Abstract
Mammalian spermatozoa reach the ability to fertilize only after they complete a complex series of physical-chemical modification, the capacitation. Recently, the endocannabinoid-binding type-1cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) channel have been proposed to play a key role in the control of capacitation. In particular CB1, acting via a Gi protein/cAMP/PKA pathway, maintains low cAMP levels in early stages of post ejaculatory life of male gametes. By this way it promotes the maintenance of membrane stability, thus avoiding the premature fusion of plasma membrane (PM) and outer acrosome membrane (OAM), which is mandatory for the exocytosis of acrosome content. TRPV1, on the contrary, becomes active during the latest stages of capacitation, and allows the rapid increase in intracellular calcium concentration that leads to the removal of the F-actin network interposed between PM and OAM, leading to their fusion and, ultimately, to the acrosome reaction.
Keywords: capacitation, endocannabinoid system, spermatozoa, transient receptor potential vanilloid 1 (TRPV1), type-1cannabinoid receptor (CB1)
Mammalian spermatozoa, immediately after ejaculation, are unable to fertilize the oocytes. In fact, male gametes acquire the full fertilizing competence only after residing for a period that ranges from hours to days (depending on the species), within the female genital tract. Here, after the removal of seminal plasma, a complex series of morpho-functional modifications, collectively known as “capacitation,” occurs due to the interaction of sperm cells with the female environment. The capacitation process is completed when sperm cells become able to recognize the oocyte and to extrude the content of acrosomal vescicle (acrosome reaction, AR), thus penetrating the zona pellucida (ZP) and reaching the oocyte membrane.
Since their discovery in the 50s, the molecular mechanisms involved in capacitation and AR have attracted much attention, with a particular interest on the well-balanced dialog between activating and inhibiting factors that drive the modification of the whole biochemical asset and signal transduction machinery. Recently, it has been proposed that the endocannabinoid system (ECS) could play a key-role in the control of sperm physiology.1-3 In line with this hypothesis, the relevance of ECS in the physiopathology of male reproduction is supported by its conservation along the evolutionary axis, from echinoderm (sea urchin4) to amphibians (frog5) and mammals (mouse,5 bull,6 boar,2 Human1). Also the finding that the assumption of tetrahydrocannabinol (THC), the main psychoactive ingredient of cannabis (Cannabis sativa), negatively affects sperm motility, metabolism and fertility,7,8 has further supported this view.
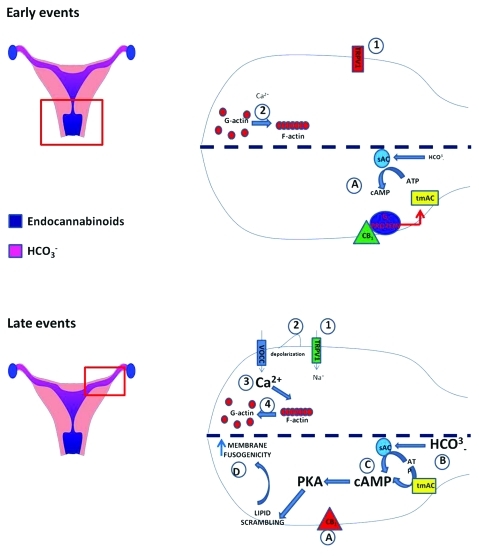
In this context, we have recently proposed that the endocannabinoid-binding type-1cannabinoid receptor (CB1)9 and transient receptor potential vanilloid 1 (TRPV1)10 channel could participate in the modulation of spermatozoa maturation (Fig. 1).
Figure 1.
Immediately after ejaculation (early events) TRPV1 receptors are located in post-equatorial area and are functionally inactive (1). The slowly increasing [Ca2+]i allows G-actin polymerization, thus creating a diaphragm between PM and OAM (2). cAMP concentration is maintained at a low level by CB1 located in the post-equatorial area, which actively inhibits tmAC, and by a low intracellular concentration of bicarbonate (A). Progressively (late events) TRPV1 translocates in the acrosomal area and becomes active (1). Its opening triggers a membrane depolarization wave (2) and, as a consequence, the recruitment of VOCCs which, in turn, promote a dramatic increase in [Ca2+]i (3). The latter event causes a fast depolymerization of F-actin network (4), thus allowing the contact between PM and OAM. At the same time, CB1 migrates in the equatorial area and becomes inactive (A). This event, along with the markedly increased intracellular concentration of bicarbonate (B), promotes the rise in cAMP levels (C) that, via a PKA-dependent pathway, causes the activation of lipid scrambling and increases membrane fusogenicity (D).
In particular CB1, a Gi/o protein coupled receptor, seems to be involved in an integrated dialog with bicarbonate, that finely regulates membranes dynamics. In early stages of post ejaculatory life (as it happens in uterus), sperm cells are exposed to a high concentration of endocannabinoids and to low levels of bicarbonate. As a result, the intracellular concentration of cAMP is maintained low through the stimulation of CB1, that inhibits the activity of the trans-membrane isoform of adenylyl cyclase (tmAC). In this state, the membranes are in a highly stable asymmetrical organization: the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are concentrated in the inner leaflet, and the choline phospholipids sphingomyelin (SM) and phosphatidylcholine (PC) in the outer leaflet. This asymmetry is established and maintained by the action of several translocating enzymes with differing phospholipid specificities, the activity of which is modulated by PKA-dependent phosphorylation.
As the spermatozoa progress along the female genital tract, they are gradually exposed to lower concentrations of endocannabinods and higher concentrations of bicarbonate (oviduct).11 This condition is associated with the migration of CB1 in the equatorial region of the sperm, where its activity progressively decreases. The reduced CB1 activity and the presence of high levels of bicarbonate, which activate a soluble isoform of adenylyl cyclase (sAC), cause a rise in intracellular cAMP levels. Interestingly, an autocrine/paracrine inhibitory feedback seems to exist between intracellular cAMP levels and CB1 localization. In fact, CB1 modulates the endogenous tone of cAMP and, in turn, this second messenger seems to control receptor translocation. Over time, the increased cAMP, acting via a PKA-dependent pathway, leads to PM phospholipids redistribution and to the rupture of the inner and outer leaflets asymmetry (“lipid scrambling”). This event causes an increased sperm membrane fluidity and disorder, and sustains a cholesterol efflux driven by soluble protein acceptors, from the anterior sperm head. Concomitantly, the antigenic mosaic modifies its organization in order to allow the specific localization of molecules involved in signal transduction, as well as the reorganization of membrane microdomains (i.e., portions that contain larger amounts of cholesterol, sphingomyelin, gangliosides, phospholipids with saturated long-chain acyl chains, and proteins such as GPI anchored proteins, caveolin and flotillin),12,13 where the transduction signaling machinery is segregated. As a consequence, plasma membrane (PM) and outer acrosome membrane (OAM) progressively increase their ability to fuse with each other (the so called “fusogenicity”), which is mandatory for the completion of AR. A result of this integrated dialog is the promotion of a capacitative status of spermatozoa and the control of the PM and OAM ability to fuse at the right time, thus avoiding lack of responsiveness to ZP and premature loss of acrosome integrity. The latter event can be prevented, during capacitation, by the calcium-dependent polymerization of G-actin, that leads to the formation of an F-actin network that acts as a diaphragm between PM and OAM. This structure has important functions in signal transduction,14 and acts as a mechanical shelter against the premature fusion of sperm head membranes.15 Its removal at the right moment (i.e., when the physiological stimulus is received) is controlled by the endocannabinoid/endovanilloid-binding transient receptor potential vanilloid 1 (TRPV1) channel. The latter protein, in fact, is inactive at early stages of capacitation, and is located over the post-equatorial area of the sperm head. As the acquisition of fertilizing ability progresses, TRPV1 translocates from the post-equatorial to the anterior region of the sperm head, thus becoming active. TRPV1 is a non-selective cationic channel, and its activation triggers a membrane depolarization wave and, as a consequence, the opening of voltage-operated calcium channels (VOCC). Then, the subsequent fast elevation of intracellular calcium concentration causes several biochemical events and, particularly, the depolymerisation of F-actin and the disappearance of the actin network. At this time, the contact between PM and OAM, and thus AR, becomes possible.
In conclusion, our present investigation has improved our understanding of the role of the ECS in the complex events leading to sperm-oocyte recognition. In the female genital tract, endocannabinoid-binding receptors seem a crucial element to modulate sperm membrane response to environmental stimuli, and to regulate sperm functioning in a spatio-temporal manner. Besides the possible physiological role of these receptors in controlling sperm acquisition of fertilizing ability, it is tempting to suggest that they may represent a novel platform, on which new diagnostic and therapeutic strategies may be developed to treat male infertility and to improve sperm management and storage.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18118
References
- 1.Rossato M, Popa FI, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor CB1 whitc activation inhibits motility, acrosome reaction and mitochondrial function. J Clin Endocrinol Metab. 2005;90:984–91. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- 2.Maccarrone M, Barboni B, Paradisi A, Bernabò N, Gasperi V, Pistilli MG, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci. 2005;118:4393–404. doi: 10.1242/jcs.02536. [DOI] [PubMed] [Google Scholar]
- 3.Schuel H, Burkman LJ. A tale of two cells: endocannabinoid-signaling regulates functions of neurons and sperm. Biol Reprod. 2005;73:1078–86. doi: 10.1095/biolreprod.105.043273. [DOI] [PubMed] [Google Scholar]
- 4.Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci USA. 1994;91:7678–82. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobellis G, Cacciola G, Scarpa D, Meccariello R, Chianese R, Franzoni MF, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol Reprod. 2006;75:82–9. doi: 10.1095/biolreprod.106.051730. [DOI] [PubMed] [Google Scholar]
- 6.Gervasi MG, Osycka-Salut C, Caballero J, Vazquez-Levin M, Pereyra E, Billi S, et al. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS One 2011; 11;6(2):e16993. [DOI] [PMC free article] [PubMed]
- 7.Morgan DJ, Muller CH, Murataeva NA, Davis BJ, Mackie K. Delta-9-Tetrahydrocannabinol (Δ(9) -THC) Attenuates Mouse Sperm Motility and Male Fecundity. Br J Pharmacol. 2011;••• doi: 10.1111/j.1476-5381.2011.01506.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badawy ZS, Chohan KR, Whyte DA, Penefsky HS, Brown OM, Souid AK. Cannabinoids inhibit the respiration of human sperm. Fertil Steril. 2009;91:2471–6. doi: 10.1016/j.fertnstert.2008.03.075. [DOI] [PubMed] [Google Scholar]
- 9.Barboni B, Bernabò N, Palestini P, Botto L, Pistilli MG, Chiarini M, et al. Type-1 cannabinoid receptors reduce membrane fluidity of capacitated boar sperm by impairing their activation by bicarbonate. PLoS ONE. 2011;6:e23038. doi: 10.1371/journal.pone.0023038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernabò N, Pistilli MG, Mattioli M, Barboni B. Role of TRPV1 channels in boar spermatozoa acquisition of fertilizing ability. Mol Cell Endocrinol. 2010;323:224–31. doi: 10.1016/j.mce.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Lewis SE, Maccarrone M. Endocannabinoids, sperm biology and human fertility. Pharmacol Res. 2009;60:126–31. doi: 10.1016/j.phrs.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganization during capacitation. Int J Dev Biol. 2008;52:473–80. doi: 10.1387/ijdb.082583bg. [DOI] [PubMed] [Google Scholar]
- 13.Botto L, Bernabò N, Palestini P, Barboni B. Bicarbonate induces membrane reorganization and CBR1 and TRPV1 endocannabinoid receptor migration in lipid microdomains in capacitating boar spermatozoa. J Membr Biol. 2010;238:33–41. doi: 10.1007/s00232-010-9316-8. [DOI] [PubMed] [Google Scholar]
- 14.Bernabò N, Berardinelli P, Mauro A, Russo V, Lucidi P, Mattioli M, et al. The role of actin in capacitation-related signaling: an in silico and in vitro study. BMC Syst Biol. 2011;5:47. doi: 10.1186/1752-0509-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263–8. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]