Abstract
Sex differences in cocaine addiction warrants further research focused on examining the growing population of female cocaine addicts. As demonstrated in both clinical and preclinical research, females are more susceptible to drug relapse with anxiety being a contributing factor. In support of this, a recent clinical study from our laboratory highlights the importance of menstrual cycle phase and anxiety at treatment admission for cocaine addiction on treatment retention. In support of these trends in the clinical population, the purpose of the present study was to design an animal model to directly test the role of circulating hormone levels during cocaine withdrawal. To directly measure the influence of estrogen on anxiety-like behavior during early stages of withdrawal, both ovariectomized and intact female rodent models were employed. The elevated-plus maze and elevated-zero maze were used to assess anxiety-like behavior. Recent evidence in male rodents highlights a potential role for the delta opioid-receptor (DOR) system in the modulation of cocaine withdrawal-induced anxiety. In addition to the evaluation of hormonal effects, a potential anxiolytic specific for DOR was tested for its efficacy in females withdrawn from cocaine. Our results support the use of DOR agonists as a potential anxiolytic in females and highlight the importance of estrogen and other circulating hormones during all phases of cocaine addiction.
Keywords: behavior, cocaine, estrogen, opiate receptors
Cocaine addiction is a growing public health concern for the female population since the percentage of female cocaine users is on the rise. Studies comparing male and female addicts have shown sex differences in all aspects of drug abuse history, including age of first use, progression to dependence, and propensity to relapse following drug abstinence (reviewed in Becker and Hu, 2008). Typically, women report shorter abstinence periods between cocaine use with longer periods of use after abstinence (Kosten et al., 1996; Gallop et al., 2007). Women are also more likely to report stress, anxiety, negative emotions, and interpersonal problems prior to drug relapse, whereas men are more likely to seek the hedonic aspects of the drug (Back et al., 2005). Supporting evidence from animal models of drug self-administration demonstrate that females are more sensitive to the reinforcing and behavioral-activating effects of cocaine and exhibit an increase in vulnerability during the acquisition and relapse/reinstatement phases (Carroll et al., 2002; Lynch et al., 2002; Lynch and Taylor, 2004). Therefore, cocaine-withdrawal induced anxiety may be more debilitating for females and may contribute to their increased propensity to relapse.
Overall, females are more vulnerable to depression and anxiety (Kendler et al., 2001; Lynch et al., 2002) with evidence supporting the notion that fluctuations in estrogen and progesterone during the menstrual cycle are related to depression and anxiety susceptibility (Rupprecht and Holsboer, 1999). A recent retrospective clinical study from our laboratory demonstrated potential links between menstrual cycle phase likelihood to enter treatment, menstrual cycle phase and anxiety measures at admission, and anxiety and cocaine urinalysis at admission in a population of cocaine-addicted females (Ambrose-Lanci et al., 2009). This study revealed that factors such as menstrual cycle phase, anxiety and urinalysis at admission may all be contributing factors to treatment retention in the female population.
Previous studies examining anxiety-like behaviors in female rodent models have shown that normal hormone cycles can influence mood and affect. Specifically, it has been reported that low levels of estradiol result in increased anxiety-like behavior (Mora et al., 1996). Females show less anxiety- and depression-like behavior during proestrous and estrous when estradiol levels are high, as compared to diestrous when estradiol levels are low (Mora et al., 1996; Diaz-Veliz et al., 1997a, 1997b; Frye et al., 2000; Marcondes et al., 2001). The purpose of the present study was to extend previous clinical and preclinical findings through the use of a rodent model to better understand the mechanisms underlying the impact of circulating hormones. Specific focus was placed on the critical window during cocaine withdrawal when negative emotions such as anxiety may contribute to a female’s increased likelihood to relapse.
In addition to the impact of circulating hormones on withdrawal, the present study also examined the efficacy of the delta opioid receptor (DOR) agonist, SNC-80, as a potential anxiolytic for females during withdrawal. Recent evidence supports the role for the opioid receptor system, specifically the DOR, in modulating emotional state. DOR and enkephalin (the endogenous DOR ligand) knock-out mice demonstrate anxiogenic-like behaviors, suggesting a modulatory role for DOR on anxiety (Konig et al., 1996; Filliol et al., 2000). Interestingly, the DOR antagonist, naltrindole, causes anxiogenic-like effects due to decreased signaling through DOR (Marin et al., 2003). In males, SNC-80 produced anxiolytic-like effects similar to the effects of classical therapeutics for anxiety (Perrine et al., 2006) and was able to decrease anxiety-like behavior following withdrawal from chronic cocaine use (Perrine et al., 2008). The effects of SNC-80 on anxiety-like behavior in female rats following cocaine administration has yet to be reported.
In the present study, both ovariectomized (OVX) and intact female rodents were used to assess the influence of circulating estrogen on anxiety-like behaviors to test the hypothesis that lower estrogen levels correlate with an increase in anxiety-like behavior as measured by the elevated-plus maze (EPM) and elevated-zero maze (EZM). The ability of SNC-80, a DOR agonist, to alleviate cocaine withdrawal-induced anxiety in female rats was also examined.
MATERIALS AND METHODS
Animals
All animal care and procedures were conducted in compliance with the guidelines set forth by the Institutional Animal Care and Use Committee of Thomas Jefferson University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adequate measures were taken to minimize pain and discomfort. OVX female Sprague Dawley rats (OVX between 36 and 49 days) and intact female Sprague-Dawley rats (~60 days) were purchased from Harlan Laboratories (Indianapolis, IN) and allowed to acclimate to the animal facility prior to experimental manipulation. A total of 75 OVX and 83 intact females were used in these experiments. Animals were housed three to a cage and maintained on a 12 hr light/dark cycle (lights on at 7 am) with free access to food and water.
Drug Preparation
Cocaine HCl (generously provided by NIH/NIDA) was dissolved in saline (0.9%) to achieve 15 mg/ml or 20 mg/ml concentrations. SNC-80 (Tocris Bioscience, Ellisville, MO) was dissolved in saline (0.9%) and HCl to achieve a 5 mg/ml concentration. Both cocaine and SNC-80 were injected at a volume of 1 ml/kg body weight. 17β-estradiol was dissolved in sesame oil to achieve a 10 µg/ml concentration (Sigma Chemical Co., St Louis, MO).
Drug Administration
Chronic cocaine and hormone administration in OVX females
Two weeks after OVX, rats were randomly assigned to either cocaine or saline treatment groups. This timeline placed the OVX animals at the same age as intact females at the commencement of treatment. Animals were handled and weighed 2–3 days prior to drug treatment and on each day prior to injections. Animals received single daily i.p. injections of cocaine (20 mg/kg) or saline (1 ml/kg) for 14 days followed by a 24 hr withdrawal period before undergoing behavioral testing on the EZM. The cocaine withdrawal regimen is based on previous studies in male rodent models of cocaine addiction (Rudoy and Van Bockstaele, 2007; Perrine et al., 2008).
Animals were given one injection of 17β-estradiol in sesame oil vehicle (10 µg 17β-estradiol s.c.) or sesame oil vehicle (s.c.) 48 hr before behavioral testing on Day 13 of treatment. Treatment groups were as follows (see Table I): chronic saline with vehicle (Sal-V), chronic saline with 17β-estradiol (Sal-E), chronic cocaine with vehicle (Coc-V), and chronic cocaine with 17β-estradiol (Coc-E). This regimen of estradiol administration has been shown to be effective at producing physiological plasma levels of estradiol and eliciting anti-anxiety behavior (reviewed in (Walf and Frye, 2005). A subset of animals was subject to locomotor activity testing for control purposes.
TABLE I.
OVX Model Treatment Table
| Chronic Drug Treatment | Hormone Treatment | |
|---|---|---|
| Sal-V | Saline | Vehicle |
| Sal-E | Saline | Estrogen |
| Coc-V | Cocaine | Vehicle |
| Coc-E | Cocaine | Estrogen |
Binge cocaine administration in intact females
Animals were handled and weighed 2–3 days prior to drug treatment and on each day prior to injections. Rats were administered binge-pattern (3 injections/day at 1 hr intervals) cocaine (15 mg/kg i.p.) or saline (1 ml/kg i.p.) for 14 days followed by a 48 hr withdrawal period. Following the 48 hr withdrawal period, rats were subjected to one of two behavioral tests for anxiety, the EPM or EZM.
For the experiments designed to test the effect of SNC-80, animals received a challenge dose of SNC-80 (5 mg/kg) or vehicle (saline) 1 hr before behavioral maze testing. Dose and timing of SNC-80 administration was based on previous studies (Perrine et al., 2006, 2008). Both SNC-80 and saline were administered subcutaneously (s.c.). Treatment groups were as follows (see Table II): chronic saline with saline challenge (Sal-Sal), chronic saline with SNC-80 challenge (Sal-SNC-80), chronic cocaine with saline challenge (Coc-Sal), and chronic cocaine with SNC-80 challenge (Coc-SNC-80). A subset of animals was subject to locomotor activity testing for control purposes.
TABLE II.
Intact Model Treatment Table
| Chronic Drug Treatment | Challenge | |
|---|---|---|
| Sal-Sal | Saline | Saline |
| Sal-SNC-80 | Saline | SNC-80 |
| Coc-Sal | Cocaine | Saline |
| Coc-SNC-80 | Cocaine | SNC-80 |
Behavioral Measures for Anxiety-like Behavior
Elevated-Plus Maze (EPM)
The elevated-plus maze, a well established method to measure anxiety in rodents, was employed as a measure of anxiety-like behavior (Pellow et al., 1985; Handley and McBlane, 1993; Dawson and Tricklebank, 1995). The EPM exploits the rodent’s contradictory nature to explore but avoid open space, bright light and height. The maze consists of four arms made of black Plexiglass (~50 cm long × 10 cm wide). The maze was elevated ~50 cm above the ground (Handley and McBlane, 1993). The arms form the shape of a plus sign, with two opposite arms being enclosed (closed arm; 40 cm high walls) and two arms remaining open. A ledge (0.5 cm high) was present around the perimeter of the open arms of the maze which has been shown to increase the pharmacological reliability of this test (Fernandes and File, 1996). Testing was conducted in a dimly lit room with a constant illumination on the open arms of the maze. At the start of the 5-min testing session, each rat was placed on the same open arm facing the center of the maze. The maze was cleaned with 5% ethanol and dried after each testing session. Time spent in the open arm was used as the output measure for this maze since known anxiolytics result in an increase in time spent in open arms (Pellow et al., 1985) and known anxiogenic drugs decrease time spent in open arms (Basso et al., 1999).
Elevated-Zero Maze (EZM)
The EZM is a modification of the EPM that is also a reliable and sensitive model of anxiety-like behavior in rodents (Shepherd et al., 1994). The EZM consists of a black ABS plastic annular platform (~120 cm diameter) elevated ~70 cm above the ground. It is divided into four equal quadrants which are ~20cm wide: two open and two closed. The two open quadrants are opposite each other and are surrounded by a 1-cm “lip”. The two closed quadrants are enclosed by walls (~27 cm high) on both the inner and outer edges of the platform. The circular nature of the EZM abrogates the issues concerning the interpretation of time spent in the center square inherent to the EPM, while allowing the animal to have uninterrupted exploration during behavioral testing (Shepherd et al., 1994). Behavior testing on the EZM was conducted in the same manner as the EPM described above. Pharmacological control studies were conducted to test the validity of behavioral tests.
Locomotor activity
Following behavioral testing, locomotor activity was assessed in a subset of animals from each treatment group to determine whether treatment influenced locomotor activity during withdrawal. Animals were placed in a home cage environment within the Home Cage Video Tracking System (Med Associates, St. Albans, Vermont) which includes a sound-attenuating cubicle, video tracking interface, computer interface card, and Activity Monitor 5 software (Med Associates). The locomotor activity was recorded for 5 min.
Chemiluminescent immunoassay for estradiol levels
Immediately after behavioral and locomotor testing, animals were anesthetized using isofluorane and sacrificed by decapitation and trunk blood was collected on ice for analysis of blood estradiol level. Blood was centrifuged at 4°C and serum collected and frozen at −20°C. Blood samples were sent to the Clinical Chemistry Laboratory at Thomas Jefferson University Hospital and analyzed for estradiol level using chemiluminescent immunoassays with The Access Immunoassay System.
Data Analysis
Behavioral measures for anxiety-like behavior
Anxiety-like behavior was assessed by calculating the amount of time spent in the open arms and reported as a percentage of the total time on the maze. Full entry into open or closed arms was counted when all four paws crossed the plane of a new arm. The initial time spent on the open arm of the maze was subtracted before calculating percent open arm time. Data was expressed as a percentage [(open arm time (s)/total time (s)) × 100] and each group was reported as average open arm time ± Standard Error of the Mean (S.E.M.) This experimental design consists of two independent variables generating continuous data for the dependent variable, therefore, a two-way analysis of variance (ANOVA) with statistical significance set at p < 0.05 was used for statistical analysis.
Behavior was scored by an experimenter present in the room and recorded on a DVD for subsequent analysis by a trained scorer that was blinded to treatment group. Each scorers’ average for the data set was reported as average open arm time ± Standard Deviation (S.D.) To determine inter-rater reliability, a Pearson correlation was performed to compare real time data collection to data scored from recorded tests, with statistical significance set at p < 0.05.
Locomotor activity
Mean distance traveled was calculated from the average of total distance traveled in 1-min increments. Mean ambulatory distance traveled per minute of testing (cm/min) was then averaged within treatment groups. These data were analyzed using two-way ANOVA with statistical significance set at p < 0.05.
Estradiol levels
Mean blood estradiol levels were calculated for each group and compared using t-tests with statistical significance set at p < .05.
RESULTS
Validation of OVX Model: Analysis of Body Weight and Blood Estradiol Level
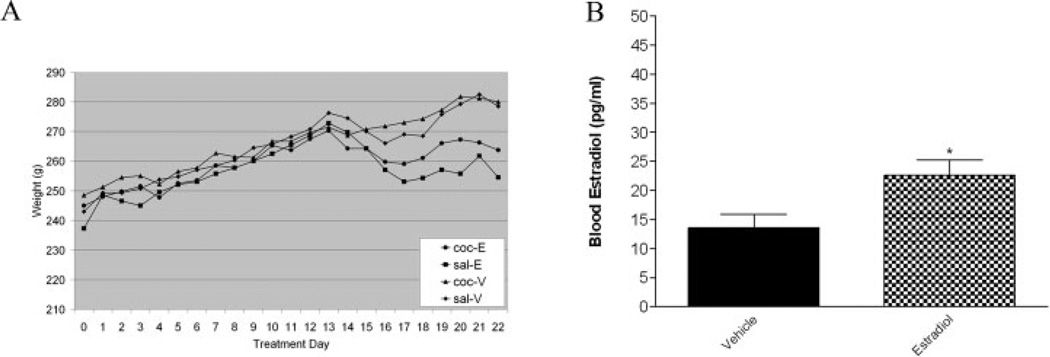
Ovariectomy removes the primary source of circulating estrogen creating a model of estrogen depletion. A group of OVX animals administered estrogen can be compared to OVX animals without estrogen replacement in order to study behavioral changes. To ensure that the effects of estrogen have been attenuated, controls were conducted before further analysis using this animal model. It is well established that estrogen depletion due to OVX causes an increase in body weight (Davidge et al., 2001). To validate that OVX reduced estrogen function, body weight was tracked for the duration of the study. Repeated measure ANOVA crossing hormone and time demonstrated a main effect of hormone (Fig. 1A) (F(1,12) = 8.698, p < .05, n = 4/ treatment group) on body weight, indicating that independent of drug treatment, animals administered estradiol (Sal-E and Coc-E) had reduced body weight compared to vehicle controls (Sal-V and Coc-V).
Fig. 1.
Body weight and blood estradiol level were used as measures of OVX efficiency. A: Independent of drug treatment, animals administered estradiol on treatment Day 13 demonstrated a reduction in body weight compared to vehicle controls. A repeated measure ANOVA crossing hormone and time demonstrated a main effect of hormone (p < .05, n = 4/ treatment group). B: Estradiol-treated OVX rats had significantly higher blood estradiol levels (pg/ml) compared to vehicle-treated controls (p < .05, n = 10–13/ treatment group).
Blood estradiol level was also assessed to confirm differences in blood estradiol level among the groups. Estradiol primed OVX females (Sal-E and Coc-E) had significantly higher plasma estradiol concentrations compared to vehicle treated OVX females (Sal-V and Coc-V) (Fig. 1B) (t = 2.520, p = .0199, n = 10–13/ treatment group). There was also no difference in estradiol levels between groups administered saline vs. cocaine (t = .2619, p = .7960, n = 9–13/ treatment group). These results validate that OVX surgery successfully depleted circulating estradiol levels which were restored by estradiol replacement.
Relationship between blood estradiol level and anxiety-like behavior in OVX females during early cocaine withdrawal
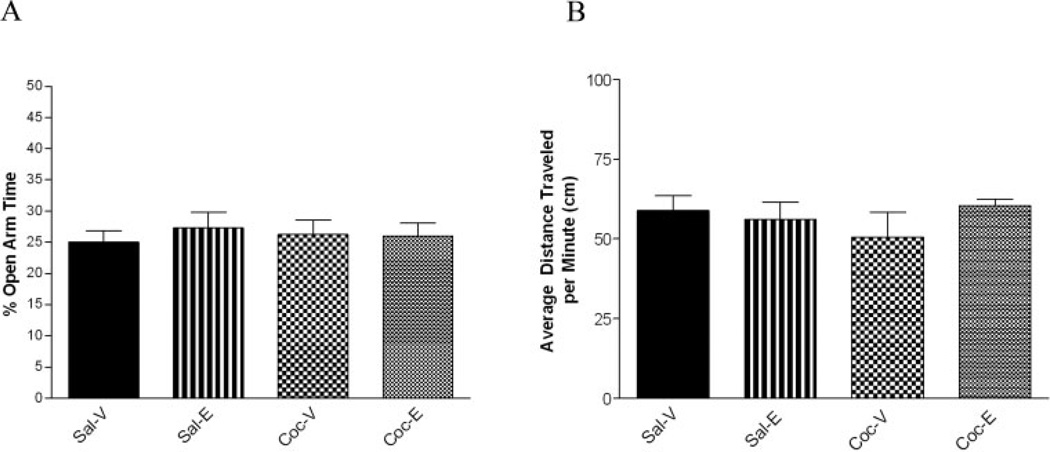
In the OVX model, the ability of estradiol to alleviate anxiety-like behavior during early withdrawal from chronic cocaine administration was assessed using the EZM. Percent time spent in the open arms of the EZM was reported as a measure of anxiety-like behavior. Percent time spent in the open arms was averaged within each of the four treatment groups and means were compared using two-way ANOVA. The means for each group were as follows: (Ms ± SEM) Sal-V (25.023 ± 2.361); Sal-E (27.286 ± 2.361); Coc-V (26.297 ± 2.082); Coc-E (25.999 ± 2.208). Statistical analysis revealed no main effects of drug F(1,58) = .001, p = .969, hormone F(1,58) = .130, p = .720, or drug*hormone interaction F(1,58) = .614, p = .437 (n = 14–16/ treatment group)(Fig. 2A). Inter-rater reliability was established using Pearson correlation (r = .890, p < .01) between both scorers (Ms ± SD 22.54 ± 8.46 and 18.91 ± 8.19).
Fig. 2.
Anxiety-like behavior and locomotor activity of OVX females during cocaine withdrawal. A: Percent time spent in the open arms of the EZM was reported as a measure of anxiety-like behavior. Two-way ANOVA revealed no main effect of drug, hormone, or drug *hormone interaction (n = 14–16/ treatment group). B: Following behavioral testing, locomotor activity was compared among groups. Two-way ANOVA revealed no main effect of drug, hormone, or drug *hormone interaction (n = 6–7/ treatment group).
Locomotor activity was assessed immediately following behavioral testing to evaluate the effect of treatment group on locomotion. As expected, two-way ANOVA revealed no main effect of drug F(1,21) = .126, p = .727, hormone F(1,21) = .384, p = .542, or drug*hormone interaction F(1,21) = .1.194, p = .287 (n = 6–7/ treatment group) (Figure 2B).
Relationship between blood estradiol level and anxiety-like behavior in intact females during early phase cocaine withdrawal
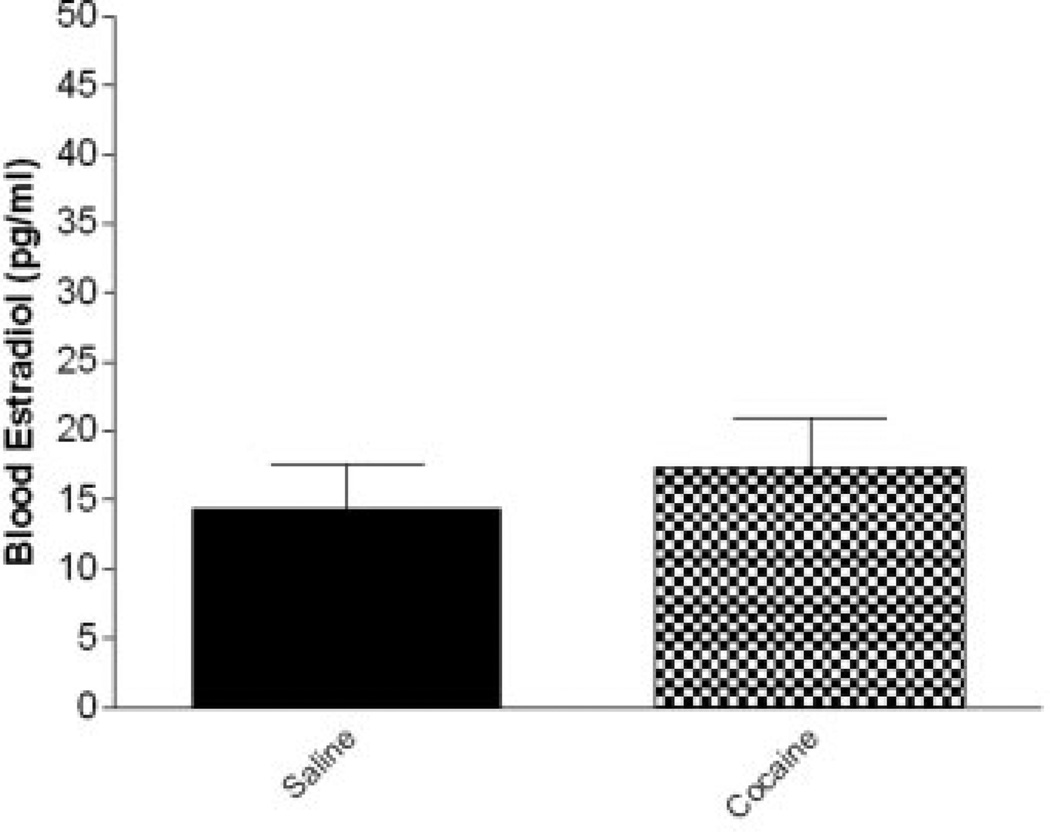
In order to evaluate the influence of estradiol on anxiety-like behavior in a model more physiologically related to human cocaine addicts, intact female rats were tested. Blood estradiol levels of these intact females were within the physiological range (0–40 pg/ml; Ms = 15.83 ± 13.065). No significant differences in average blood estradiol level between animals treated chronically with cocaine or saline were observed: (Ms ± S.D.) Cocaine (17.3800 ± 13.67), Saline (14.38 ± 12.74); t(29) = −.634, p = .535) (n = 15–16/ treatment group) (Fig. 3).
Fig. 3.
Comparison of blood estradiol level between drug treatment groups in intact females. An unpaired t-test revealed no difference in average blood estradiol level between groups (n = 15–16/ treatment group).
Percent time spent in the open arms was reported as a measure of anxiety-like behavior. The Pearson correlation conducted to determine whether blood estradiol level had a direct impact on anxiety-like behavior did not reach statistical significance (n = 31,r = .141, p = .449). There was also no correlation between these two variables when analyzed within cocaine (n = 15, r = .081, p = .775) or saline (n = 31,r = .144 p = .594) treatment groups.
Effect of SNC-80 on cocaine-withdrawal induced anxiety in intact female rats
SNC-80 was administered to evaluate its potential anxiolytic effects in females during withdrawal. The influence of SNC-80 on anxiety-like behavior was evaluated in intact female rats during early withdrawal from chronic cocaine using the EPM and EZM.
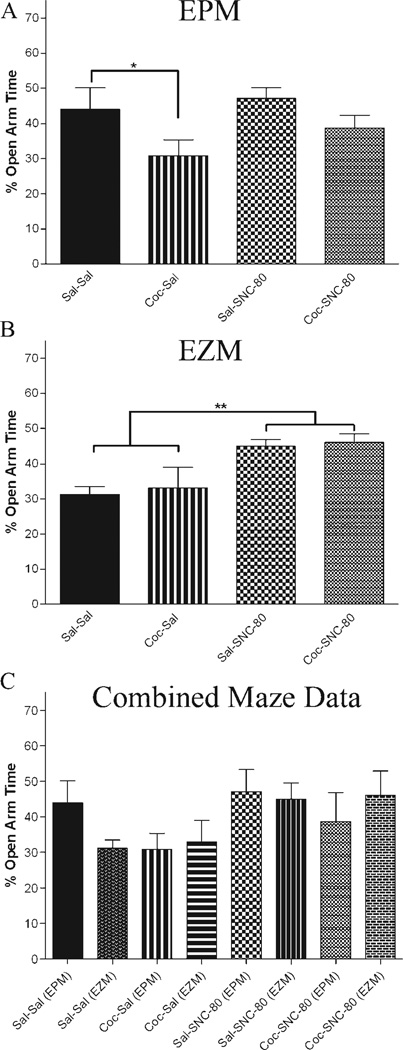
Percent time spent in the open arms was averaged within each of the four treatment groups and means were compared using two-way ANOVA. For animals tested on the EPM, the means for each group were as follows, (Ms ± SEM) Sal-Sal (44.00 ± 4.94); Sal-SNC-80 (47.17 ± 4.94); Coc-Sal (30.835 ± 4.04); Coc-SNC-80 (38.732 ± 4.42). Two-way ANOVA revealed a main effect of treatment condition on anxiety-like behavior (F (1, 15) = 5.507, p < .05) whereby cocaine administration resulted in a significant decrease in open arm time representing an increase in anxiety-like behavior. There was no main effect of the challenge condition (F (1, 15) = 1.445, p = .248) or drug*challenge interaction (F (1, 15) = .264, p = .614) (n = 4–6/ treatment group) (Fig. 4A). Inter-rater reliability was established using Pearson correlation (r = .958, p < 0.01) between both scorers (Ms ± SD 39.12 ± 11.14 and 39.51 ± 11.45).
Fig. 4.
Influence of SNC-80 on anxiety-like behavior on intact females during cocaine withdrawal. Behavior was tested using the EPM and EZM. Anxiety-like behavior was reported as percent time spent in the open arms and statistical significance was determined using two-way ANOVA. A: EPM data revealed a significant decrease in open arm time in animals treated with cocaine compared to saline controls (p < .05). No effect of SNC-80 challenge was demonstrated (n = 4–6/treatment group). B: Females tested using the EZM showed no effect of drug treatment on anxiety-like behavior; however, a main effect of the challenge condition was observed (p < .001) with SNC-80 causing an increase in open arm time (n = 6–10/treatment group). C: Combined analysis using maze type as a potential factor influencing behavioral outcome revealed no main effect of drug treatment or maze; however, SNC-80 administration increased time spent in the arm (p < 0.1).
For animals tested using the EZM, the means for each group were as follows: (Ms ± SEM) Sal-Sal (31.27 ± 3.23), Sal-SNC-80 (45.00 ± 4.17), Coc-Sal (33.04 ± 3.61) Coc-SNC-80 (46.08 ± 3.61). Two-way ANOVA revealed no main effect of treatment condition on anxiety-like behavior (F (1, 28) = .151, p = .700). However, a main effect of the challenge condition was observed (F (1, 28) = 13.299, p < .001) with SNC-80 demonstrating anxiolytic effects. There was no main effect of drug*challenge interaction (F (1, 28) = .009, p = .925) (n = 6–10/ treatment group) (Fig. 4B). Interrater reliability was established using Pearson correlation (r = .945, p < 0.01) between both scorers (Ms ± SD 37.99 ± 11.90 and 32.97 ± 9.98).
As reported above, results varied depending on the maze used for behavioral testing. For further analysis, data was combined with the addition of maze type (EPM vs. EZM) as a factor and a two-way ANOVA was performed. Interestingly, there was no main effect of treatment (F (1, 43) = .2.489, p = .122) or maze (F (1, 43) = .202, p = .655) when combined data was analyzed. However, there was a main effect of the challenge condition (F (1, 43) = 10.143, p < .01) with SNC-80 demonstrating anxiolytic effects. When analyzing interaction effects, no drug*challenge (F(1,43) = .115, p = .736), challenge*maze (F(1,43) = 1.748, p = .193) or treatment*challenge*maze interactions (F(1,43) = .209, p = .650) were noted. However an interaction of treatment* maze was observed (F(1,43) = 4.238, p < .05). The means for each group are graphically depicted in Figure 4C. An overall anxiolytic effect for SNC-80 is demonstrated.
Effect of treatment and challenge on locomotor activity during drug administration and withdrawal
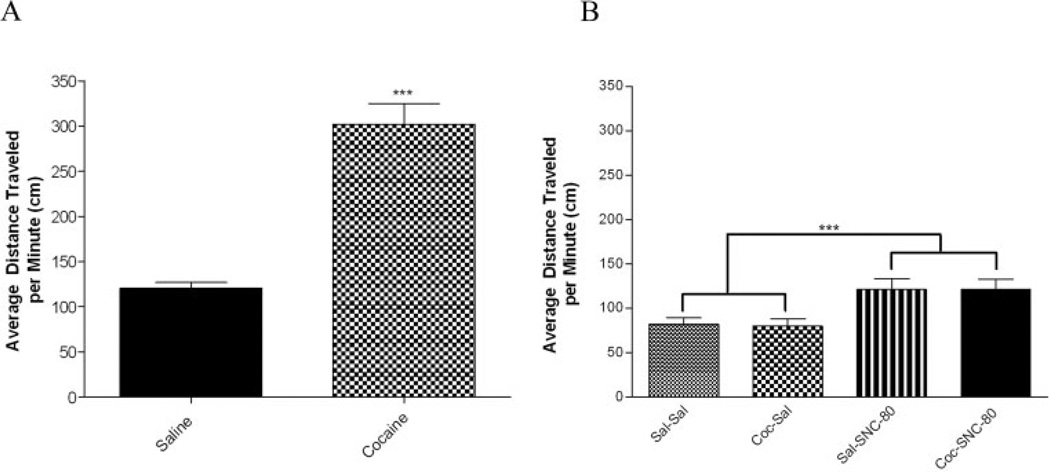
Locomotor activity is reported as mean ambulatory distance traveled averaged for each treatment group (Ms ± SEM). Following the final injection on Day 12 of cocaine or saline administration, animals were placed in an activity monitor for locomotor assessment. Mean ambulatory distance traveled was compared between groups using a t-test. As expected, cocaine treated animals displayed a statistically significant increase in locomotor activity compared to saline treated animals (t(30) = 7.659, p < .0001, n = 16/treatment group) (Fig. 5A).
Fig. 5.
Locomotor assessment of intact cocaine-treated females. Locomotor activity was assessed using mean ambulatory distance traveled during treatment (A) and during cocaine withdrawal (B). A: Cocaine caused a significant increase in locomotor activity during drug treatment (p < .001, n = 16/treatment group). B: Animals administered SNC-80 challenge exhibited a significant increase in locomotor activity (p < .001, n = 6–10/ treatment group) as evaluated using two-way ANOVA. The cocaine-induced increase in locomotor activity during treatment was ameliorated during withdrawal.
Locomotor activity was also assessed during cocaine withdrawal following behavioral testing. The mean ambulatory distance traveled for each treatment groups are as follows: Sal-Sal (82.27 ± 23.89); Sal-SNC-80 (121.04 ± 30.21); Coc-Sal (79.92 ± 23.63); Coc-SNC-80 (121.22 ± 32.67). Two-way ANOVA revealed a main effect of challenge (F(1, 28) = 16.483, p < .001) but not for drug treatment (F(1,28) = .012, p = .913). The interaction effect of drug*challenge was not significant (F(1, 28) = .016, p = .899) (n = 6–10/ treatment group) (Fig. 5B).
DISCUSSION
The present study is a first step in elucidating the relationship between cocaine withdrawal and circulating hormone levels in two female rodent models of cocaine withdrawal. These results extend previous analysis regarding the role of circulating hormone levels during cocaine withdrawal by showing that increased estrogen level was not correlated to a decrease in anxiety-like behavior in the present animal models. However, the DOR agonist, SNC-80, was able to alleviate anxiety in females. The present findings highlight the need for further studies focused on evaluating differences between males and females during cocaine withdrawal and in regards to behavioral testing.
Methodological Considerations
To our knowledge this is the first study focused on the effects of chronic cocaine treatment on anxiety-like behavior in female rodent models. As mentioned in the methods section, treatment paradigms used were based on previous work testing cocaine withdrawal-induced anxiety and estradiol’s influence on anxiety. These results demonstrate the complexity associated with the study of female models and lays the ground work for future studies determined to understand the differences inherent to male and female animal models of addiction.
Behavioral Tests
Females are often not included in behavioral experiments as it is thought that their fluctuating hormones render the results of such experiments too variable. However, it is essential to elucidate the influence of sex differences on behavior, as there is evidence to support that sex may influence behavioral tests (Johnston and File, 1991). Our data are in agreement with these studies as our behavioral data support complex effects on anxiety-related behaviors in female rodent models.
Data from the EPM revealed a significant increase in anxiety-like behavior during cocaine withdrawal compared to saline controls; however, there was no significant change in anxiety-like behavior following administration of an SNC-80 challenge dose. While the EZM did not show a significant effect of chronic cocaine on anxiety-like behavior, a statistically significant decrease in anxiety-like behavior was observed following administration of SNC-80. Although both mazes are designed to test anxiety-like behavior in rodents by measuring open arm time, the present data demonstrate that there are inherent differences in these similar tests. In male rodent models, cocaine-withdrawal induced anxiety has been consistently detected (Perrine et al., 2008), further supporting the influence of sex on cocaine withdrawal and behavioral testing.
It is also possible that sex differences in cocaine seeking behavior may affect behavioral testing during cocaine withdrawal. A recent study reported that cocaine seeking behavior was influenced by sex in a time-dependent manner during long-term withdrawal. Interestingly, estrous cycle phase influenced this cocaine seeking behavior during self-administration, early, and long-term cocaine withdrawal (Kerstetter et al., 2008). If female rats have a greater drive for cocaine-seeking while on the maze, a subsequent increase in overall exploration could affect behavioral outcome measures. This may be an underlying factor contributing to the inability of exploratory tests of anxiety to reliably show cocaineinduced anxiety in females. Future studies utilizing the defensive burying paradigm for testing anxiety-like behavior in females may be beneficial.
Testing Hormonal Effects in Female Rats
Both OVX and intact female models were used in the present study. OVX females were used as a first step in trying to elucidate the role of estradiol in anxiety-like behavior during cocaine withdrawal. Comparing OVX animals to animals given estrogen replacement provides a direct way to examine the influence of estradiol on anxiety-like behavior during cocaine withdrawal. However, disruption of natural hormone cycles is a confounding factor. Intact females were also used as a model of normal hormone fluctuation, which more closely mimics the natural hormonal state in female cocaine addicts. Estrous cycle phase was not taken into account since we were interested in determining whether circulating blood estradiol level during behavioral testing would directly correlate to changes in anxiety-like behavior. However, estradiol level being a continuous variable posed a challenge when attempting to correlate directly with behavioral measures.
The inability of estrogen replacement to elicit an effect on anxiety-like behavior in the OVX model may be due to the length of estrogen administration. The animal models used in the present study focused on the level of blood estradiol during the withdrawal period. However, the presence of estradiol at all phases of the addiction process may be essential for eliciting behavioral changes during withdrawal. A recent study by Pandaranandaka et al. investigated the influence of chronic estrogen on anxiety-like behavior in naive animals. In this study, OVX females in the estrogen supplementation group were administered daily injections of 17β-estradiol (10 µg/kg, s.c.) for four weeks following OVX. This treatment paradigm resulted in an increased time spent in the open arms of the EPM indicative of the anxiolytic properties of estrogen (Pandaranandaka et al., 2006). Chronic hormone replacement may enhance the physiological relevance of using an OVX model for the study of estrogen’s effect on sex difference in cocaine addiction. In addition, future studies should also take into account the dual influence of estrogen and progesterone since progesterone is also involved in influencing emotional state (Mora et al., 1996). Studies focusing on hormonal influences throughout the addiction process may lead to hormone based treatments for the clinical population.
DOR Agonists as Potential Therapeutic Agents During Cocaine Withdrawal
This is the first study to test the efficacy of SNC-80 as a potential anxiolytic during cocaine withdrawal in female rats. Based on the results of the combined analysis using EPM and EZM data, SNC-80 was able to alleviate anxiety-like behavior.
Consistent with previous findings, SNC-80 caused an increase in locomotor activity (Spina et al., 1998; Perrine et al., 2008). Despite this potential caveat, the use of DOR agonists are promising since they have fewer negative side effects and lower abuse potential compared to other opiates (Jutkiewicz, 2006). Future studies are warranted in order to further examine the therapeutic potential of SNC-80 in female rodent models.
Previous studies from our laboratory provide ultrastructural evidence for intracellular trafficking of DOR during early withdrawal phases (Ambrose-Lanci et al., 2008). In light of the present study, it is possible that SNC-80 is able to mediate its anxiolytic effect by activating the functional DOR remaining on the plasma membrane during cocaine withdrawal. Clinical observations report that cocaine addicts often use opiates to alleviate the negative symptomology often associated with cocaine withdrawal, emphasizing a potential role for the opioid system during this critical period (Gawin 1991; O’Brian 2001). DOR agonists may be beneficial as a therapeutic for addiction, anxiety and depression, and other opioid-related disease states, such as pain.
Could Hormone Therapy Benefit a Subset of Female Cocaine Addicts?
Reports from the National Cancer Institute state that the two most prescribed hormone replacement drugs, Premarin and Prempro, dropped from 61 million prescriptions in 2001 to 21 million in 2004. This is most likely due to the controversy that has surrounded hormone replacement therapy (HRT) since the release of the results from the Women’s Health Initiative. However, more recent research on the benefits of HRT has focused on estrogen’s effect during the “critical period” defined as early phases of the menopause transition before an estrogen withdrawal state is experienced. As more research is conducted, it is becoming apparent that individualized care is the best way to manage the menopause transition as well as many medical issues including drug addiction. In terms of addiction, specifically cocaine addiction, there is a need for individually tailored treatment programs for women. Currently, 83% of facilities offer specially designed programs for addiction with only 32% tailored for adult women and only 14% for pregnant/ postpartum women (SAMHSA, 2006). Females will benefit from programs that acknowledge the influence of circulating hormone levels and other issues, such as anxiety, on the transition from drug use to abstinence.
CONCLUSIONS
The present study highlights the need for further research focusing on the contribution of circulating hormones and DOR agonists on cocaine withdrawal-induced anxiety in females and discusses the caveats inherent to research committed to understanding sex differences. Future clinical research focused on these areas is essential to aid in tailoring treatment programs for individual needs and to uncover the potential benefits of pharmacotherapy utilizing both anxiolytics and hormone replacement therapy during cocaine withdrawal.
ACKNOWLEDGMENTS
We would like to thank Dr. Ellen Unterwald and Dr. Shane Perrine for their assistance with the drug administration and behavioral methodologies. We would also like to thank Lauren Stamps for her technical assistance and behavioral scoring.
Contract grant sponsor: National Institutes of Health; Contract grant number: DA# 009082-10A2 (to E.V.B.); Contract grant sponsor: National Institutes of Health; Contract grant number: F31DA#021981 (to L.M.A.L.).
REFERENCES
- Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ. Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain Res. 2008;1021:92–102. doi: 10.1016/j.brainres.2008.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose-Lanci LM, Sterling RC, Weinstein SP, Van Bockstaele EJ. The influence of intake urinalysis, psychopathology measures, and menstrual cycle phase on treatment outcome. The American Journal on Addictions. 2009;18(2):1–6. doi: 10.1080/10550490902772710. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the "anxiogenic-like" effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: Phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang Y, Stewart KG. A comparison of ovariectomy models for estrogen studies. Am J Physiol Regul Integr Comp Physiol. 2001;280:R904–R907. doi: 10.1152/ajpregu.2001.280.3.R904. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: Influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav. 1997a;58:637–642. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Dussaubat N, Mora S. Ketanserin effects on rat behavioral responses: Modifications by the estrous cycle, ovariectomy and estradiol replacement. Pharmacol Biochem Behav. 1997b;57:687–692. doi: 10.1016/s0091-3057(96)00394-2. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gallop RJ, Crits-Christoph P, Ten Have TR, Barber JP, Frank A, Griffin ML, Thase ME. Differential transitions between cocaine use and abstinence for men and women. J Consult Clin Psychol. 2007;75:95–103. doi: 10.1037/0022-006X.75.1.95. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant -like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Gawin FH, Rounsaville BJ, Kleber HD. Cocaine abuse among opioid addicts: Demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-hr/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plusmaze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Marin S, Marco E, Biscaia M, Fernandez B, Rubio M, Guaza C, Schmidhammer H, Viveros MP. Involvement of the kappa-opioid receptor in the anxiogenic-like effect of CP 55,940 in male rats. Pharmacol Biochem Behav. 2003;74:649–656. doi: 10.1016/s0091-3057(02)01041-9. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- O’Brian C. Drug Addiction and Drug Abuse. In: Hardman JG, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th edition. New York: McGraw Hill; 2001. pp. 633–636. [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav. 2006;87:828–835. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1119–1129. doi: 10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey of Substance Abuse Treatment Services. 2006 [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Spina L, Longoni R, Mulas A, Chang KJ, Di Chiara G. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 1. Locomotion, rearing and stereotypies in intact rats. Behav Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]