Abstract
Exocytosis is the process of fusion of a membrane-bound vesicle with the cell membrane and subsequent release of the vesicle content to the outside. It is now widely accepted that SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins are key components in the molecular machinery of exocytosis. SNARE proteins on the vesicle membrane selectively form complexes with specific SNAREs on the cell membrane. In a variant of exocytosis, called compound exocytosis, secretory vesicles still fuse with the cell membrane but vesicle-to-vesicle fusion enhances secretory output. Two types of compound exocytosis occur, either vesicles fuse with each other and then fuse with the cell membrane, or a vesicle fuses with the cell membrane and then becomes a target for further vesicles to fuse with it. It is expected that SNAREs are important for vesicle-to-vesicle fusion but the mechanism(s) that control these processes is unknown. In our recent paper (Behrendorff et al. 2011) we provide evidence that VAMP8 (a Q-SNARE) is essential in regulating compound exocytosis. Here we discuss the implications of our findings with reference to a new model for the control of vesicle-to-vesicle fusion.
Keywords: exocytosis, granule, Secretion
Compound exocytosis
Membrane-to-membrane fusion has to be tightly regulated and SNARE proteins, located on both membranes, are part of the molecular machinery that underpin this regulation. A SNARE complex of syntaxin1, SNAP25 and VAMP supports neuronal exocytosis and analogous complexes are thought to be fundamental to all membrane fusion both fusion of intracellular compartments and fusion with the plasma membrane.1 So for any type of exocytosis, in any cell type, we would expect similar SNARE complexes forming part of the machinery that regulate fusion.2 One example of diversity in exocytic processes is a mechanism of vesicle-to-vesicle fusion called compound exocytosis. This mechanisms is found in some neurones, mast cells and acinar cells and in all cases is thought to be a way of enhancing secretory output.3 Why this occurs is a matter of speculation. In mast cells the antigen-evoked response triggers the exocytosis of all the secretory vesicles (granules) and compound exocytosis provides a mechanism where deeper lying granules can readily release their content without having to be transported to the cell membrane.4 A similar explanation may be important but for a different reason in acinar cells. In these cells regulated exocytosis only occurs at the apical cell membrane which is a spatially complex region that circumscribes a minor proportion of the total cell area.5 Given it is such as small area this will lead to spatial competition for granule access to fuse with this limited apical membrane. Without a process of compound exocytosis repeated rounds of granule fusion would require that fused granules be endocytosed, these endocytic compartments would need to be cleared from the sub-membrane area and then new granules would need to be transported to the cell membrane. Thus granule-to-granule fusion provides a mechanism to avoid the necessity for these steps and enhance secretory output.3 The physiology of exocrine tissues means that they need to secrete high outputs but only for short periods of time; for example the pancreas secretes enzymes at each meal. Thus each burst of secretion is followed by long periods of quiescence during which the processes of endocytosis and transport of new granules can take place.
How is Compound Exocytosis Regulated?
A distinction is drawn between compound exocytosis where granules fuse with each other prior to fusion with the plasma membrane and an alternative where a primary granule fuses with the cell membrane that then becomes the target for secondary granule fusion, termed sequential compound exocytosis.3 The evidence for sequential compound exocytosis is clear in pancreatic acinar cells where larger fusion events, expected if there was prior granule-to-granule fusion, are never seen.6,7 The imaging methods used in these papers could readily identify larger events and, since they are never seen, this strongly supports the idea of sequential compound exocytosis.
Evidence for granule-to-granule fusion prior to fusion with the cell membrane comes from mast cells. Here cell capacitance measurements show larger capacitance increases than expected for fusion of a single granule indicating granules have fused together.4 However, the authors use other indirect data to suggest that granule-to-granule fusion is actually dependent on an intermediate step that involves granules fusing with the cell membrane and then recapture of multigranular structures.4 If these recaptured multigranular structures do account for the large capacitance increases then the distinction between the two models becomes less clear. In both cases granule fusion to another granule does not take place until one granule has fused with the plasma membrane.8
Speculation as to how sequential compound exocytosis might be regulated center on the idea that after fusion of the primary granule it somehow becomes competent for subsequent fusion with secondary granules. This would provide a ready explanation as to why granule-to-granule fusion only occurs after fusion of primary granules. In support of this idea there is evidence for movement of SNAREs from the plasma membrane into the primary granule membrane. Evidence for SNARE redistribution into the primary granule includes immunostaining of SNAP25 in pancreatic β cells,9 SNAP23 in mast cells8 and syntaxin 2 in pancreatic acinar cells.10 However, translocation of SNAREs does not prove they play a role in compound exocytosis. Only for SNAP23 in mast cells is there functional data that supports a role for this SNARE in mediating subsequent fusion of secondary granules.8 Furthermore, the data shed no light on the composition of the SNARE complex that controls granule-to-granule fusion. By analogy to the neuronal SNARE complex, it would be expected that Q and R SNAREs located on the membranes of the fusing granules, are required to mediate fusion.1
Our Evidence that VAMP8 is a Crucial SNARE in Regulating Granule-to-Granule Fusion
We chose to study the potential role of a granule SNARE, VAMP8 because there was already good evidence that the knockout mice had a deficit in secretion from pancreatic acinar cells,11 VAMP8 was shown to be present on the granules and overexpression of VAMP8 constructs inhibited secretion12 and finally there was apparently less granule-to-granule fusion as measured in electron micrographs.13 To further these studies we employed live-cell imaging and developed a new assay for the identification of secondary granule fusion.14 To date the identification of primary granule and secondary granule fusion has been based on the spatial location of the fused granules. The presumption being that if granules deeper in the cell were seen to fuse these was likely to be secondary fusion events. However, it is impossible to rule out that invaginations of the cell membrane could dive deep into the cell and thus an apparent secondary fusion event could really be a primary event. In our new assay we track pH changes within the lumen of the granules during fusion. All secretory granules are acidic and granule fusion releases the protons into the outside space. In this way fusion of a genuine secondary granule leads to acidification of the primary granule and this is readily tracked with a pH sensitive dye (HPTS).15
Our functional data using VAMP8 knockout mice show no change in primary exocytic fusion event numbers or kinetics but an almost complete loss of secondary fusion events.14 This explains the deficit in secretion previously observed and provides strong evidence that VAMP8 is a crucial SNARE in mediating granule-to-granule fusion. In immunoprecipitation experiments we further show that VAMP8 is part of a SNARE complex that includes syntaxin3 (also found on the granule membranes) and SNAP23. We show this SNARE complex is distinct from a complex where syntaxin 2 immunoprecipitated VAMP2 (a granule SNARE) and SNAP23.
A New Model for the Molecular Control of Compound Exocytosis
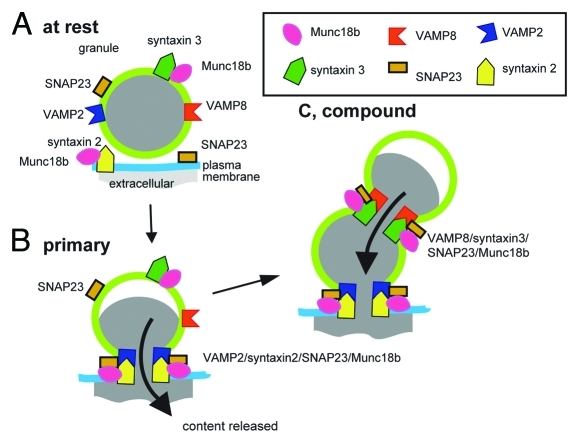
Figure 1 summarizes our new model for SNARE-mediated granule-to-granule fusion and places this complex as distinct from the SNARE complex that regulates fusion of the primary granules. The remaining unresolved problems in our model are:
Figure 1.
Model based on work in pancreatic acinar cells, where different SNARE complexes regulate primary granule fusion with the cell membrane and granule-to-granule fusion.
• how does the primary granule signal fusion competence?
• and since the granule-to-granule SNARE complex we propose includes three proteins, VAMP8, syntaxin3 and SNAP23 that are all present on the granule membranes, how is granule-to-granule fusion within the cell prevented?
There are three possible mechanisms.
First, although SNAP23 has been shown to be present on the granule membranes16 it may be at a sub-threshold concentration and require enrichment through movement from the plasma membrane into the primary granule membrane. This model would be consistent with the localization and functional data from mast cells8 and would present a conserved model for the control of compound exocytosis.
Second, both primary and secondary fusion processes could be regulated by the sec1/Mun18like (SM) proteins.1 We showed that Munc18b was in a binary complex with syntaxin2 and in a binary complex with syntaxin3; possibly holding the SNAREs in a “closed” conformation.1,14 After stimulation the syntaxins formed ternary complexes with VAMPs and SNAP23. Stimulation also affected Munc18b binding to the syntaxins, which while still present, were apparently reduced. Specific modulation of the syntaxin3/VAMP8/SNAP23 complex by Munc18b could therefore confer fusion competence on the primary granule.
Third, fusion competence of the primary granule may be controlled by an as yet other unidentified mechanisms. There are multiple changes associated with primary granule fusion that include changes to the luminal constituents (loss of content, alkalinisation, etc) and changes to the granule structure such as reorganization of the surrounding F-actin cytoskeleton.
In conclusion, our paper describes a crucial role for VAMP8 and identifies a SNARE complex in granule-to-granule fusion that is distinct from the SNARE complex mediating primary granule fusion. Future work is needed on the control mechanisms that “signal” primary granule fusion and its competence to fuse with secondary granules.
Acknowledgments
This work was supported by an Australian Research Council Grant DP110100642 (to PT) and a National Health and Medical Research Council Grant APP1002520 (to PT and HYG).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18058
References
- 1.Südhof TC, Rothman JE. Membrane fusion: Grappling with snare and sm proteins. Science. 2009;323:474–7. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, et al. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996;7:2019–27. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickett JA, Edwardson JM. Compound exocytosis: Mechanisms and functional significance. Traffic. 2006;7:109–16. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez de Toledo G, Fernandez JM. Compound versus multigranular exocytosis in peritoneal mast cells. J Gen Physiol. 1990;95:397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larina O, Thorn P. Ca2+ dynamics in salivary acinar cells: Distinct morphology of the acinar lumen underlies near-synchronous global Ca2+ responses. J Cell Sci. 2005;118:4131–9. doi: 10.1242/jcs.02533. [DOI] [PubMed] [Google Scholar]
- 6.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, et al. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3:253–8. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 7.Thorn P, Fogarty KE, Parker I. Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc Natl Acad Sci USA. 2004;101:6774–9. doi: 10.1073/pnas.0400336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Z, Turner C, Castle D. Relocation of the t-snare snap-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–48. doi: 10.1016/S0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Hatakeyama H, Okado H, Miwa A, Kishimoto T, Kojima T, et al. Sequential exocytosis of insulin granules is associated with redistribution of snap25. J Cell Biol. 2004;165:255–62. doi: 10.1083/jcb.200312033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickett JA, Campos-Toimil M, Thomas P, Edwardson JM. Identification of snares that mediate zymogen granule exocytosis. Biochem Biophys Res Commun. 2007;359:599–603. doi: 10.1016/j.bbrc.2007.05.128. [DOI] [PubMed] [Google Scholar]
- 11.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, et al. A role of vamp8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell. 2004;7:359–71. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Weng N, Thomas DDH, Groblewski GE. Pancreatic acinar cells express vesicle-associated membrane protein 2-and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J Biol Chem. 2007;282:9635–45. doi: 10.1074/jbc.M611108200. [DOI] [PubMed] [Google Scholar]
- 13.Cosen-Binker LI, Binker MG, Wang CC, Hong WJ, Gaisano HY. Vamp8 is the v-snare that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–51. doi: 10.1172/JCI34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrendorff N, Dolai S, Hong W, Gaisano HY, Thorn P. Vesicle-associated membrane protein 8 (vamp8) is a snare (soluble n-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem. 2011;286:29627–34. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139:1711. doi: 10.1053/j.gastro.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, et al. Supramaximal cholecystokinin displaces munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]