Abstract
Purpose
A generic product must meet the standards established by the Food and Drug Administration (FDA) to be approved for marketing in the USA. FDA approves a generic product for marketing if it is proved to be therapeutically equivalent to the reference product. Bioequivalence (BE) between a proposed generic product and its corresponding reference product is one of the major components of therapeutic equivalence. These approvals may be delayed if the BE portion of the submission is determined to be deficient. Many of these BE deficiencies recur commonly and can be avoided.
Method
We conducted a survey of the BE submissions to abbreviated new drug applications (ANDAs) over years 2001 to 2008 to identify the most commonly occurring BE deficiencies.
Results
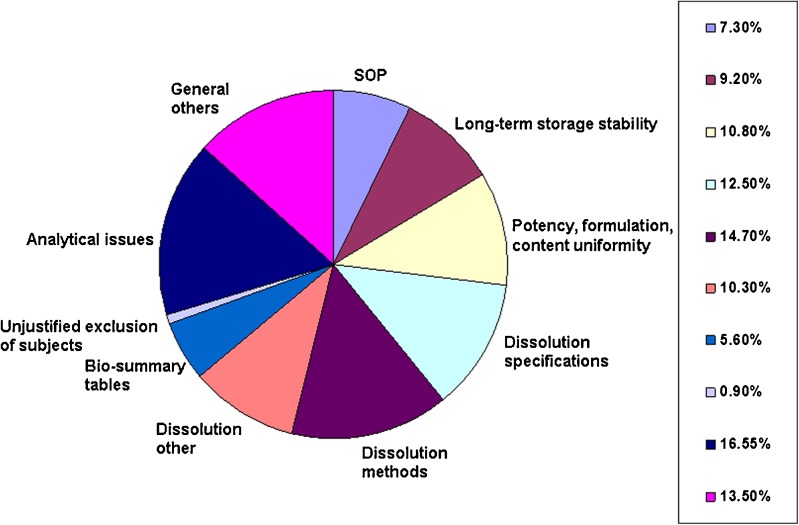
Recurring deficiencies are found in a majority of the ANDAs reviewed by FDA’s Division of Bioequivalence. The most common deficiencies were the two deficiencies related to dissolution (method and specifications) found in 23.3% of the applications and analytical method validation and/or report found in 16.5% of the applications. The approval of generic drugs would be greatly accelerated if these deficiencies could be avoided.
KEY WORDS: ANDA, bioequivalence, common deficiency, FDA
INTRODUCTION
Since the passage of Hatch-Waxman Amendment in 1984, FDA has approved more than 10,000 generic drugs. The shift from branded drugs to generics steadily increases every year. Presently, generic drugs account for about 65% of all prescriptions dispensed in the USA [1]. To market a prescription or over-the-counter generic drug, an abbreviated new drug application (ANDA) must be submitted to FDA’s Office of Generic Drugs (OGD). The OGD decides whether a certain generic product is therapeutically equivalent to its corresponding Reference Listed Product (RLD). To be deemed therapeutically equivalent to the corresponding reference product, the generic must provide evidence that the generic is pharmaceutically equivalent to the corresponding RLD, adequately labeled, manufactured in compliance with current good manufacturing practice regulations, and bioequivalent to the RLD [2].
Pharmaceutically equivalent drug products are formulated to contain the same amount of active ingredient in the same dosage form and to meet compendial or other applicable standards (i.e., strength, quality, purity, and identity) [2]. Bioequivalence (BE) is defined as the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study [2].
Thus, reports providing data from BE studies, which are conducted to compare the rate and extent of drug absorption in vivo for a generic and corresponding reference product, are one of the critical components of ANDA submissions. Together with the determination of pharmaceutical equivalence, establishing BE allows a regulatory conclusion of therapeutic equivalence. A therapeutically equivalent generic product is interchangeable with the RLD. The Division of Bioequivalence (DBE) in the OGD reviews BE studies in applications received for new generic drug products.
For ANDA BE submissions that contain the results of in vivo studies, the four major study report components are the following: in vitro dissolution testing, bioanalytical methodology, clinical study reports, and statistical analysis. The DBE reviews each of these components, identifies any deficiencies in the submission and provides recommendations to the ANDA applicants. As it takes time for the applicant to correct these deficiencies, the presence of these deficiencies often delays the generic drug’s approval. In an effort to improve the quality of ANDA submissions and the review process, the DBE implemented General Bioavailability and Bioequivalence (BA/BE) Guidance (October 2000) [3], Food Effect Bioavailability and Fed Bioequivalence Studies Guidance (December 2002) [4], and Electronic Bio-summary Tables (October 2007) [5] in addition to the creation of the on-line Dissolution Methods Database (November 2005) [6] along with posting the Bioequivalence Recommendations Specific Products (May 2007) [7]. Although, there has been an improvement in the overall quality of ANDA submissions with the employment of these guidance and the Dissolution Methods Database, there are still some recurring deficiencies which may be associated with the one or more of the components of the BE portion of the application.
The objective of this study is to identify common deficiencies in the BE section of ANDAs that may unnecessarily prolong the ANDA review process. We believe that such delays could be decreased by providing information to the industry about common BE deficiencies, in order that these can be avoided in the future ANDA submissions. In addition, we also aim to accumulate a large pool of ANDA deficiency data (8 years) to understand how giving feedback to the generic industry can assist the industry in submission of higher quality ANDA applications.
METHODS
Internal FDA databases were searched from January 2001 to December 2008 for the most commonly occurring BE deficiencies in ANDA submissions. The dates were chosen because 2001 was the first full year after the initial posting of the BE Guidance, and 2008 was the last year for which all submissions received at least one first-cycle BE review. For the purpose of this evaluation, we focused solely on those ANDAs that contained in vivo BE studies with pharmacokinetic (PK) end point, including solid oral dosage forms, transdermal drug delivery systems, chewing gums, and suspensions for oral or parenteral delivery. The common deficiencies identified are categorized into the following:
Pharmacokinetic (PK) repeats: the criteria for selection of samples for re-analysis are considered not objective, unscientifically sound or potentially biased toward favorable bioequivalence outcome.
Standard Operating Procedure (SOP): the bioanalytical SOPs used in the application were not submitted.
Long-term storage stability: the long-term frozen storage stability data were not submitted or not enough to cover the whole biological sample storage time period.
-
Potency, content uniformity, and formulation: the potency or content uniformity data for the test product was not submitted.
The following formulation deficiencies are commonly found in the ANDA applications:- The information on colorant or flavor used in the test formulation submitted was submitted.
- One or more excipients are over the limit in the inactive ingredient guide.
- Based on the maximum daily dose of the drug and formulation, the intake of iron from the drug may exceed the limit of 5 mg per day.
Dissolution specification: the in vitro dissolution testing specifications were not proposed or not as recommended by DBE.
Dissolution method: the dissolution method used in the application is not optimal or not consistent as that suggested by DBE.
- Dissolution other: dissolution deficiencies that cannot be categorized into specification or method:
- Failure to submit individual dissolution data for each of the 12 units of test and reference products.
- Incomplete dissolution testing (for example, lacking dissolution data in multimedia for extended release products and alcohol dose dumping data for certain products).
- Failure to provide information on dissolution testing date and site address
- High variability in dissolution data.
Bio-summary tables: the 16 summary tables for BE studies (available to the industry on OGD’s website) are not submitted or incomplete.
Unjustified exclusion of subjects: Subjects are excluded from statistical analysis without proper justification.
- Analytical issues: deficiencies related with analytical method validation or analytical report:
- Insufficient submission of analytical raw data from the study runs of all the subjects.
- Incomplete bioanalytical report (for example, missing dilution integrity data, stock stability data, absolute recovery data).
- Lacking chromatograms for 20% of study subjects.
- General other: deficiencies that do not fall into any of the categories above:
- Dropping subjects who are assumed outliers from statistical analysis without adequate justification.
- Improper submission or missing of electronic data files which are required for statistical analysis.
- Inadequate information on the failed bioequivalence study.
RESULTS
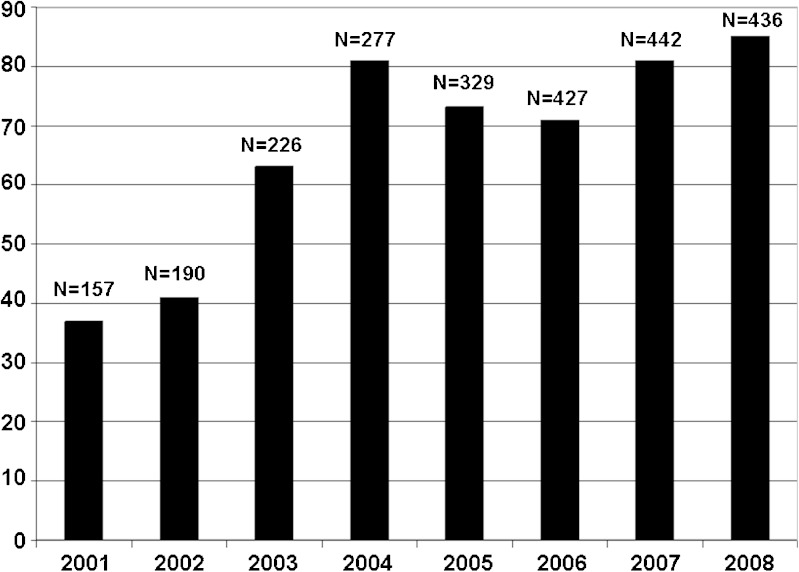
To identify the common BE deficiencies in the ANDA submissions since the implementation of General BA/BE Guidance in October 2000, we collected data from a total of 2,484 ANDA submissions that contained in vivo BE studies, submitted from January 2001 to December 2008. One or more deficiencies over the years 2001 to 2008 were found in most of the applications (Fig. 1).
Fig. 1.
Number (N) and percentage of applications containing BE studies with PK end point with one or more deficiencies over the years 2001 to 2008
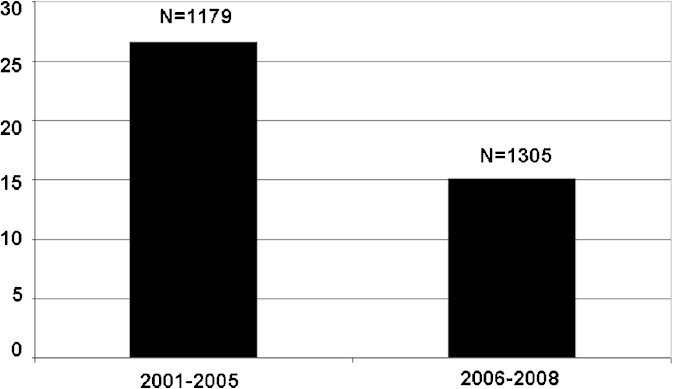
To improve the quality of ANDA submissions and expedite the review process, the OGD has implemented many new Guidance for Industry and publicly available databases. The FDA on-line dissolution methods database was created in November 2005. The database provides information about the in vitro dissolution methods to be tested for incorporation into a drug product’s stability and quality controls program. Dissolution is intended to predict the in vivo, i.e., pharmacokinetic, behavior of the drug product. Since the implementation of this database, the percentage of applications with dissolution method deficiencies decreased dramatically: from 26.6% (year 2001–2005) to 15.1% (year 2006–2008) (Fig. 2). However, the percentage of ANDA submissions with dissolution specifications deficiencies rose from 2001 to 2008 (data not shown). Dissolution specifications are usually decided upon final evaluation of the dissolution data submitted. The most common dissolution specification deficiency is that FDA recommends a more stringent specification for the test product compared with what proposed by the firm. It is concluded from the data presented in Fig. 2 that the establishment of on-line dissolution methods database has helped greatly in improving the quality of the ANDA submissions thus accelerate the approval of generic drugs.
Fig. 2.
Number (N) and percentage of applications containing BE studies with PK end point with dissolution method deficiencies calculated by years 2001 to 2005 and 2006 to 2008
It should be noted that prior to the beginning of 2003, if the applicant proposed different dissolution specifications compared with those recommended by FDA, the DBE would send a letter recommending the appropriate dissolution specifications, but would not identify this letter as a “deficiency letter” per se. In early 2003, the DBE made a procedural change, and began to send deficiency letters following initial review of the dissolution data associated with a particular ANDA. Therefore, since 2003, a discrepancy between FDA’s proposed dissolution specifications and an applicant’s proposed dissolution specifications is counted as a BE deficiency with an application. This procedural change explains the increased percentage of ANDAs with deficiencies since 2003, as shown in Fig. 1.
In addition to the bioanalytical and dissolution deficiencies described above, a number of other general deficiencies are generally noted in BE submissions (Fig. 3). Many common bioanalytical deficiencies occurred in the various applications submitted from 2001 to 2008. Most frequently, these deficiencies include lack of SOPs, no data showing long-term stability of drug substance in frozen samples of biological fluid, and incomplete sets of bioanalytical raw data. FDA is considering including submission of bioanalytical SOPs, documentation of long-term frozen storage stability, and receipt of full bioanalytical raw data sets as criteria for receiving ANDAs for review. Most of these general deficiencies occur repeatedly, and it is highly likely that many of these commonly occurring deficiencies are avoidable.
Fig. 3.
Percentage of deficiencies in each category, calculated on polled data in the ANDA applications containing BE studies with PK end point from 2001 to 2008
In summary, we hope that publication of this information will help the pharmaceutical industry to take steps toward eliminating recurring problems with BE submissions. Submission of acceptable, complete and well-organized BE submissions to ANDAs will be of great value to the generic drug industry as well as the American public, who will benefit from the earlier availability of generic drugs.
Footnotes
Disclaimer
This article reflects the views of the author and should not be construed to represent FDA’s views or policies.
REFERENCES
- 1.Generic Pharmaceutical Association. http://www.gphaonline.org/media/press-releases/2009/02/12/gpha-congratulates-president-elect-barack-obama-and-new-congress-and (assessed 2011, May 2)
- 2.Approved products with therapeutic equivalence evaluations. 31st edition. Washington, DC: US Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Pharmaceutical Science, Office of Generic Drugs, 2009. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf (assessed 2011, May 2)
- 3.U.S. Food and Drug Administration Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products – General Considerations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf
- 4.U.S. Food and Drug Administration Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070241.pdf
- 5.Model Bioequivalence Data Summary Tables. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM120957.pdf
- 6.U.S. Food and Drug Administration External Dissolution Methods Database: http://www.accessdata.fda.gov/scripts/cder/dissolution/index.cfm
- 7.U.S. Food and Drug Administration Guidance for Industry: Bioequivalence Recommendations for Specific Products: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075207.htm