharm. 2010;392(1–2):218–23. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]