Abstract
Objective
Assays for T cell receptor excision circles (TREC) have been utilized in human, primate, and mouse models as a measure of thymic activity, but no comparable assay has been described in artiodactyls. We describe the development of the porcine signal joint (sj) TREC assay, and provide a likely reason for previous difficulties in its identification in artiodactyls.
Design and Methods
Utilizing the homology between the known genomic sequences in sjTREC in human and mouse, polymerase chain reaction (PCR) primers were derived for the putative porcine sjTREC. Primers from the ψJα side of the sjTREC were derived from the known porcine sequence.
Results
The sjTREC in two artiodactyls, swine and sheep, was identified using forward primers from the ψJα region, and reverse primers from the putative δ-rec region. Unlike in the detection of primate TRECs, initially the use of similar primers close to the δ-rec failed to yield the sjTREC product. Marching about 800 basepairs into δ-rec, primers derived from a homology region between human and mouse led to the detection of sjTREC. Comparing sjTREC amongst the species revealed highest homology between the two artiodactyls. A quantitative PCR (QPCR) assay of porcine sjTREC was also developed.
Conclusion
Identification and analysis of the sjTREC sequences in two artiodactyls suggested why previous attempts at cloning the pig TREC using known sjTREC sequences were unsuccessful. The development of the porcine signal joint TREC assay should enable a more direct quantification of thymic activity in porcine models of transplant biology.
Keywords: T-cell receptor excision circle, Signal joint TREC, Pig, T cell
Swine serve as an excellent large animal model for studies of transplantation immunology. Studies conducted in our laboratory with partially inbred miniature swine have shown a critical role for the thymus/recent thymic emigrants (RTEs) in solid organ allograft tolerance (1–5). Understanding thymic activity during organ transplantation may help us to modify transplantation protocols to optimize graft survival and promote tolerance. T-cell receptor (TCR) excision circle assay has been used in human, primate, and mouse studies to quantify thymic activity (6–16). We investigated whether a reliable TREC assay can be established in the miniature swine.
For generation of a functional TCR α chain during differentiation, the δ locus which lies within the α locus needs to be excised. One particular rearrangement event within the TCR α/δ locus, occurring between recombination signal sequence (RSS) of the δ-rec region, which lies 5′ to the variable δ region, and the RSS of the ψJα region, which lies 3′ to the constant δ region and upstream of joining α region, produce a unique signal joint TREC sequence (6,17,18). sjTREC analysis has been reported and used to study thymic activity in mice and non-human primates, but not in artiodactyls (6,8,9,12–14,18–20).
The application of primers and polymerase chain reaction (PCR) conditions used for human sjTREC detection facilitated the discovery of non-human primate sjTREC sequences (8,9,14). Also, the high homology between the human and the non-human primate TCR sequences of the δ-rec and ψJα regions was another advantage in the generation of non-human primate sjTREC. Previously, similar approaches were used in our laboratory and by other groups working with large animal models but were unsuccessful in detecting the TREC in any artiodactyl species. Possibilities that might explain this are as follows: (1) poor sequence homology between the human and the artiodactyl δ-rec and ψJα regions; (2) large sequence deletions/additions in the artiodactyl δ-rec/ψJα regions which could drastically alter PCR conditions; (3) absence/very low frequency of the use of the δ-rec/ψJα rearrangement in the artiodactyl αβ T-cell lineage, thus precluding its detection.
We report the detection of the signal joint TREC in two artiodactyls: pig and sheep. Initial attempts at cloning the sjTREC using primers close to the δ-rec region failed, but marching about 800 basepairs (bp) into the δ-rec region enabled detection of the sjTREC. We also developed a reliable quantitative PCR (QPCR) assay for quantifying sjTREC molecules as markers of porcine thymic activity.
RESULTS
Porcine Signal Joint TREC Sequence
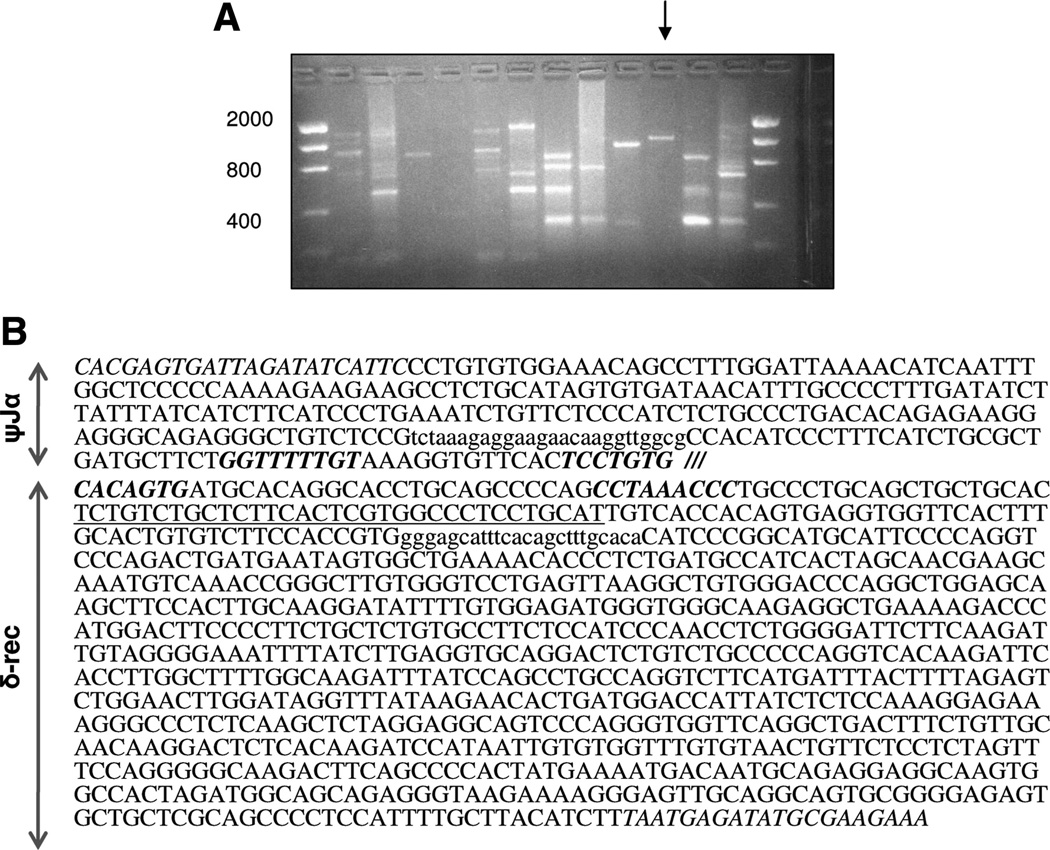
In swine, only the sequence between the constant region of the δ locus to the constant region of the α locus has been sequenced and published (21). The variable region of the α locus to the constant region of the δ locus is still unsequenced. However, given that there is a [Psi]Jα region, it was hypothesized that at least one δ-rec region should exist mediating the rearrangement of the α locus. Initial primers, designed in the proximal δ-rec RSS region based on homology among human, primate, and mouse sequences, did not amplify the expected PCR products. Even on altering PCR conditions, the porcine or sheep TREC could not be obtained. Therefore, using the known TCR A/D loci sequences in human and mouse, δ-rec primers were designed in an area approximately 800 bp from the RSS of δ-rec. An expected band of approximately 1100 bp was obtained by using these δ-rec primers (Fig. 1A). This band was found to be the signal joint TREC in the pig. The presence of the δ-rec/ψJα recombination sites together in the sequence confirmed the formation of the sjTREC in the pig, suggesting that a population of αβ T-cell lineage does undergo δ-rec/ψJα recombination. The sequence of the sjTREC in the pig is shown in Figure 1(B).
FIGURE 1.
Identification of the porcine sjTREC. (A) Multiple combinations of primers derived from the δ-rec and ψJα regions of human and mouse were used for polymerase chain reaction (PCR). A primer combination with an expected sjTREC product of approximately 1150 bp was noted as a single band (arrow). This product was reproduced in thymic genomic DNA from three miniature swine. The isolated final PCR product of 1167 bp was found to contain the signal joint TREC sequence. (B) Porcine sjTREC sequence is shown. The RSS sites of the δ-rec and ψJα regions are separated by /// symbol, with RSS italicized and in bold. The ψJα and δ-rec primers are italicized, but not in bold. Primer sequences used for quantitative PCR (QPCR) are shown in lower case, and the QPCR probe is underlined.
sjTREC Can Be Detected in Sheep
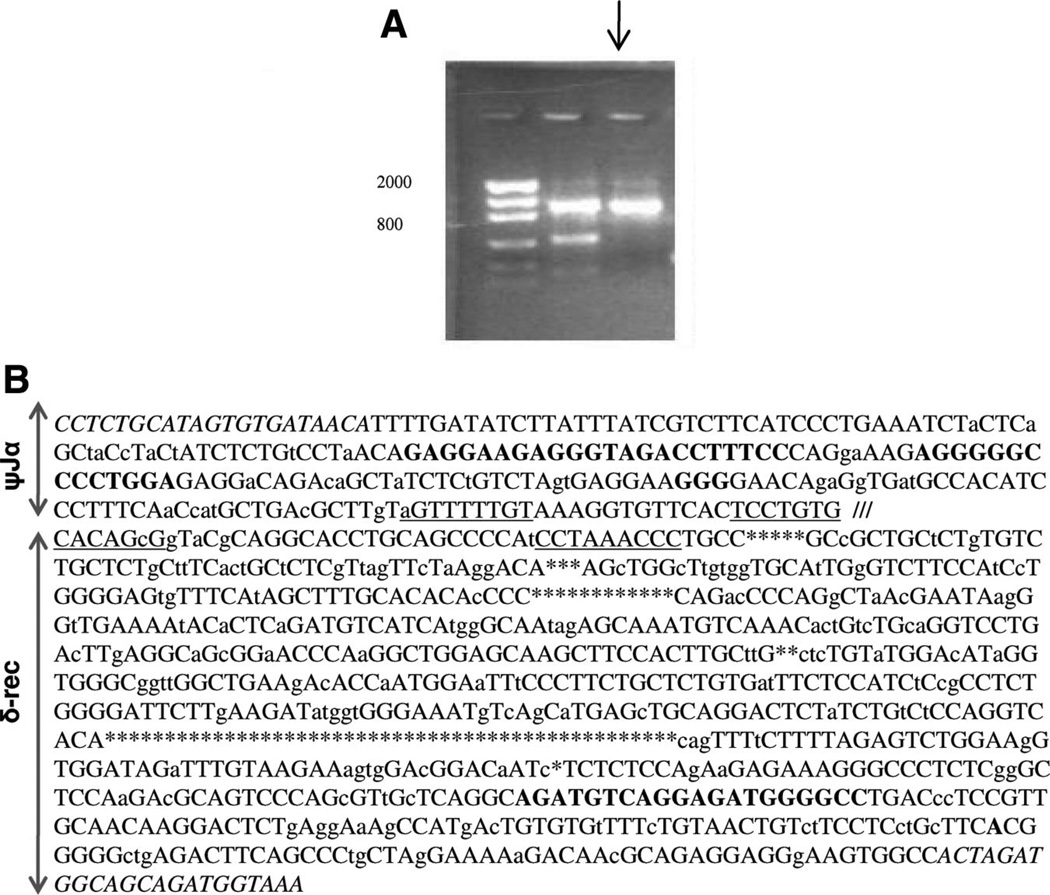
Using primers derived from the known sjTREC sequence in pig, sheep sjTREC was detected as a PCR product of 1155 bp (Fig. 2A). The sheep sequence was 12 bp shorter than the pig sjTREC. As expected, the sjTREC between the pig and the sheep showed areas of significant homology, especially around the RSS sites of δ-rec and ψJα (88% homology), as determined by the standard National Center for Biotechnology Information (NCBI) Blast program. Across various segments of the sjTREC, 79.2% of the sequence showed homology of more than 75% between the pig and sheep sjTREC (Fig. 2B).
FIGURE 2.
sjTREC can also be isolated in sheep. (A) Using primers derived from the porcine sjTREC, an expected band of approximately 1150 bp was obtained by PCR of sheep peripheral blood genomic DNA (arrow). (B) Sheep sjTREC sequence is shown, drawing comparison to a homologous segment of the porcine sjTREC. Nucleotides differences between the sheep and pig sjTREC are shown in small case letter text; black capital text represents identical sequences between the two species. Areas of highest homology were at the δ-rec and ψJα RSS sites (underlined). The RSS sites of the δ-rec and ψJα regions are separated by /// symbol. Areas in which there were nucleotides missing in the sheep sjTREC are shown with * symbol. Areas where nucleotides were seen in the sheep sjTREC but absent in the porcine sjTREC are shown in bold. δ-rec and ψJα primers are italicized.
Comparison of the Artiodactyls sjTRECs to Human, Baboon, and Mouse Sequences
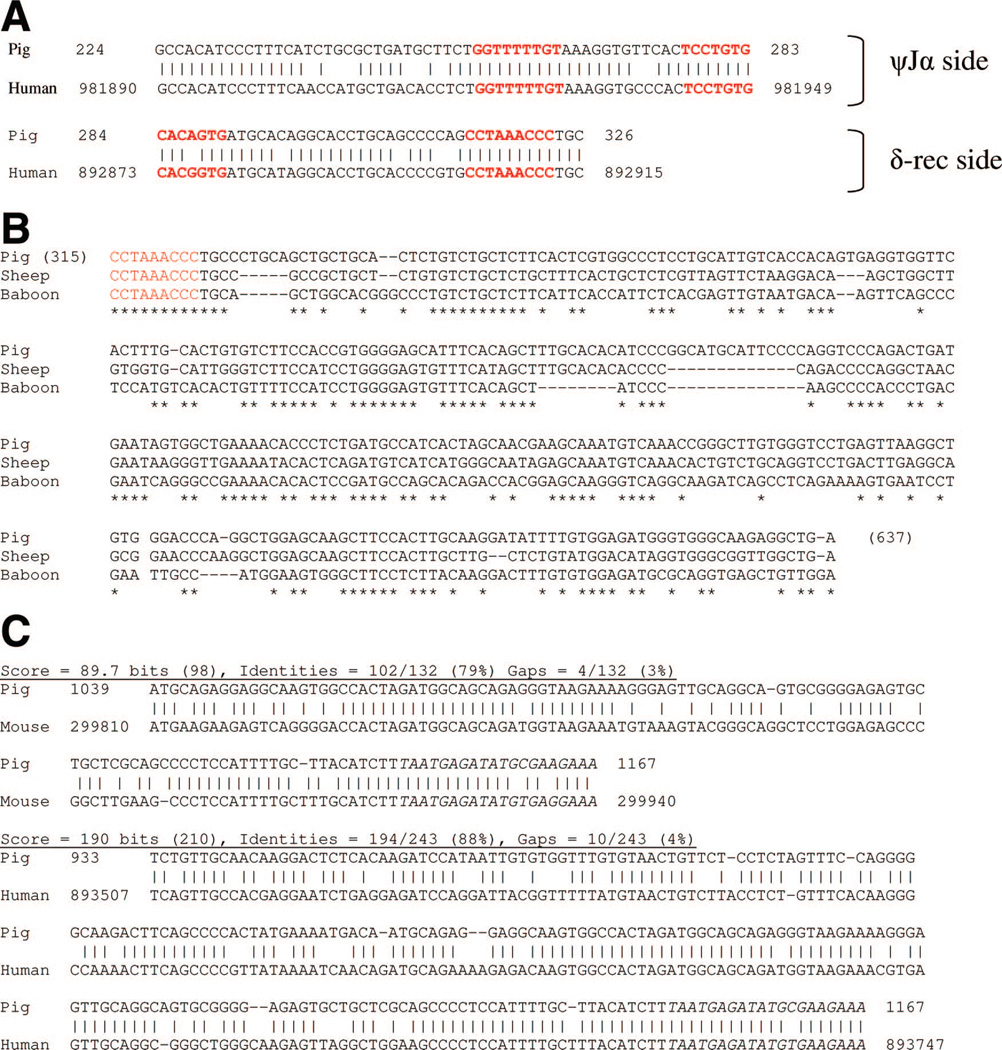
Using the NCBI Blast program, a comparison of the porcine sjTREC sequence to the human (GI 11640533) and mouse TCR αδ (GI 15795314) locus further confirmed that the PCR amplified product was the porcine sjTREC. In both species compared with the porcine sjTREC, there was approximately 89,000 bp gap between the regions of homology of the ψJα and δ-rec regions of the TCRA/D locus, indicating that the sequenced porcine product was the signal joint TREC (Fig. 3A). The highest area of homology was approximately 85% to 88%, seen at the RSS sites of δ-rec and ψJα. Comparison of the regions around the RSS for the human and pig are shown in Figure 3(A).
FIGURE 3.
Comparison of pig and sheep sjTREC with human, primate, and mouse sequences. (A) Porcine sjTREC shows homology to alignments of different parts of the human TCRA/D loci confirming the cloned product is a signal joint TREC. Standard nucleotide blast program was utilized to align the porcine sjTREC product to the TCRA/D loci of human (accession GI 11640553; website: blast.ncbi.nlm.nih.gov/Blast.cgi). Basepairs 1 to 283 of the porcine product corresponded to a different region of the TCRA/D locus compared with basepairs 284 to 1167. This division, which correlates to the corresponding ψJα and δ-rec regions of the porcine PCR product, matches exactly to the respective ψJα and δ-rec regions in human TCRA/D locus. RSS sites are shown in red text. (B) Blast program used to compare the proximal δ-rec region in pig, sheep, and baboon failed to provide any alignment scores or sequences. Therefore, a representative Clustal alignment of the proximal δ-rec region is shown. The nonamer of the RSS of δ-rec in the pig, sheep, and baboon is shown in red text. Note the 320 bp from the nonamer sequence show poor homology between the three species, suggesting why cloning of the sjTREC in artiodactyls using this portion of the δ-rec in human, primates, and mouse has been unsuccessful. (C) Comparing porcine and sheep sjTRECs to corresponding sequences from human, mouse, and baboon showed that the pig sequence was most homologous to the sheep. Segments of the sjTREC sequence with the highest homology between pig to human and pig to mouse are shown. Note that the highest homology among the species was at the RSS sites. The distal end of δ-rec region in the sheep and pig were also homologous to the corresponding areas in the other species. This explained why primers derived from this area enabled the detection of the sjTREC in sheep and pig.
In comparing the pig and sheep sjTREC to the human sequence, overall, a greater homology was observed between the pig and sheep sjTREC alignments (79.2% of the sequence with areas of homology >75%) than the pig and baboon alignments (59% of the sequence with areas of homology >75%). But evidently, for a non-coding region of genomic DNA, the δ-rec and the ψJα regions are relatively highly conserved among the compared species.
Upon assessment of the δ-rec region near its RSS, we noted that there was poor homology in comparing the artiodactyls sequence to that of primate/human and mouse sequences. In fact, the standard NCBI blast program failed to show any matches in this area comparing the pig or sheep sjTREC to mouse, human, or baboon (Papio hamadryas). A representative figure comparing the proximal δ-rec region of the sjTRECs in the pig and sheep with the baboon (Papio hamadryas) sjTREC (recently cloned in our laboratory, in publication) is shown in Figure 3(B). The Clustal program alignment in this area showed poor homology between the pig and sheep sjTREC to the baboon sequence. In fact, the next area of highest homology between the pig to the human, primate and mouse TCR A/D loci, approximately 85%, was seen at 800 bp into the δ-rec region from where the δ-rec primers were designed to obtain the sjTREC (Fig. 3C). This explains why primers derived from this portion of the δ-rec helped to obtain the sjTREC in both sheep and pig, whereas previous attempts at cloning this segment from more proximal sites in δ-rec were unsuccessful.
sjTREC Can Be Detected in the Thymus, Spleen, Lymph Node, and Peripheral Blood Mononuclear Cells
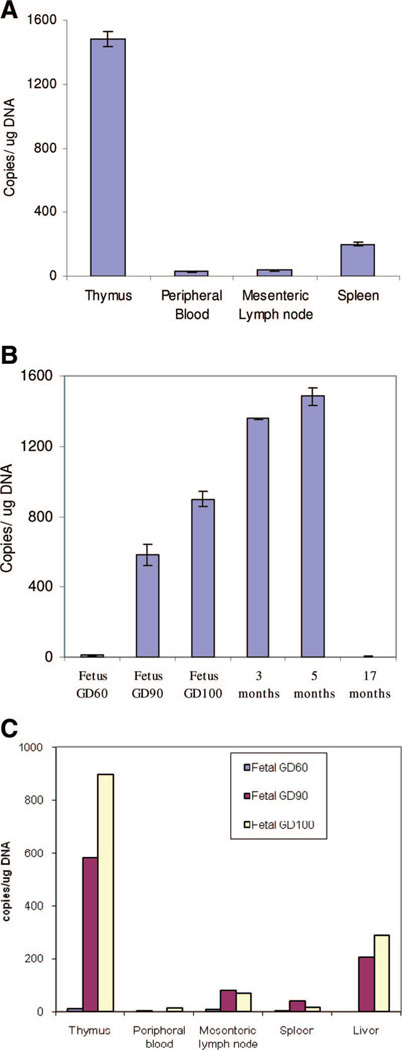
In addition to the thymus, we tested whether sjTREC could also be detected in the secondary lymphoid organs and the periphery. A QPCR assay was developed to enable reliable quantification of sjTREC levels in a given tissue. In a 5-month-old pig, the sjTREC signal was reliably quantified in all of these tissues (Fig. 4A). The results showed that the sjTREC levels were the highest in the thymus, followed by the spleen, and lymph node. This is expected given the high percentage of RTEs that home to the secondary lymphoid organs in a young animal.
FIGURE 4.
Porcine sjTREC could be detected in secondary lymphoid organs and in peripheral blood. (A) sjTREC values from the thymus, spleen, lymph node, and peripheral blood of a 5-month-old pig were reliably quantified. (B) Porcine sjTREC quantitative polymerase chain reaction (QPCR) assay of thymic tissue from different aged animals correlated with known age-related thymic activity. In fetal life, with increasing gestational day, the thymic sjTREC values increased dramatically; a trend that continued in early postnatal life. But in the 17-month-old animal, a marked drop in sjTREC values was noted. (C) sjTREC could be detected in the lymphoid organs and peripheral tissues of the miniature swine fetus. sjTREC values in fetuses of three different gestational days (see legend, upper right corner) suggested increasing thymic activity in late gestational fetal life, and a homing of recent thymic emigrants to the liver in porcine fetal life.
sjTREC Levels Correlate With the Expected Age-Related Thymic Activity
To assess whether sjTREC QPCR could be used as a measure of age-related thymic activity, sjTREC levels from the thymus of 3 fetuses, 2 young animals, and 1 old animal were measured (Fig. 4B). In fetus from gestation day (GD) 60, low sjTREC levels were detected, but the levels increased dramatically in the near term fetus GD 105. The increasing trend in sjTREC levels continued through the early postnatal life. A 3-month-old animal had nearly threefold higher sjTREC levels in the thymus compared with a near-term fetus (Fig. 4B). But a 17-month-old pig thymus showed low levels of sjTREC. These values correlated with the known age-related thymic involution process in the pig and support the findings of a dramatic increase in thymic activity in late fetal and early postnatal life of the miniature swine.
Porcine sjTREC Can Be Detected in Fetal Tissues
DNA from thymus, spleen, mesenteric lymph node, and peripheral blood of three fetuses with the following approximate GDs was isolated: 60, 90, and 105 (a near-term fetus). sjTREC molecules could be reliably detected in porcine fetal tissues (Fig. 4C). A substantial increase was seen in fetal thymic activity with increasing gestation age. In addition, the increase in thymopoesis corresponded with an increase in sjTREC levels observed in the secondary lymphoid organs and the periphery. Interestingly, we found relatively higher sjTREC levels in fetal liver from GD 90 and 105 than from the secondary lymphoid organs.
DISCUSSION
Thymic function and regeneration can be critical to the recovery of T-cell immunity in situations of intensive chemotherapy, radiation, bone marrow transplantation, and in disease conditions such as HIV (10,23–33). Studies in animal models also indicate a role for the thymus in organ transplantation (2,4,5). Thymic activity has been measured previously through indirect methods such as analysis of thymic volume through computed tomography, and phenotyping of naive T cells in the circulation (34,35). TCR excision circles are byproducts of the required rearrangements of the TCR genes during T-cell differentiation (11,18). They contain excised pieces of genomic DNA from the TCR locus, with RSSs. The signal joint TREC is one such by-product resulting from the rearrangement of the TCRA/D locus. TRECs are episomal, stable, do not replicate, and thus get diluted during T-cell proliferation. TREC assays have been reported in mouse, human, and non-human primates, and careful interpretation of TREC levels in these systems has led to insights into thymic activity (9,12,14,19,31,32,36–41).
The detection of the sjTREC in sheep and pig showed that the T cells also undergo δ-rec/ψJα recombination as one way to eliminate the δ locus during differentiation. Despite the high homology (>80%) found in the RSS region of δ-rec in the sequences of human, mouse, and primates, initial primers were unsuccessful in amplifying a porcine sjTREC under various PCR conditions. However, using primers from an area of homology approximately 800 bp away from the δ-rec RSS, a PCR product signifying the porcine sjTREC was obtained. These findings suggested that a reason why TREC has not been reported in artiodactyls might be that outside the RSS, the δ-rec sequence in the artiodactyls is different from the human, primate, and mouse sequences. But further into the δ-rec, there is a region of high homology between mouse and human sequences. Confirming the expectation that this region may also be homologous in artiodactyls, δ-rec primers derived from this area enabled the discovery of the sjTREC in sheep and pig.
A comparison of the sjTREC sequence of the pig to the sheep, human, and mouse showed that the highest regions of homology were at the recombination signal sites, with the highest level of homology seen between the two artiodactyls. Even though this area is a pseudogene, its conservation across these species suggests the important use of this specific recombination in further differentiation of the αβ T cell in the thymus. Of note, the sjTREC assay in the pig may not show comparable levels of sjTREC values with those seen in humans and primates. This may be partly due to the relatively large population of γδ T cells seen in artiodactyls.
Using QPCR, evaluation of sjTREC levels in the thymus of a fetus, young, and old animals showed a dramatic increase in thymopoesis in late fetal and early postnatal life, and then a remarkable decline in thymic activity (Fig. 4B). A 5-month-old pig had approximately 1500 copies of sjTREC/µg thymic DNA compared with a 17-month-old pig with almost undetectable levels of sjTREC in its thymus. This attests to the process of thymic involution in the miniature swine occurring rapidly in a period of 1 year. Evaluating sjTREC values in miniature swine during the first year of postnatal life would provide a more detailed understanding of thymic activity and involution, and may provide clues into central tolerance mechanisms in this large animal model of organ transplantation.
We also tested whether porcine sjTREC assay can be used to study fetal thymopoesis. In the miniature swine, gestation is approximately 114 days. At GD 50, bilateral thymic streaks in the neck are evident, and thymopoesis begins after this time point. In analyzing sjTREC in fetuses of three different gestational ages, there was a substantial increase in thymic activity with increasing fetal age. By comparing the near-term fetus to a young animal, there was an increase in sjTREC levels, which corresponded with an increase of sjTREC levels in the secondary lymphoid organs. Together, these data suggest that there is an increase and a reorganization of the RTEs pool in fetal and early postnatal life of the Massachusetts General Hospital (MGH) miniature swine. Interestingly, higher levels of sjTREC were seen in the liver of a near-term fetus compared with its spleen, lymph node, and peripheral blood (Fig. 4C). Although outside the scope of this article, future studies investigating this finding may provide clues into fetal immunity and organization of the RTE pool.
MATERIALS AND METHODS
Animal Tissue Samples
All animals were housed at the Transplantation Biology Research Center, MGH, Boston, MA. Biopsies of the thymus, lymph node, spleen, and liver were done by the MGH staff. Peripheral blood for detection of the signal joint TREC analysis in the sheep was obtained by staff veterinarian, Dick Hurley. All procedures were performed in accordance with the protocols approved by the MGH Research Committee.
Cell Isolation
Peripheral blood mononuclear cells (PBMC) were obtained from blood through centrifugation of a Histopaque gradient. The buffy coat was removed and cells were washed in Hanks salt solution. DNA was extracted from PBMC using Invitrogen DNeasy Qiagen kit. DNA concentration was determined by a UV spectrophotometer by measuring optical density at 260 nm and the concentration value was compared with the nanodrop and Hoechst dye spectrophotometers. All samples were checked twice before QPCR reaction and fell within 5% error margin. Cells from animal tissues were obtained from biopsy samples. The tissue was homogenized mechanically using a filter membrane, and DNA was isolated by using the Invitrogen DNeasy tissue isolation kit.
Sequencing sjTREC and the Putative δ-rec Region
In the pig, an 1167 bp fragment of interest was sequenced using DNA from thymic tissues of three young animals: 15,289, 16,789, and 16,550. PCR included 10 pmol of δ-rec primer: 5′-TTTCTTCGCATATCTCATTA-3′, 10 pmol of ψJα primer: 5′-CACGAGTGATTAGATATCATTC-3′, 250 ng of thymic DNA, 0.5 µL Hot start Taq polymerase (Qiagen), 1 µL of 10 mM dNTP, 5µL of 10× buffer, in a 50µL total reaction volume. PCR conditions were as follows: 95°C×15 min, followed by 94°C×30 s, 51°C×30 s, and 72°C×60 s, for 35 cycles. A final extension step of 6 min at 72°C was added. Reactions were run using a PTC-100 Programmable Thermal Controller (MJ Research, Inc.). The PCR reaction product was run on a 2% agarose gel and the DNA was isolated from the gel using a Geneclean spin kit (Q-BIOgene). The PCR band of the size of interest was isolated and cloned into a pCR2.1 TOPO TA cloning vector (Invitrogen). For each animal, five to six clones were sequenced using M13 forward and reverse primers to obtain the sequence of the putative porcine TREC. These primers flank the inserted clone. Sequencing was done at the MGH DNA core facility.
sjTREC in the sheep was obtained using DNA from PBMC of a single animal. The product was obtained using the same reaction conditions but with primers internal to the porcine sjTREC; δ-rec primer: 5′-TTTACCATCTGCTGCCATCTAGT-3′, and ψJα primer: 5′-CCTCTGCATAGTGTGATAACA-3′. The PCR product was cloned into pCR2.1 TA cloning vector, and the sequence from five positive clones was analyzed.
QPCR and Porcine sjTREC Analysis
From the 1167 bp band of the porcine sjTREC, primers were designed for QPCR reaction, with the expected PCR product containing the RSS of the δ-rec and ψJα regions. In a total volume of 25 µL, each reaction included 800 nM of ψJα forward primer: 5′-TCTAAAGAGGAAGAACAAGGTTGGCG-3′, 800nM of δ-rec reverse primer: 5′-TGTGCAAAGCTGTGAAATGCTCCC-3′, 200 nM of labeled probe: 5′-/56-FAM/ ATGCAGGAGGGCCACGAGTGAAGAGCAGACAGA/36-TAMSp/-3′. Other reagents included 0.25 µL Hot start Taq polymerase (Qiagen), 2 µL 10 mM dNTPs, 150 ng of DNA template, 2.5 µL of 10× Hot start Taq buffer. The cycling conditions were as follows: 95°C×15 min, followed by 50 cycles of the steps: 94°×30 s, 55°C×60 s, 72°C×30 s. All QPCR reactions and quantitative analyses were performed using a Stratagene.
MxPRO 3500
For quantifying sjTREC molecules, a standard curve of sjTREC molecules was established using a cloned plasmid containing 1167 bp porcine sjTREC. A known number of plasmid molecules was serially diluted 10-fold to obtain a standard curve that could determine anywhere from 10 to 10,000,000 molecules. Standards and samples were run in triplicates, and each experiment was performed twice. For each QPCR experiment, gel electrophoresis of the QPCR products and the standard samples was performed to confirm that a single product of the expected size was being amplified.
ACKNOWLEDGMENTS
The authors acknowledge Christene Huang and Smita Sihag for critical review of the manuscript; John Hanekamp for reviewing sjTREC clone data; Isabel Hanekamp and Bob Wilkinson for technical support; and Rebecca Wark for expert secretarial assistance.
This work was supported by the American College of Surgeons Fellowship, American Society of Transplant Surgeons, Roche Scientific Research Fellowship, Ruth L. Kirschstein National Research Scholarship Award (5 F32 AI066699-02) (P.V.).
Footnotes
This work was performed in Transplantation Biology Research Center, Massachusetts General Hospital, Boston, MA.
The authors declare no conflicts of interest.
P.V. participated in research design, writing of the manuscript, research performance, data analysis, and analytical tools. A.T. participated in research performance and data analysis. K.Y. participated in research design and analytical tools. D.H.S. participated in research design, writing of the manuscript, data analysis, and analytical tools.
REFERENCES
- 1.Sachs DH, Leight GJ, Cone S, et al. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ierino FL, Yamada K, Lorf T, et al. Mechanism of tolerance to class I-mismatched renal allografts in miniature swine: Regulation of interleukin-2 receptor alpha-chain expression on CD8 peripheral blood lymphocytes of tolerant animals. Transplantation. 1998;66:454. doi: 10.1097/00007890-199808270-00007. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine: II. Effect of steroids and age on the induction of tolerance to class I mismatched renal allografts. Transplantation. 1999;67:458. doi: 10.1097/00007890-199902150-00020. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature swine. III. Surgical manipulation of the thymus interferes with stable induction of tolerance to class I-mismatched renal allografts. Transplantation. 1999;67:1112. doi: 10.1097/00007890-199904270-00005. [DOI] [PubMed] [Google Scholar]
- 6.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 7.Harthi AL, Marchetti LG, Steffens CM, et al. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA) J Immunol Methods. 2000;237:187. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti LA, Lewin SR, Zhang L, et al. Age-dependent changes in T cell homeostasis and SIV load in sooty mangabeys. J Med Primatol. 2000;29:158. doi: 10.1034/j.1600-0684.2000.290309.x. [DOI] [PubMed] [Google Scholar]
- 9.Sodora DL, Douek DC, Silvestri G, et al. Quantification of thymic function by measuring T cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. Eur J Immunol. 2000;30:1145. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 11.Hazenberg MD, Verschuren MC, Hamann D, et al. T cell receptor excision circles as markers for recent thymic emigrants: Basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001;79:631. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 12.Sempowski GD, Gooding ME, Liao HX, et al. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 13.Sodora DL, Milush JM, Ware F, et al. Decreased levels of recent thymic emigrants in peripheral blood of simian immunodeficiency virus-infected macaques correlate with alterations within the thymus. J Virol. 2002;76:9981. doi: 10.1128/JVI.76.19.9981-9990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry TJ, Moniuszko M, Creekmore S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected non-human primates. Blood. 2003;101:2294. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 15.Wang XH, Hsu C, Wang Y, et al. Phenotype of genetically regulated thymic involution in young BXD RI strains of mice. Scand J Immunol. 2006;64:287. doi: 10.1111/j.1365-3083.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- 16.Van den Dool C, de Boer RJ. The effects of, age, thymectomy, and HIV Infection on alpha and beta TCR excision circles in naive T cells. J Immunol. 2006;177:4391. doi: 10.4049/jimmunol.177.7.4391. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg MD, Borghans JA, de Boer RJ, et al. Thymic output: A bad TREC record. Nat Immunol. 2003;4:97. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson BD, Douek DC, Killian S, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 19.Fang HT, Khatissian RE, Monceaux V, et al. Disease progression in macaques with low SIV replication levels: On the relevance of TREC counts. Aids. 2005;19:663. doi: 10.1097/01.aids.0000166089.93574.5a. [DOI] [PubMed] [Google Scholar]
- 20.Richardson MW, Sverstiuk AE, Silvera P, et al. T-cell receptor excision circles (TREC) in SHIV 89.6p and SIVmac251 models of HIV-1 infection. DNA Cell Biol. 2004;23:1. doi: 10.1089/104454904322745880. [DOI] [PubMed] [Google Scholar]
- 21.Uenishi H, Hiraiwa H, Yamamoto R, et al. Genomic structure around joining segments and constant regions of swine T-cell receptor alpha/delta (TRA/TRD) locus. Immunology. 2003;109:515. doi: 10.1046/j.1365-2567.2003.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezrich JD, Haller GW, Arn JS, et al. Histocompatible miniature swine: An inbred large-animal model. Transplantation. 2003;75:904. doi: 10.1097/01.TP.0000054839.43852.BF. [DOI] [PubMed] [Google Scholar]
- 23.Douek DC, Koup RA, McFarland RD, et al. Effect of HIV on thymic function before and after antiretroviral therapy in children. J Infect Dis. 2000;181:1479. doi: 10.1086/315398. [DOI] [PubMed] [Google Scholar]
- 24.Hazenberg MD, Otto SA, Cohen Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 25.Yasunaga J, Sakai T, Nosaka K, et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: Its implications in the immunodeficient state. Blood. 2001;97:3177. doi: 10.1182/blood.v97.10.3177. [DOI] [PubMed] [Google Scholar]
- 26.Al-Harthi L, Landay A. Immune recovery in HIV disease: Role of the thymus and T cell expansion in immune reconstitution strategies. J Hematother Stem Cell Res. 2002;11:777. doi: 10.1089/152581602760404586. [DOI] [PubMed] [Google Scholar]
- 27.Franco JM, Rubio A, Martinez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 28.Hazenberg MD, Otto SA, de Pauw ES, et al. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood. 2002;99:3449. doi: 10.1182/blood.v99.9.3449. [DOI] [PubMed] [Google Scholar]
- 29.Storek J, Joseph A, Dawson MA, et al. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002;73:1154. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 30.Poulin JF, Sylvestre M, Champagne P, et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003;102:4600. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 31.Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Barfield R, Benaim E, et al. Prediction of T-cell reconstitution by assessment of T-cell receptor excision circle before allogeneic hematopoietic stem cell transplantation in pediatric patients. Blood. 2005;105:886. doi: 10.1182/blood-2004-04-1405. [DOI] [PubMed] [Google Scholar]
- 33.Fu YW, Wu DP, Cen JN, et al. Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients—a study in Chinese patients. Eur J Haematol. 2007;79:138. doi: 10.1111/j.1600-0609.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 34.Kolte L, Strandberg C, Dreves, et al. Thymic involvement in immune recovery during antiretroviral treatment of HIV infection in adults; comparison of CT and sonographic findings. Scand J Infect Dis. 2002;4:668. doi: 10.1080/00365540210147705. [DOI] [PubMed] [Google Scholar]
- 35.Harris JM, Hazenberg MD, Poulin JF, et al. Multiparameter evaluation of human thymic function: Interpretations and caveats. Clin Immunol. 2005;115:138. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez M, Martinez C, Ercilla G, et al. Clinical factors influencing T-cell receptor excision circle (TRECs) counts following allogeneic stem cell transplantation in adults. Transpl Immunol. 2006;16:52. doi: 10.1016/j.trim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Farge D, Henegar C, Carmagnat M, et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arthritis Rheum. 2005;52:1555. doi: 10.1002/art.21036. [DOI] [PubMed] [Google Scholar]
- 38.Ye P, Kirschner DE, Kourtis AP. The thymus during HIV disease: Role in pathogenesis and in immune recovery. Curr HIV Res. 2004;2:177. doi: 10.2174/1570162043484898. [DOI] [PubMed] [Google Scholar]
- 39.Pirovano S, Notarangelo LD, Malacarne F, et al. Reconstitution of T-cell compartment after in utero stem cell transplantation: Analysis of T-cell repertoire and thymic output. Haematologica. 2004;89:450. [PubMed] [Google Scholar]
- 40.Douek DC. Thymic output and HIV infection: On the right TREC. Immunity. 2004;21:744. doi: 10.1016/j.immuni.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Svaldi M, Lanthaler AJ, Dugas M, et al. T-cell receptor excision circles: A novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br J Haematol. 2003;122:795. doi: 10.1046/j.1365-2141.2003.04482.x. [DOI] [PubMed] [Google Scholar]