Abstract
High temperature influences plant development and can reduce crop yields. We examined how ambient temperature influences reproductive development in the temperate cereals wheat (Triticum aestivum) and barley (Hordeum vulgare). High temperature resulted in rapid progression through reproductive development in long days, but inhibited early stages of reproductive development in short days. Activation of the long-day flowering response pathway through day-length-insensitive alleles of the PHOTOPERIOD1 gene, which result in high FLOWERING LOCUS T-like1 transcript levels, did not allow rapid early reproductive development at high temperature in short days. Furthermore, high temperature did not increase transcript levels of FLOWERING LOCUS T-like genes. These data suggest that genes or pathways other than the long-day response pathway mediate developmental responses to high temperature in cereals. Transcriptome analyses suggested a possible role for vernalization-responsive genes in the developmental response to high temperature. The MADS-box floral repressor HvODDSOC2 is expressed at elevated levels at high temperature in short days, and might contribute to the inhibition of early reproductive development under these conditions. FLOWERING PROMOTING FACTOR1-like, RNase-S-like genes, and VER2-like genes were also identified as candidates for high-temperature-responsive developmental regulators. Overall, these data suggest that rising temperatures might elicit different developmental responses in cereal crops at different latitudes or times of year, due to the interaction between temperature and day length. Additionally, we suggest that different developmental regulators might mediate the response to high temperature in cereals compared to Arabidopsis (Arabidopsis thaliana).
Rising average global temperatures are predicted to decrease yield in staple food crops, such as cereals (Lobell and Field, 2007; Asseng et al., 2011). At higher temperatures wheat (Triticum aestivum) and barley (Hordeum vulgare) progress rapidly through early stages of reproductive development. This accelerated pace of development reduces grain number (Fischer, 1985; Rawson and Richards, 1993). Changed average temperature can also alter the timing of flowering in temperate cereals and further reduce yield by increasing the risk of frost or heat damage to developing grains. Despite progress in identifying genes controlling the developmental response of cereals to day length and low temperature (see Distelfeld et al., 2009; Greenup et al., 2009), genes controlling developmental responses to high temperature have not been identified. In this study we examine how high temperature and day length cues interact to control early reproductive development in cereals and identify candidates for temperature-responsive developmental regulators.
The molecular mechanisms controlling developmental responses to temperature have been studied in Arabidopsis (Arabidopsis thaliana), where FLOWERING LOCUS T (FT) mediates acceleration of flowering by high temperature as well as playing a key role in the long-day flowering response (Blázquez et al., 2003; Balasubramanian et al., 2006; Turck et al., 2008). FT transcript levels are influenced by temperature, with higher transcript levels and correspondingly earlier flowering occurring at higher temperatures (Blázquez et al., 2003; Balasubramanian et al., 2006). Loss of FT function abolishes the promotion of flowering by high temperature (Balasubramanian et al., 2006) and overexpression of FT is sufficient to eliminate delay of flowering at low temperature (Blázquez et al., 2003). Up-regulation of FT and acceleration of flowering at high temperature occur independently of upstream components of the long-day flowering response pathway, such as CONSTANS (Blázquez et al., 2003; Balasubramanian et al., 2006).
FT orthologs have been identified in wheat and barley: FT-like1 (FT1; Turner et al., 2005; Yan et al., 2006). In these cereals, FT1 mediates the long-day flowering response, as it does in Arabidopsis, but it is not known whether FT1 also mediates the flowering response to high temperature. It has been shown that, in wheat and barley, the rate of reproductive development at high temperature can be modified by light quality or day length and by the ability of plants to correctly perceive day length. For example, in a barley cultivar described as long-day responsive, or rapid flowering in long days, the shoot apex progresses more rapidly from vegetative to reproductive development as temperature increases from 15°C to 25°C (Aspinall, 1969). This acceleration of development only occurs if this cultivar is grown in florally inductive far-red light. Similarly, in other barley cultivars that flower rapidly in long days, awn emergence occurs more rapidly as temperature increases. Again, this acceleration of development only occurs if plants are grown in florally inductive long days (Ellis et al., 1988). In contrast, in barley cultivars described as being day neutral or unable to correctly perceive long days, the transition from vegetative to reproductive development and awn emergence does not occur more rapidly as temperature increases from 15°C to 25°C, irrespective of light source or day length (Aspinall, 1969; Ellis et al., 1988). Although not all of these studies were performed with plants of defined genotype, barley cultivars that are described as long-day responsive or day neutral typically carry variant alleles of a single gene, PHOTOPERIOD1 (PPD1), that is required for up-regulation of FT1 in long days (Turner et al., 2005). This suggests that, in barley and wheat, activation of the long-day flowering response pathway and expression of FT1 might play a role in promotion of reproductive development by high temperature.
In this study we use wheat and barley lines carrying known alleles of flowering time genes to examine the effect of high temperature on development and gene expression in cereals. We show that the effect of temperature on reproductive development is day-length dependant. High temperature results in rapid progression through reproductive development in long days, but inhibits early stages of reproductive development in short days. Unlike Arabidopsis, FT1 transcript levels are not influenced by temperature. Instead, genes in the vernalization response pathway offer potential candidates for developmental regulators controlling the response to temperature in cereals.
RESULTS
Reproductive Development Occurs Rapidly at High Temperature in Long Days
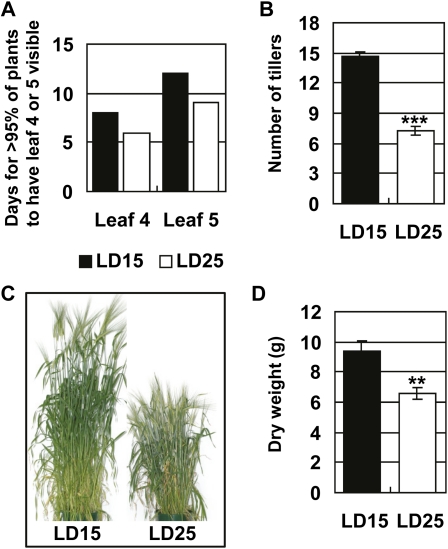
Barley and wheat crops in southern and eastern Australia experience mean maximum temperatures between 15°C and 25°C. Within this temperature range barley and wheat plants are able to complete normal development: producing phenotypically normal vegetative and reproductive organs. We examined the effect of constant temperatures of 15°C or 25°C on growth of vernalized barley plants (cultivar Sonja) in long days. Temperature affected the rate of growth. Leaves on the main stem emerged in fewer calendar days at 25°C than at 15°C (Fig. 1A). Tillers emerged more rapidly at 15°C than at 25°C, so that plants with the same number of leaves visible on the main stem had more tillers at 15°C than at 25°C (Fig. 1B). Temperature also affected plant architecture. Plants grown at 25°C were shorter (Fig. 1C), and had smaller final biomass than plants grown at 15°C (Fig. 1D).
Figure 1.
Ambient temperature affects plant growth. Vernalized barley plants were grown at 15°C or 25°C in long days. A, The number of days for >95% of plants in a cohort of 60 to have leaf 4 or leaf 5 visible on the main stem. B, Average tiller number per plant in plants with leaf 8 visible on the main stem. C, Plants at maturity. D, Average dry weight per plant in mature plants. LD15, Long days and 15°C; LD25, long days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: **, P < 0.01; ***, P < 0.001. [See online article for color version of this figure.]
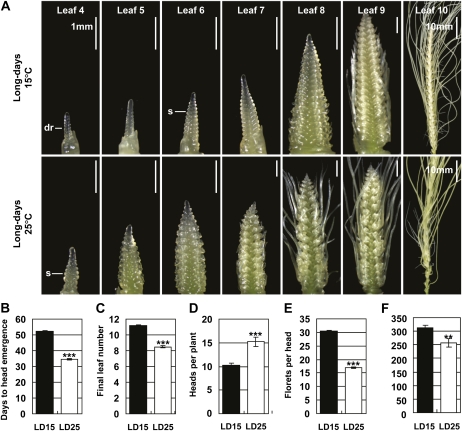
We then examined the effect of temperature on reproductive development. The morphology of the shoot apex was examined as each leaf became visible on the main stem. At each leaf stage examined plants had reached a more advanced stage of reproductive development at 25°C compared to 15°C (Fig. 2A). At 25°C heads emerged in fewer calendar days and fewer leaves were produced on the main stem (Fig. 2, B and C). Due to premature senescence of some tillers at 15°C, plants grown at 25°C made more heads than plants grown at 15°C, despite the faster emergence of tillers at 15°C than at 25°C (Fig. 2D). At 25°C plants produced fewer primordia and fewer florets per head (Fig. 2, A and E). Thus, although plants grown at 25°C made more heads than plants grown at 15°C, total floret number per plant was lower at the higher temperature (Fig. 2F).
Figure 2.
Reproductive development occurs rapidly at high temperature in long days. Vernalized barley plants were grown at 15°C or 25°C in long days. A, Representative image of the main shoot apex as leaves 4 to 10 became visible on the main stem. dr indicates double ridges, the first visible sign of floral development; S, expanding spikelet primordium. The scale bar shows 1 mm unless otherwise indicated. B, Average calendar days to head emergence. C, Average final number of leaves on the main stem. D, Average number of heads per plants. E, Average number of florets per head. F, Average total number of florets per plant. LD15, Long days and 15°C; LD25, long days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: **, P < 0.01; ***, P < 0.001. [See online article for color version of this figure.]
Early Reproductive Development Is Inhibited at High Temperature in Short Days
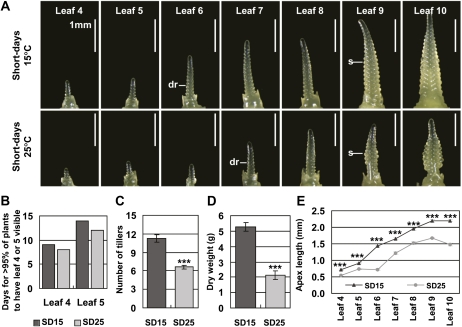
Growth and development were then examined at 15°C or 25°C in short days. As with plants grown in long days, leaf emergence occurred in fewer calendar days at 25°C compared to 15°C (Fig. 3B). Plants also had fewer tillers (Fig. 3C) and smaller biomass (Fig. 3D) at 25°C compared to 15°C. In contrast to long-day-grown plants, in short days at leaf stages 4 to 8 plants had reached a more advanced stage of reproductive development at 15°C compared to 25°C (Fig. 3A). Visible swelling of floral primordia occurred around the time of appearance of leaf 9 at both temperatures, although the pattern of development of floral primordia along the apex was different (Fig. 3A). Plants grown at 25°C in short days had a shorter shoot apex and fewer primordia at all leaf stages examined than plants grown at 15°C (Fig. 3, A and E). Plants did not reach head emergence at either temperature. In a separate experiment plants were grown in oscillating temperatures under noninductive light. As with plants grown at constant temperatures, plants reached a more advanced stage of reproductive development at lower temperatures at all leaf stages examined (Supplemental Fig. S1, A and B). Wheat plants grown in short days at 15°C and 25°C also reached a more advanced stage of reproductive development at the lower temperature when plants were compared at the same leaf stage (Supplemental Fig. S1D).
Figure 3.
Early reproductive development is inhibited by high temperature in short days. Vernalized barley plants were grown at 15°C or 25°C in short days. A, Representative image of the main shoot apex as leaves 4 to 10 became visible on the main stem. dr indicates double ridges, the first visible sign of floral development; S, expanding spikelet primordium. The scale bar shows 1 mm. B, The number of days for >95% of plants in a cohort of 60 to have leaf 4 or leaf 5 visible on the main stem. C, Average tiller number per plant in plants with leaf 8 visible on the main stem. D, Average total dry weight per plant in plants with leaf 10 visible on the main stem. E, Average length of the main shoot apex for plants in A. SD15, Short days and 15°C; SD25, short days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: ***, P < 0.001. [See online article for color version of this figure.]
Activation of the Long-Day Flowering Response Pathway Does Not Allow Rapid Early Reproductive Development at High Temperature in Short Days
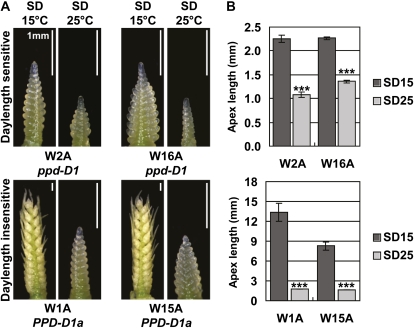
Since long days are required for rapid early reproductive development at high temperature in cereals, we asked whether activation of the long-day flowering response pathway changes the progression of early reproductive development at high temperature in plants grown in short days. Wheat lines that require long days to flower rapidly were compared to near-isogenic sibling lines that flower rapidly in both short and long days due to mutant alleles of the TaPPD1 gene that cause constitutive expression of TaFT1 (see “Materials and Methods”). Although reproductive development was faster in plants carrying the mutant TaPPD1 allele compared to plants carrying a wild-type allele, plants compared at the same leaf stage had reached more advanced stages of reproductive development at 15°C compared to 25°C irrespective of TaPPD1 genotype (Fig. 4, A and B). Similar results were obtained using a barley mutant, MAT-a.8, which flowers rapidly and expresses HvFT1 in short days (Supplemental Fig. S2, A and B). In MAT-a.8 mutants, plants compared at the same leaf stage reached more advanced stages of reproductive development at 15°C compared to 25°C (Supplemental Fig. S2C). Later stages of reproductive development were comparable at 15°C and 25°C (Supplemental Fig. S2C) and heads emerged in fewer calendar days at higher temperatures (Supplemental Fig. S2, D and E).
Figure 4.
Activation of the long-day flowering response pathway does not allow rapid early reproductive development at high temperature in short days. Early reproductive development was assayed in near-isogenic wheat lines varying for alleles of the PPD1 gene. Lines W2A and W16A carry wild-type PPD1 alleles (ppd-D1). These lines require long days to flower rapidly (day-length sensitive). Lines W1A and W15A carry a mutant PPD1 allele (PPD-D1a) that allows plants to flower rapidly in both short and long days (day-length insensitive). A, Representative image of the main shoot apex from plants grown at 15°C or 25°C in short days until leaf 8 was visible on the main stem. Scale bar shows 1 mm. B, Average length of the main shoot apex for plants in A. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: ***, P < 0.001. [See online article for color version of this figure.]
Transcript Levels of Genes in the Long-Day Flowering Response Pathway Are Not Significantly Changed at High Temperature
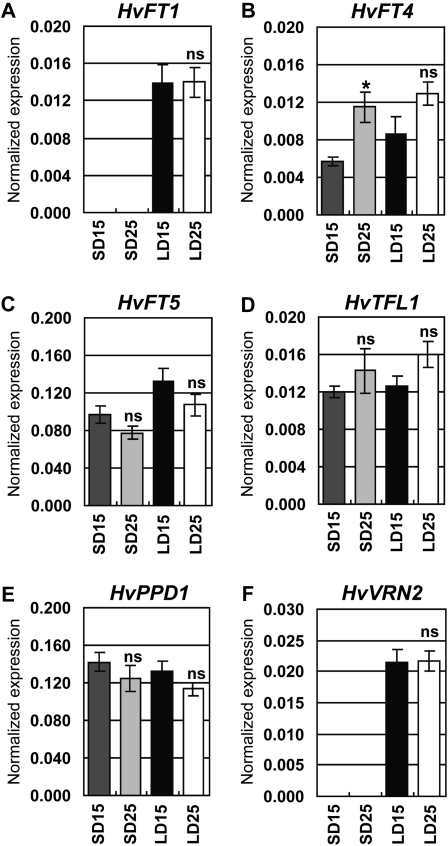
We used quantitative reverse transcription (qRT)-PCR to examine transcript levels of genes likely to control the long-day flowering response in cereals. Transcript levels were assayed in vernalized barley plants (cultivar Sonja) grown at 15°C or 25°C in short or long days until leaf 4 was visible on the main stem. At this stage of development the shoot apex was more advanced at 25°C compared to 15°C in long-day-grown plants and less advanced at 25°C compared to 15°C in short-day-grown plants (Supplemental Fig. S3). HvFT1 was expressed in long but not short days (Fig. 5A). In long days HvFT1 transcript levels did not differ between the two temperature treatments. Two FT-like genes, HvFT4 and HvFT5, were expressed in short and long days (Fig. 5, B and C). HvFT4 transcript levels were higher at 25°C compared to 15°C (2-fold, P < 0.05) in short days. HvFT5 transcript levels did not differ significantly between temperature treatments. HvFT2 transcript levels were low (Supplemental Fig. S4A) and the HvFT3 gene is deleted in the cultivar examined. Another member of the phosphatidylethanolamine-binding protein gene family, TERMINAL FLOWER1, was expressed at similar levels in short and long days and transcript levels were not significantly influenced by temperature (Fig. 5D). Transcript levels of HvPPD1 were not significantly influenced by day length or temperature (Fig. 5E). HvVERNALIZATION2 (HvVRN2) transcript was detectable in long days but was not influenced by temperature (Fig. 5F).
Figure 5.
Transcript levels of genes in the long-day flowering response pathway. A to F, qRT-PCR assay of expression levels. Expression is shown relative to ACTIN. SD15, Short days and 15°C; SD25, short days and 25°C; LD15, long days and 15°C; LD25, long days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Students t test: *, P < 0.05. ns, Not significantly different.
Transcript Levels of Some Vernalization-Responsive Genes Are Changed at High Temperature
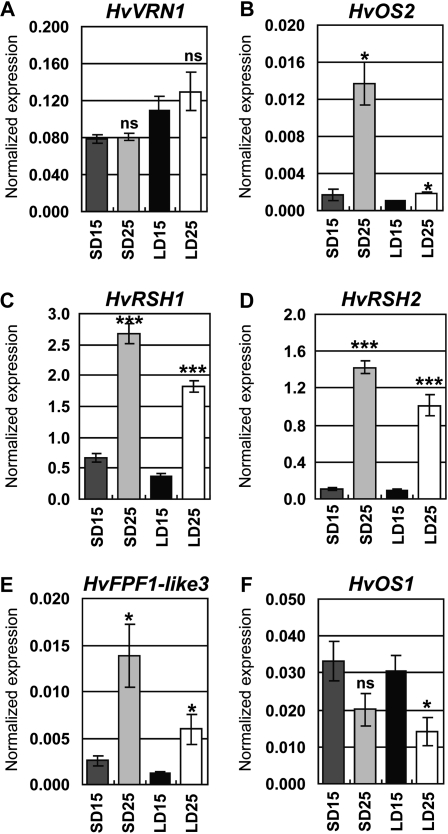
The transcript levels of genes in the vernalization response pathway (HvVRN1 and HvODDSOC2) were examined in vernalized barley plants (cultivar Sonja) grown at 15°C or 25°C in short or long days until leaf 4 was visible on the main stem. HvVRN1 transcript levels were similar in short and long days and not significantly changed by temperature (Fig. 6A). HvODDSOC2 is a MADS-box repressor of elongation and flowering that is down-regulated in vernalized plants (Greenup et al., 2010). HvODDSOC2 transcript levels were low in plants grown in long days and in plants grown at 15°C in short days, but increased (8-fold, P < 0.05) in short days at 25°C, where development of the shoot apex is slowest (Fig. 6B). HvODDSOC2 transcript levels were also examined in nonvernalized barley plants (cultivar Sonja) grown at average temperatures of 12°C, 17°C, or 22°C under noninductive light until leaf 2 was visible on the main stem. Under these conditions, where plants have not made the transition to reproductive development at any temperature, HvODDSOC2 transcript levels were higher in plants grown at 22°C than in plants grown at 12°C (Supplemental Fig. S5).
Figure 6.
Transcript levels of genes in the vernalization response pathway. A to F, qRT-PCR assay of expression levels. Expression is shown relative to ACTIN. SD15, Short days and 15°C; SD25, short days and 25°C; LD15, long days and 15°C; LD25, long days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: *, P < 0.05; ***, P < 0.001. ns, Not significantly different.
Transcript levels of genes that might act downstream of HvODDSOC2 (Greenup et al., 2010) were also examined. Transgenic barley plants overexpressing HvODDSOC2 show increased transcript levels of RNase-S-like (HvRSH) genes and reduced transcript levels of FLOWERING PROMOTING FACTOR1 (FPF1)-like genes (Greenup et al., 2010). Transcript levels of HvRSH1 and 2 were higher at 25°C in both short and long days (Fig. 6, C and D). Transcript levels of HvFPF1-like1 and HvFPF1-like2 were low and were not significantly influenced by temperature (Supplemental Fig. S4, B and C). HvFPF1-like1 and HvFPF1-like2 transcript levels were also not influenced by day length, although higher transcript levels have previously been observed in vernalized barley plants in long days compared to short days (Greenup et al., 2010). Transcript levels of a third FPF1-like gene, HvFPF1-like3, were higher at 25°C in both short and long days (Fig. 6E). Transcript levels of an HvODDSOC2-like gene with unknown function, HvODDSOC1, were lower (2-fold decrease, P < 0.05) at 25°C in long days (Fig. 6F).
Transcript levels of MADS-box genes belonging to the SHORT VEGETATIVE PHASE-like, SEPALLATA-like, FRUITFUL (FUL)-like, and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1)-like gene families were also examined in the different temperature and day-length treatments. The three SHORT VEGETATIVE PHASE-like genes examined, VEGETATIVE TO REPRODUCTIVE TRANSITION2, BARLEY MADS1 (HvBM1), and HvBM10 did not show changes in transcript levels at the different temperatures (Supplemental Fig. S4, D–F). A FUL-like gene (HvBM8) and a SOC1-like gene (HvSOC1) also did not show changes in transcript levels at the different temperatures (Supplemental Fig. S4, H and I). Transcript levels of another FUL-like gene (HvBM3) and a SEPALLATA-like gene (HvBM7) were lower at 25°C in both short and long days (Supplemental Fig. S4, G and J).
HvODDSOC2 Might Slow Early Reproductive Development at High Temperature
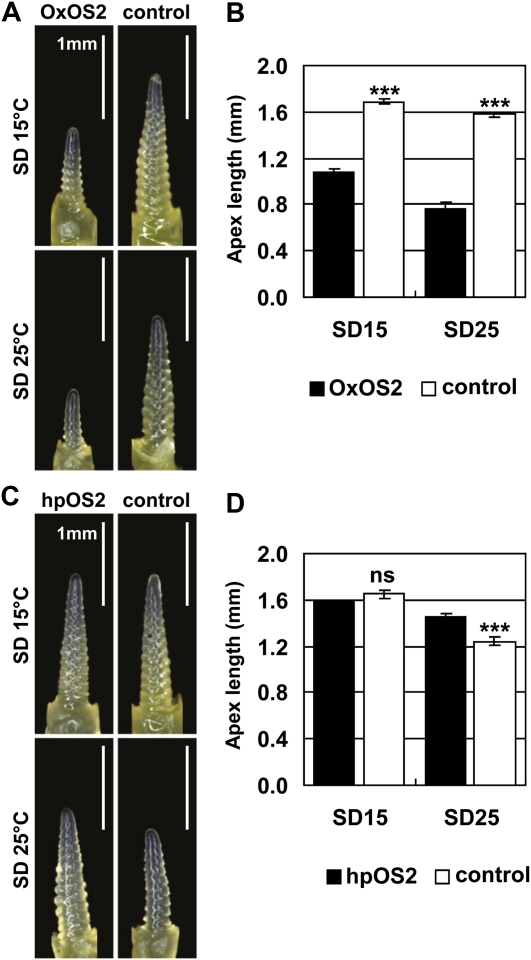
To examine the role of HvODDSOC2 in early reproductive development at high temperatures, we grew transgenic barley lines with altered HvODDSOC2 transcript levels at 15°C or 25°C in short days. In a line in which HvODDSOC2 is expressed under the control of the Zea mays UBIQUITIN promoter (OxOS2-20 in Greenup et al., 2010), early reproductive development was less advanced compared to a nontransgenic sibling control line at both 15°C and 25°C (Fig. 7, A and B). This is consistent with the effect of this transgene in plants grown at 20°C (Greenup et al., 2010). In a line in which HvODDSOC2 transcript levels are reduced by an RNAi hairpin construct (hpHvOS2-2 in Greenup et al., 2010), early reproductive development was not significantly different between the RNAi line and a nontransgenic sibling control line at 15°C, but at 25°C the RNAi line was more advanced than the nontransgenic sibling control line (Fig. 7, C and D).
Figure 7.
HvODDSOC2 might slow early reproductive development at high temperature. Transgenic barley lines with altered transcript levels of the HvODDSOC2 gene were grown at 15°C or 25°C in short days. A, Representative images of the main shoot apex from a trangenic line overexpressing HvODDSOC2 (OxOS2) and a nontransgenic sibling control line. Leaf 7 was visible on the main stem at 15°C and leaf 8 was visible on the main stem at 25°C. B, Average length of the main shoot apex from plants in A. C, Representative images of the main shoot apex from a transgenic line in which HvODDSOC2 transcript levels are reduced by an RNAi hairpin construct (hpOS2) and a nontransgenic sibling control line. Leaf 7 was visible on the main stem at 15°C and leaf 8 was visible on the main stem at 25°C. D, Average length of the main shoot apex from plants in C. SD15, Short days and 15°C; SD25, short days and 25°C. Error bars show se. Asterisks indicate the 25°C treatment is significantly different from the 15°C treatment; P values of Student’s t test: ***, P < 0.001. ns, Not significantly different. [See online article for color version of this figure.]
Microarray Analysis of Gene Expression in Barley Plants Grown at Different Temperatures
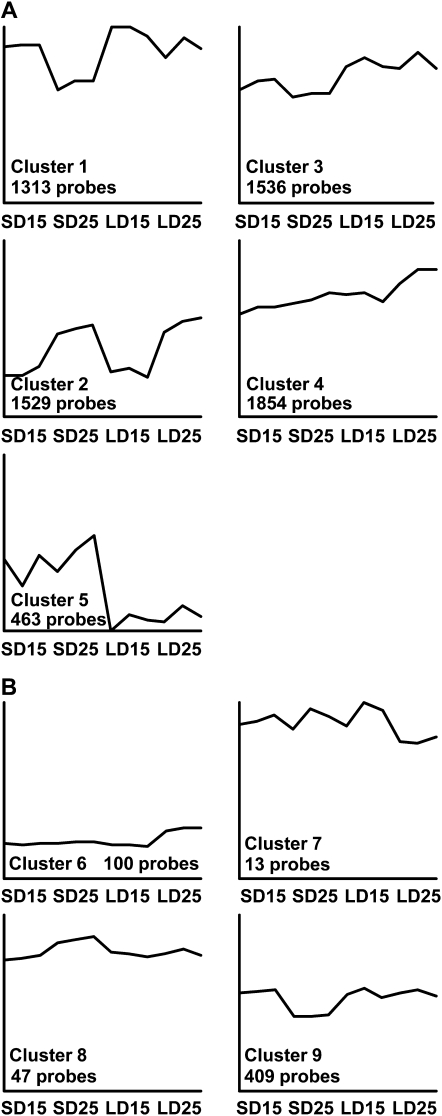
To identify other genes that might regulate early reproductive development at high temperature we used the Agilent 4x44K barley gene expression microarray to examine gene expression in vernalized barley plants (cultivar Sonja) grown at 15°C or 25°C in short or long days until leaf 4 was visible on the main stem. Temperature and day length both influenced the transcriptome (Supplemental Figs. S6 and S7). Pairwise comparisons were used to identify probes that were expressed at different levels in the contrasting day-length and temperature treatments. A total of 7,541 probes that were differentially regulated (empirical Bayes test, no minimum fold cut) in at least one pairwise comparison were used for cluster analysis to define groups with common expression patterns. Five large clusters were identified (Fig. 8A). These clusters contain probes that respond to temperature irrespective of day length (clusters 1–4) or day length irrespective of temperature (cluster 5; Supplemental Data Set S1, clusters 1–5). Smaller clusters of probes that responded only to specific combinations of temperature and day length were also identified (Supplemental Data Set S1, clusters 6–14). Of particular interest to this study are probes with expression patterns that might explain the developmental observations made. For example, genes that show changed transcript levels at high temperature only in long days might contribute to the acceleration of reproductive development (clusters 6 and 7; Tables I and II). Conversely genes that show changed transcript levels at high temperature only in short days might contribute to the inhibition of early apex development observed under these conditions (clusters 8 and 9; Tables III and IV). Cluster 6 includes a VER2-like gene (Table I; Supplemental Fig. S4). VER2 has previously been suggested to play a role in reproductive development in wheat (Yong et al., 2003). Cluster 9 (Table IV) includes a CBL-interacting protein kinase. Otherwise no clear candidates for temperature-responsive developmental regulators were identified.
Figure 8.
PAMSAM cluster analysis of differentially expressed genes. Graphs show the expression pattern of the mediod (representative gene). Y axes represent the Log2 expression value of the cluster mediod. Clusters 1 to 4 contain probes that are influenced by temperature irrespective of day length. Cluster 5 contains probes that are influenced by day length irrespective of temperature. Clusters 6 to 9 contain probes that respond to specific combinations of temperature and day length. SD15, Short days and 15°C; SD25, short days and 25°C; LD15, long days and 15°C; LD25, long days and 25°C.
Table I. Genes up-regulated ≥2-fold at 25°C in long days (cluster 6).
Also shown are data for a VER2-like gene that might act as a temperature-responsive developmental regulator (up-regulated 1.7-fold).
| Gene or Gene Family | Possible Roles | Agilent Probes | Fold Change | P Value |
| PR-1 pathogenesis-related proteins | Defense, stress | A_13_P414930 | 3.1 | 7.08E-04 |
| A_13_P127895 | 3.0 | 6.19E-05 | ||
| A_13_P414930 | 2.9 | 8.67E-05 | ||
| Nicotianamine synthase | Iron acquisition | A_13_P561894 | 3.0 | 1.95E-03 |
| Metallothionein-like proteins | Iron acquisition | A_13_P437101 | 2.7 | 6.38E-06 |
| A_13_P358072 | 2.0 | 4.50E-05 | ||
| Basic helix-loop-helix transcription factor (HvIRO2) | Iron acquisition | A_13_P073926 | 2.5 | 1.23E-04 |
| Putative proteinase inhibitors | Unknown | A_13_P118080 | 2.2 | 2.36E-06 |
| A_13_P436156 | 2.0 | 1.18E-02 | ||
| Proton-dependant oligopeptide transport family protein | Peptide transport | A_13_P163175 | 2.1 | 6.74E-07 |
| Possible O-methyltransferase | Enzymatic methylation | A_13_P518164 | 2.1 | 6.05E-05 |
| Legumain-like Cys protease | Unknown | A_13_P234334 | 2.0 | 2.01E-05 |
| 32-kD jasmonate-induced protein, jacalin-like lectin (similar to TaVER2) | Defense, signal transduction, development | A_13_P074401 | 1.7 | 1.43E-04 |
Table II.
Genes down-regulated ≥2-fold at 25°C in long days (cluster 7)
| Gene or Gene Family | Possible Roles | Agilent Probes | Fold Change | P Value |
| Unknown, possible bacterial | Unknown | A_13_P566739 | 0.3 | 5.10E-05 |
| Unknown | Unknown | A_13_P035571 | 0.4 | 1.94E-05 |
| PSI P700 chlorophyll a apoprotein | Photosynthesis | A_13_P302582 | 0.5 | 1.25E-03 |
| RNA polymerase β-subunit chloroplastic | RNA synthesis | A_13_P148770 | 0.5 | 1.22E-04 |
| Ferredoxin, chloroplastic | Photosynthesis | A_13_P569554 | 0.5 | 4.47E-05 |
| A_13_P279264 | 0.5 | 2.35E-04 | ||
| Light-harvesting chlorophyll a/b-binding protein | Photosynthesis | A_13_P120480 | 0.5 | 1.75E-03 |
| Unknown | Unknown | A_13_P585079 | 0.5 | 5.64E-04 |
| Neutral ceramidase | Unknown | A_13_P350797 | 0.5 | 1.88E-03 |
| Unknown, kinesin like | Unknown | A_13_P026186 | 0.5 | 6.36E-06 |
Table III.
Genes up-regulated ≥2-fold at 25°C in short days (cluster 8)
| Gene or Gene Family | Possible Roles | Agilent Probes | Fold Change | P Value |
| Possible profilin | Unknown | A_13_P439331 | 2.6 | 1.33E-02 |
Table IV.
Genes down-regulated ≥2-fold at 25°C in short days (cluster 9)
| Gene or Gene Family | Possible Roles | Agilent Probes | Fold Change | P Value |
| Papain-like Cys proteinase | Unknown | A_13_P519309 | 0.3 | 2.71E-04 |
| A_13_P120300 | 0.3 | 4.69E-05 | ||
| Carbonic anhydrase | Photosynthesis | A_13_P542232 | 0.4 | 5.48E-04 |
| Similar to Z. mays anther-specific Pro-rich protein APG (hydrolase) | Unknown | A_13_P042346 | 0.5 | 1.28E-03 |
| Similar to CBL-interacting Ser/Thr protein kinases | Calcium sensing, signal transduction | A_13_P139015 | 0.5 | 3.47E-04 |
| Similar to meiosis 5 protein | Meiosis | A_13_P132070 | 0.5 | 5.84E-06 |
| Gln-dependent Asn synthetase | A_13_P087221 | 0.5 | 5.98E-04 | |
| Unknown | Unknown | A_13_P521034 | 0.5 | 6.80E-07 |
| Histone H2B | A_13_P116250 | 0.5 | 8.37E-04 | |
| Histone H2A | A_13_P117940 | 0.5 | 2.98E-06 | |
| A_13_P441376 | 0.5 | 1.31E-06 | ||
| A_13_P118055 | 0.5 | 1.49E-05 | ||
| PR-1 pathogenesis-related protein | Defense, stress | A_13_P304427 | 0.5 | 9.82E-03 |
DISCUSSION
In temperate cereal crops, the duration of early phases of reproductive development has a large effect on grain number. Here we show that early reproductive development in wheat and barley is controlled by an interaction between temperature and day length. In long days plants reach more advanced stages of reproductive development relative to vegetative development (leaf emergence) at 25°C compared to 15°C, and reach maturity in fewer calendar days at the higher temperature. This pattern of development is associated with lower floret number at the higher temperature (Fig. 2). In short days high temperature has the opposite effect on early reproductive development. Plants compared at the same leaf stage reached more advanced stages of reproductive development at lower temperatures (Fig. 3; Supplemental Fig. S1). Slowing of reproductive development at higher temperatures in short days did not result in production of larger numbers of primordia. It would be interesting to know whether progression toward reproduction development in long days also occurs more rapidly at 25°C compared to 15°C at very early stages of development, for example before double ridges are visible.
We found that slowing of early reproductive development relative to leaf emergence at higher temperature in light spectra that do not induce flowering occurs across a wide temperature range (Supplemental Fig. S1). This developmental pattern is also observed in either constant or oscillating temperatures and in dark-grown seedlings (Fig. 3; Supplemental Fig. S1). Our observations are consistent with another study that examined the influence of average temperatures of 15°C and 25°C on development in a series of wheat lines under different day lengths (Rawson and Richards, 1993). The combination of short days and 25°C delayed the appearance of double ridges and slowed production of primordia on the developing shoot apex. Taken together, these data suggest that the interaction between temperature and day length observed here under controlled growth conditions might also occur in field-grown plants. If this interaction does occur in the field, the impact of high temperatures on early reproductive development, and thus potentially on grain number and yield, of temperate cereal crops might vary at different times of year or at different latitudes, in a day-length-dependant manner. Moreover, if plants are unable to correctly perceive long days, as is the case for cultivated barleys that carry loss-of-function alleles of HvPPD1 (Turner et al., 2005), early reproductive development might not be accelerated by high temperatures, even in long days.
In Arabidopsis, FT mediates the long-day flowering response (see Turck et al., 2008). Changes in FT transcript levels also mediate the flowering response to high temperature (Blázquez et al., 2003; Balasubramanian et al., 2006). FT transcript levels increase at higher temperatures in short-day-grown plants, accelerating flowering (Blázquez et al., 2003; Balasubramanian et al., 2006). Here we have shown that, unlike the situation in Arabidopsis, transcript levels of the barley ortholog of FT (HvFT1) are not influenced by temperature: HvFT1 transcript levels do not increase at high temperature and high temperature is not sufficient to trigger rapid flowering in short days (Figs. 3 and 5). Mutations that increase FT1 transcript levels (alleles of PPD-D1 in wheat or the MAT-a.8 mutation in barley) do not abolish day-length-specific developmental responses to high temperature in either wheat or barley (Fig. 4; Supplemental Fig. S2). We found no clear evidence that any other known regulators of the long-day flowering response are temperature responsive, at least at the transcript level. For example, transcript levels of genes that influence HvFT1 expression, HvPPD1 and VERNALIZATION2 (HvVRN2), are not changed by high temperature (Fig. 5). Together, these data suggest that other genes are likely to mediate developmental responses to high temperature in wheat and barley.
Genes previously identified as being vernalization responsive might contribute to the developmental response to high temperature in temperate cereals. Transcript levels of the floral repressor HvODDSOC2, which are reduced by vernalization (Greenup et al., 2010), increase in vernalized barley plants grown at high temperature in short days: conditions in which early reproductive development proceeds slowly relative to vegetative development (Fig. 6). Transcript levels of MADS-box genes from other plants are responsive to wide ranges of temperatures (see Hemming and Trevaskis, 2011), and HvODDSOC2 transcript levels in nonvernalized barley seedlings vary over the range 12°C to 22°C (Supplemental Fig. S5), so HvODDSOC2 transcript levels might also change over a wide temperature range: being lower at low temperatures and higher at high temperatures, under certain conditions. Transgenic barley plants with reduced HvODDSOC2 transcript levels reached a slightly more advanced stage of reproductive development compared to nontransgenic sibling control lines when grown at high temperature in short days (Fig. 7). This is preliminary evidence that increased HvODDSOC2 transcript levels might contribute to the delay of early reproductive development under these conditions. It is also possible that increased HvODDSOC2 transcript levels might be a consequence of the delayed reproductive development in these plants, rather than a cause. However, HvODDSOC2 transcript levels are temperature responsive in nonvernalized barley plants that have not made the transition to reproductive development (Supplemental Fig. S5), suggesting that temperature can effect HvODDSOC2 transcript levels independent of development. Transcriptome analysis did not identify any other genes with transcript levels specifically changed at high temperature in short days that offer clear candidates for regulators of early reproductive development under these conditions (Tables III and IV).
Transcriptome analysis showed that transcript levels of a lectin-like gene increase in vernalized barley plants grown at high temperature in long days: conditions in which early reproductive development is proceeding rapidly relative to vegetative development (Table I). This gene is similar to a wheat gene, TaVER2. TaVER2 transcript levels increase in vernalized plants, but decrease when plants are placed at 35°C in the dark after vernalization (Yong et al., 2003). This decrease in TaVER2 transcript levels at high temperature in the dark is consistent with the low transcript levels we observed for the related barley gene in short days. TaVER2-like genes might function as vernalization-responsive promoters of flowering, as antisense lines have delayed flowering and do not respond to vernalization (Yong et al., 2003). VER2-like genes might thus play a role in acceleration of reproductive development by high temperature in long days. It is also possible that increased TaVER2 transcript levels might be a consequence of reproductive development in these plants, rather than a cause.
We identified a number of potential developmental regulators that displayed changed transcript levels at different temperatures in both short and long days. These include FPF1-like3, the RNase-S-like genes HvRSH1 and HvRSH2, HvODDSOC1, HvBM3, and HvBM7 (Fig. 6; Supplemental Fig. S4). Although the transcript levels of these genes do not change under specific combinations of temperature and day length, they might contribute to control of reproductive development at high temperature through interaction with day-length-responsive genes. For example, the combined activity of HvFT1 and HvFPF1-like3 might be required to accelerate reproductive development at high temperature. Together with HvODDSOC2 and TaVER2, these genes represent candidates for temperature-responsive developmental regulators in cereals. To understand what role these genes might play in reproductive development it will be necessary to identify plants carrying loss-of-function or variant alleles and examine the developmental response of these plants to high temperature.
Overall, we suggest that multiple genes might contribute to setting the rate of early reproductive development at high temperatures. Floral promoters, such as FPF1-like3 or VER2-like genes, might accelerate reproductive development as temperature increases in spring and early summer. The influence of these floral promoters could be counteracted by floral repressors, such as HvODDSOC2, to prevent acceleration of early reproductive development by rising temperatures when day lengths are short. This day-length-dependant inhibition of early reproductive development could help to reduce the risk of warm periods in late winter causing precocious flowering and resulting frost damage to developing floral organs. If this were true, allelic variation at temperature-responsive promoters or repressors of early reproductive development could be targeted in breeding strategies to modify the sensitivity of temperate cereal crops to high temperature.
CONCLUSION
To understand how crop yields can be maintained in a changing climate, we need to understand how reproductive development in crop plants responds to high temperatures. Here we have shown that, in the temperate cereal crops wheat and barley, high temperature and day length interact to control early reproductive development: a critical phase for determining yield. Unlike Arabidopsis, regulators of the long-day flowering response pathway do not appear to control early reproductive development at high temperature in temperate cereals. Instead, genes in the vernalization response pathway show changed transcript levels at high temperature and may interact to control early reproductive development in winter and spring. High-temperature-responsive developmental regulators would offer potential targets for breeding programs aimed at adapting the early development of cereal crops to maximize yield in a changing climate.
MATERIALS AND METHODS
Barley and Wheat Lines
The barley (Hordeum vulgare) cultivar Sonja was used to examine reproductive growth and development in short and long days and for all gene expression analysis. Sonja carries wild-type alleles of the flowering-time genes HvVRN1, HvVRN2, and HvPPD1 (genotype: Hvvrn1,HvVRN2,HvPPD1). Sonja requires vernalization and long days to flower rapidly. Other barley lines used were the mutant MAT-a.8 (eam8) and its parent cultivar Bonus [genotype: HvVRN1-1,Hvvrn2(ΔHvVRN2),Hvppd1; Dormling et al., 1966; Gustafsson et al., 1971] and transgenic lines OxHvOS2-20 and hpHvOS2-2 described in Greenup et al. (2010).
The wheat (Triticum aestivum) lines used to examine development in short days were near-isogenic lines carrying contrasting alleles of the TaPPD1 gene in the background of cultivar Sunstate. These lines were generated by recurrent crossing to Sunstate to the fourth backcross generation then selection of homozygotes. Lines W1A and W2A carry dominant alleles of TaVRN-1 on the B and D genomes and a recessive allele of TaVRN-1 on the A genome. W1A carries the TaPPD-D1a allele, which allows plants to flower rapidly in both short and long days (Beales et al., 2007; genotype: Tavrn-A1,TaVRN-B1,TaVRN-D1,TaPPD-D1a). W2A carries a Tappd-D1 allele derived from the cultivar Extra Early Black Hull, which causes plants to require long days to flower rapidly (genotype: Tavrn-A1,TaVRN-B1,TaVRN-D1,Tappd-D1). Lines W15A and W16A carry dominant alleles of TaVRN-1 on all three genomes and either the TaPPD-D1a allele or a Tappd-D1 allele derived from CSP00071 (genotypes: W15A TaVRN-A1,TaVRN-B1,TaVRN-D1,TaPPD-D1a and W16A TaVRN-A1,TaVRN-B1,TaVRN-D1,Tappd-D1). Supplemental Table S1 summarizes the barley and wheat genotypes, the sources of alleles, and flowering behavior.
Plant Growth Conditions
Seeds of cultivar Sonja were germinated and grown in darkness at 2°C ± 1.5°C for 9 weeks in plastic propagation trays containing pots 6.7-cm square and 10-cm height. Plants were grown in Debco seed raising mix (Debco) supplemented with 2 g/L Mini-Osmocote (Scotts Australia). At the end of the cold treatment, seedlings with the first leaf emerged from the coleoptile, an average leaf length of 12.6 cm (se 0.3 cm), and an average root number of 5.4 (se 0.1) were moved to Conviron PGC20 plant growth chambers (Conviron). Plants were grown in the chambers at constant temperatures of 15°C or 25°C ± 0.2°C and either a short-day treatment of 8-h light/16-h dark or a long-day treatment of 16-h light/8-h dark. Light was provided by 40 Philips 54W 840 cool-white fluorescent tubes (Philips Electronics Australia) and seven Sylvania Reflector 60W incandescent bulbs (Sylvania). Total light intensity was 600 μmol m−2 s−2 at plant height. Plants grown to developmental stages up to Zadoks = 14 (Zadoks et al., 1974) were grown in 6.7-cm square pots. Plants grown to developmental stages Zadoks = 15 and beyond were grown in pots 21-cm diameter and 19-cm height at a density of five plants per pot. Cold-treated seedlings were transplanted into the 21-cm diameter pots at the end of the cold treatment. Transplanted seedlings were kept in the cold for 24 h after transplantation before transfer to growth chambers. Comparisons between treatments were always performed using plants that had been grown in the same way, for example plants that had been transplanted were compared with other plants that had been transplanted. All plants were transferred from the cold treatment into growth chambers at the end of the light period. Plants were maintained in a fully watered state throughout the experiment. Supplemental fertilizer (Aquasol [Hortico Australia]) was supplied weekly at a rate of 3.2 g/L. Seeds of all other barley and wheat lines were grown as described above, except that they were cold treated for only 48 h before transfer to growth cabinets. Plants were grown until head emergence in long days or for 10 weeks in short days.
In a separate experiment plants were grown as described above in 16-h light/8-h dark except that light was provided only by 40 Philips 54W 840 cool-white fluorescent tubes, so that light in the far-red part of the emission spectrum was absent. The light spectrum provided was sufficient for normal growth and photosynthesis but was not inductive for flowering. Temperature was varied in a sine wave over each 24-h period, with the temperature maximum occurring 3 h before the end of the light period and the temperature minimum occurring at the end of the dark period. Temperature varied ±4°C around averages of 12°C, 17°C, and 22°C. Plants were grown until leaf 8 was visible on the main shoot in all treatments.
Measurements of Reproductive and Vegetative Development
Head emergence was measured as the day when the head first emerged from the sheath on the main shoot (Zadoks = 51). Leaves on the main shoot were numbered sequentially to determine final leaf number at head emergence. Heading date was measured for 37 plants and final leaf number was measured for 10 randomly selected plants from each long-day treatment. The number of heads per plant and number of florets per head was measured for 15 randomly selected plants from each long-day treatment. The rate of leaf emergence was measured for 60 plants from each treatment. Tiller number and dry weight of aboveground biomass were measured for 10 randomly selected plants from each treatment. Tiller number was counted when leaf 8 was visible on the main stem. Dry weight measurements were made at head emergence from plants from the long-day treatment and when leaf 10 had emerged on the main shoot for plants from the short-day treatments. Assay of apex morphology was performed on at least eight randomly selected plants from each treatment as each new leaf became visible on the main shoot. This allows calibration of reproductive development for different growth rates. Plants grown in long-day 25°C reached a final leaf number of 8, 9, or 10. Plants grown in long-day 15°C reached a final leaf number of 10, 11, or 12. This allowed comparison of shoot apices in both treatments up to the 10-leaf stage. Apices were isolated under a binocular dissecting microscope and then digitally photographed on a Leica M8 digital camera. Apex length was determined from the digital image. The means of treatments were compared using a Student’s t test assuming a two-tailed distribution and equal variance.
Gene Expression Analysis
Whole seedlings, minus roots, were harvested from each treatment for RNA extraction at Zadoks = 13.21 (third leaf at least 50% emerged, fourth leaf visible, and first tiller visible in 15°C ± 0.2°C treatments, 7–9 d after transfer to cabinets). Seedlings were harvested 30 min before the end of the light period to maximize the chance of detecting changes in transcript levels of genes for which transcript levels vary in a diurnal manner. RNA was extracted using the method of Chang et al. (1993) and then further purified using RNeasy columns (Qiagen). cDNA was prepared for qRT-PCR by using an oligo(T) primer (T18[G/C/A]) to prime first-strand cDNA synthesis from 2 μg of total RNA with SuperScript III reverse transcriptase enzyme (Invitrogen). qRT-PCR was performed on a Rotor-Gene 3000 real-time cycler (Corbett Research). Primer sequences are provided in Supplemental Table S2. qRT-PCR was performed using Platinum Taq DNA polymerase (Invitrogen) and SYBR green. Cycling conditions were 2 min at 94°C, 40 cycles of 15 s at 95°C, 15 s at 60°C, and 20 s at 72°C, followed by a melting-curve program (72°C–95°C with a 5-s hold at each temperature). Fluorescence data were acquired at the 72°C step and during the melting-curve program. Expression levels of genes of interest were calculated relative to ACTIN using the comparative quantification analysis method (Rotogene-5; Corbett Research), which takes into account the amplification efficiency of each primer set. Data presented are averages of four biological replicates, each biological replicate consisting of three plants. Each PCR experiment was carried out twice and similar results were obtained from both technical replicates. Data from one technical replicate are presented. Error bars show se. The means of treatments were compared using a Student’s t test assuming a two-tailed distribution and equal variance.
Microarray Analysis
Gene expression was assayed using the Agilent 4x44K barley gene expression microarray (Agilent). RNA quality assessment, probe synthesis, labeling, and hybridization to the Agilent barley chip was performed at the Australian Genome Research Facilities, following the manufacturer’s recommendations. Microarray analyses were performed on three biological replicates for each treatment. Each biological replicate consisted of three plants.
The resulting microarray data set was analyzed in R v2.7.1 using packages from Bioconductor (Gentleman et al., 2004; http://www.bioconductor.org/). Scale normalization (Smyth and Speed, 2003) was carried out using the normalizeBetweenArrays function in limma and differentially expressed probes were identified across the normalized data sets for biological replicates using linear modeling in limma (Smyth, 2005). The long-day and short-day experimental samples at each temperature were compared with each other (e.g. short-day 15 to long-day 15 and short-day 25 to long-day 25) and likewise the samples representing the different temperatures at short and long days were compared with each other (e.g. long-day 25 to long-day 15 and short-day 25 to short-day 15). In all cases multiple testing was corrected for by controlling the false discovery rate (Benjamini and Hochberg, 1995). The probes that were differentially expressed across all the treatments (empirical Bayes test, no minimum fold cutoff applied; 7,541 probes) were grouped into five clusters according to their expression pattern using the pamsam function in smida (Wit and McClure, 2004) with correlation selected as the distance measured. For principal component analysis clustering the normalized expression values of the data sets were grouped based on condition using the cluster.samples function in smida. The method chosen was pca and correlation selected as the distance measured. The clusters were plotted using the first two principal components of the principal component analysis. Hierarchical clustering of the conditions was performed using the diana function in the cluster package using correlation as the distance measured. Clusters were manually edited to create a final set of 14 clusters (Supplemental Data Set S1). A blastx search of the nonredundant protein sequence database using the largest available sequence contig corresponding to each probe was used to provide a tentative annotation for approximately 500 probes. The expression patterns of the following probes were verified by RT-PCR: HvVRN1 (A_13_P126555), HvRSH1 and HvRSH2 (A_13_P134400, A_13_P010366, A_13_P011106), HvFPF1-like3 (A_13_P417695), HvVER2-like (A_13_P074401). All microarray data have been deposited in Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/), experiment GSE33938 (GSM839112–GSM839123).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Reproductive development is slowed by high temperature in an oscillating temperature regime, in the dark and in wheat.
Supplemental Figure S2. Activation of the long-day response pathway does not allow rapid early reproductive development at high temperature.
Supplemental Figure S3. Developmental stage of plants used for gene expression analysis.
Supplemental Figure S4. Transcript levels in plants grown at 15°C or 25°C in short or long days.
Supplemental Figure S5. Transcript levels of HvODDSOC2 in nonvernalized barley plants grown at three different temperatures.
Supplemental Figure S6. Changes in gene expression in four pairwise comparisons between treatments.
Supplemental Figure S7. Principal component analysis of microarray data.
Supplemental Table S1. Barley and wheat genotypes and flowering behavior.
Supplemental Table S2. Primers used for qRT-PCR.
Supplemental Data Set S1. Complete lists of 14 clusters of differentially expressed probes.
Acknowledgments
We acknowledge excellent technical support from Xavier Sirault and Scott Berry (High Resolution Plant Phenomics Centre) and from Rosemary White, Mark Talbot, Sandra Stops, Anna Wielopolska, and Carl Davies (Commonwealth Scientific and Industrial Research Organization Division of Plant Industry). We also thank Jean Finnegan (Commonwealth Scientific and Industrial Research Organization) for critical reading of the manuscript.
References
- Aspinall D. (1969) The effects of day length and light intensity on the growth of barley. Aust J Biol Sci 22: 53–67 [Google Scholar]
- Asseng S, Foster I, Turner N. (2011) The impact of temperature variability on wheat yields. Glob Change Biol 17: 997–1012 [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. (2006) Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115: 721–733 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57: 289–300 [Google Scholar]
- Blázquez MA, Ahn JH, Weigel D. (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet 33: 168–171 [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney K. (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Dormling I, Gustafsson A, Jung HR, von Wettstein D. (1966) Phytotron cultivation of Svalof’s Bonus barley and its mutant Svalof’s Mari. Hereditas 56: 221–237 [Google Scholar]
- Ellis RH, Roberts EH, Summerfield RJ, Cooper JP. (1988) Environmental control of flowering in barley (Hordeum vulgare L.) II. Rate of development as a function of temperature and photoperiod and its modification by low-temperature vernalization. Ann Bot (Lond) 62: 145–158 [Google Scholar]
- Fischer RA. (1985) Number of kernels in wheat crops and the influence of solar radiation and temperature. J Agric Sci 105: 447–461 [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup A, Peacock WJ, Dennis ES, Trevaskis B. (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot (Lond) 103: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Talbot MJ, Dennis ES, Hemming MN, Trevaskis B. (2010) ODDSOC2 is a MADS box floral repressor that is down-regulated by vernalization in temperate cereals. Plant Physiol 153: 1062–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson A, Hagberg A, Persson G, Wiklund K. (1971) Induced mutations and barley improvement. Theor Appl Genet 41: 239–248 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Trevaskis B. (2011) Make hay when the sun shines: the role of MADS-box genes in temperature-dependant seasonal flowering responses. Plant Sci 180: 447–453 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Field CB. (2007) Global scale climate-crop yield relationships and the impacts of recent warming. Environ Res Lett 2: 1–7 [Google Scholar]
- Rawson HM, Richards RA. (1993) Effects of high temperature and photoperiod on floral development in wheat isolines differing in vernalisation and photoperiod genes. Field Crops Res 32: 181–192 [Google Scholar]
- Smyth G. (2005) Limma: linear models for microarray data. In R Gentlemen, V Carey, W Huber, R Irizarry, S Dudoit, eds, Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, New York, pp 397–420 [Google Scholar]
- Smyth GK, Speed TP. (2003) Normalization of cDNA microarray data. Methods 31: 265–273 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Wit E, McClure J. (2004) Statistics for Microarrays: Design, Analysis and Inference. Wiley, Chichester, UK, pp 167–169 [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong WD, Xu YY, Xu WZ, Wang X, Li N, Wu JS, Liang TB, Chong KC, Xu ZH, Tan KH, et al. (2003) Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2. Planta 217: 261–270 [DOI] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. (1974) A decimal code for the growth stages of cereals. Weed Res 14: 415–421 [Google Scholar]