Abstract
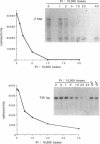
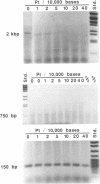
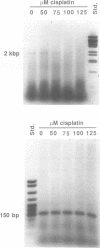
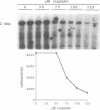
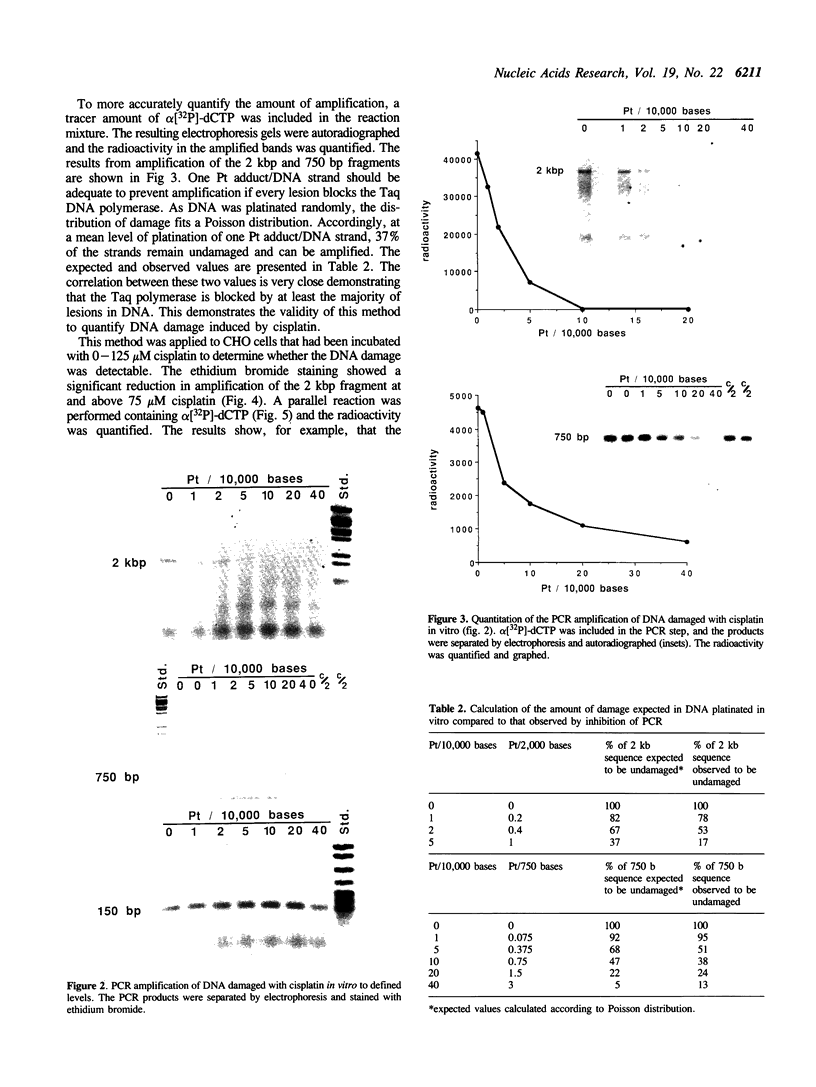
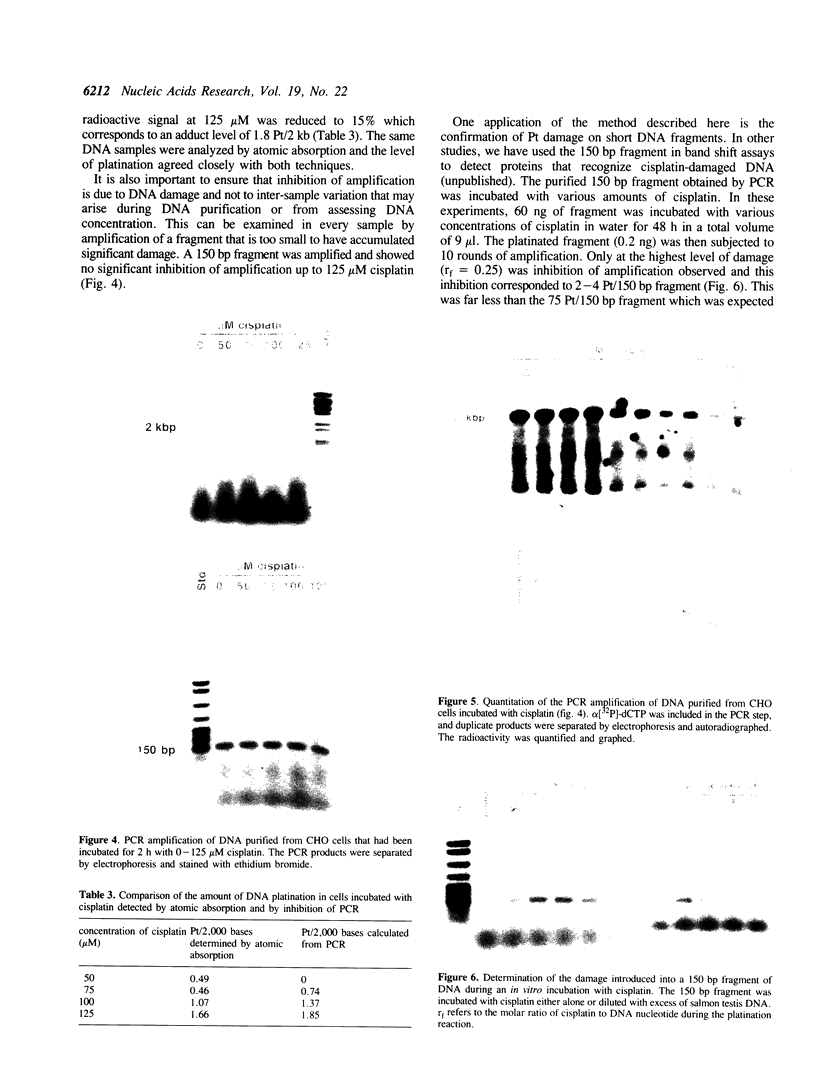
Every bulky lesion in DNA can potentially inhibit the Taq DNA polymerase and thereby decrease the amplification produced in the polymerase chain reaction. We investigated the feasibility of using this inhibition to quantify DNA lesions produced by the anticancer drug cisplatin. Products were detected by electrophoresis followed by ethidium bromide staining. Quantitation was obtained by including [32P]dCTP in the amplification reaction and subsequently assessing the incorporated radioactivity. Hamster genomic DNA was platinated in vitro to defined levels and amplified with primers that produce either a 150, 750 or 2,000 base pair fragment. The degree of inhibition of PCR agreed with the predicted level of DNA platination in each size of fragment, suggesting that the polymerase was inhibited by every cisplatin-induced lesion. This method was used to detect cisplatin-induced lesions in the adenine phosphoribosyltransferase gene of CHO cells. Cells were incubated with 0-125 microM cisplatin for 2 h, the DNA was purified and subjected to PCR. A significant decrease in amplification of the 2 kbp fragment was observed in DNA from cells incubated with cisplatin at 75 microM. The degree of inhibition agreed closely with the amount of DNA damage in the overall genome as measured by atomic absorption. No change was detected in amplification of the 150 base fragment which can therefore be used to normalize data for any variations between DNA samples. This assay has the same sensitivity as other methods currently used for the analysis of gene-specific damage. The advantage of this assay is that it obviates the need for specific endonuclease complexes to recognize and cleave DNA adducts as previously required when analyzing damage in specific genomic sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990 Aug-Sep;2(8-9):275–280. [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Govan H. L., 3rd, Valles-Ayoub Y., Braun J. Fine-mapping of DNA damage and repair in specific genomic segments. Nucleic Acids Res. 1990 Jul 11;18(13):3823–3830. doi: 10.1093/nar/18.13.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. C., James T. W. Detection of A + T-rich DNA in gels by differential fluorescence. Anal Biochem. 1983 May;131(1):205–210. doi: 10.1016/0003-2697(83)90156-2. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C., Zhen W. P., Reed E., Parker R. J., Sancar A., Bohr V. A. Gene-specific formation and repair of cisplatin intrastrand adducts and interstrand cross-links in Chinese hamster ovary cells. J Biol Chem. 1991 Apr 15;266(11):7101–7107. [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Nalbantoglu J., Phear G. A., Meuth M. Nucleotide sequence of hamster adenine phosphoribosyl transferase (aprt) gene. Nucleic Acids Res. 1986 Feb 25;14(4):1914–1914. doi: 10.1093/nar/14.4.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N., Jennerwein M. M., Eastman A. DNA repair in cells sensitive and resistant to cis-diamminedichloroplatinum(II): host cell reactivation of damaged plasmid DNA. Biochemistry. 1989 Apr 4;28(7):3120–3124. doi: 10.1021/bi00433a055. [DOI] [PubMed] [Google Scholar]
- Sorenson C. M., Eastman A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient Chinese hamster ovary cells. Cancer Res. 1988 Dec 1;48(23):6703–6707. [PubMed] [Google Scholar]
- Thomas D. C., Morton A. G., Bohr V. A., Sancar A. General method for quantifying base adducts in specific mammalian genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3723–3727. doi: 10.1073/pnas.85.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]