Abstract
The common garden ant Lasius niger use both trail pheromones and memory of past visits to navigate to and from food sources. In a recent paper we demonstrated a synergistic effect between route memory and trail pheromones: the presence of trail pheromones results in experienced ants walking straighter and faster. We also found that experienced ants leaving a pheromone trail deposit less pheromone. Here we focus on another finding of the experiment: the presence of cuticular hydrocarbons (CHCs), which are used as home range markers by ants, also affects pheromone deposition behavior. When walking on a trail on which CHCs are present but trail pheromones are not, experienced foragers deposit less pheromone on the outward journey than on the return journey. The regulatory mechanisms ants use during foraging and recruitment behavior is subtle and complex, affected by multiple interacting factors such as route memory, travel direction and the presence trail pheromone and home-range markings.
Keywords: complexity, cuticular hydrocarbons, home-range markings, route memory, synergy, Trail pheromones
The foraging behavior of ants, with its interplay between the individuals and the group, plays an important role in the study of self organization and the emergent behavior of complex systems,1,2 and has inspired the well known metaheuristic Ant Colony Optimization (ACO).2-4 However, in ACO foraging ants are usually considered to utilize a rather simple set of behavioral rules3,5,6 often limited to simply “If you find food, return to the nest laying trail pheromone” and “preferentially follow trails with more pheromone.”3,5,7 Subsequent study of ant foraging has uncovered further foraging rules and properties of the pheromone trail network.6 For example, Pharaoh’s ant deposit two types of attractive trail pheromone: a short-lived pheromone that decays within 20 min and a longer lasting pheromone that acts as an external long-term memory, allowing colonies to re-use trails laid one or two days previously.8 They also deposit repellent pheromones on branches leading to depleted food sources.9 In the ant Lasius niger, rules such as “Deposit more pheromone when food quality is higher,”10 “Deposit more pheromone if the colony is starving”11 and “Deposit more pheromone the closer you get to the food source”12 have been uncovered. However, foraging ants do not rely solely trail pheromones. L. niger foragers can form accurate route memories after just a few visits to a food source,13-15 and these route memories are followed in preference to trail pheromones when in the two conflict.13,15-17
In a recent paper,18 we allowed L. niger foragers which had already made several trips to a feeder to walk along a walkway with alternating segments marked and unmarked by naturally-laid trail pheromone. We found that the two information sources, route memory and trail pheromone, interact. Experienced ants use the presence of trail pheromone as what we termed ‘reassurance’ that they are on the correct path. Reassured, the ants walk faster and straighter. If, by chance, they do make an error and step off the path, they reduce speed, walk more sinuously, and perform more U-turns. We suggested that this might help them to get back on the right path. Furthermore, we showed that ants with a route memory greatly reduce the amount of pheromone they deposit, which we quantified by counting the number of times they dot the tip of their abdomen on the substrate, when they step off the marked path. This represents another rule used by ants for modifying pheromone deposition: “Reduce pheromone deposition if you step off a pheromone trail and have been to the food source before.” Presumably, this reduces the likelihood that nestmate ants will be diverted down the wrong path, so maintaining trail integrity and prevents an error cascade.
However, the complexity found in this experiment extended further than the interaction between trail pheromones and memory; the ants also changed their behavior in the presence of home range markings. Home range markings in L. niger consists of cuticular hydrocarbons (CHCs) secreted from tarsal glands on the feet19,20 and are passively deposited on surfaces that ants walks over.20 They are non-volatile, long lasting, and unlike trail pheromones, which lead to specific locations, CHCs are considered to be home range markings. Due to heavier ant traffic closer to the nest, a CHC gradient forms from the nest entrance outwards, defining the areas frequently visited by the colony’s foragers.21 Ants can sense CHCs on a surface and on other ants.
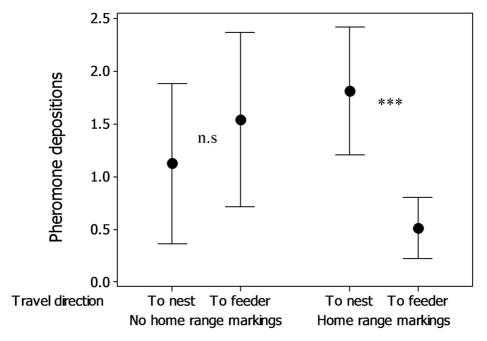
The presence of CHCs on the substrate increases aggression levels22 and reduces food discovery time23 and walking sinuosity23 in L. niger and has also been shown to increase pheromone deposition on the first return to the nest.21,23 However, by observing ants making repeated trips to a feeder, we found that this was only half the story. When walking on a substrate with home range markings but without trail pheromone, experienced ants lay less pheromone on outward journeys to a food source, and deposit more pheromone on the return journey (see Figure 1). When home range markings are not present, deposition on both the outwards and return journey is of intermediate intensity. In other words, the ants seem to have a further rule modifying pheromone deposition intensity: “If returning to a feeder on a home range marked path, deposit less pheromone.”
Figure 1.
Number of pheromone depositions by experienced ants either traveling toward the feeder or returning to the nest source on a 7cm trail section either marked or unmarked with home range markings (see Czaczkes et al. 2011 for detailed methods). When home range markings are present, outgoing ants deposit significantly less pheromone than returning ants (Generalized Linear Mixed-Effects Model, z = 3.984, p < 0.001). When home range markings are absent, pheromone deposition rates are not different between outwards and return journeys (z = 0.696, p = 0.486) (data from Czaczkes et al.18
Sensing that a trail is heavily marked by CHCs on an outward journey but unmarked by trail pheromones may indicate that the food source has been heavily exploited, and may now be depleted. In that case it would make little sense to increase recruitment of foragers on the outwards journey, as the food source may be depleted. However, on the return journey, when the ant knows there is food at the end of the trail, the colony would benefit from further recruitment to this location. Indeed, a high level of CHCs suggests that this food source was visited frequently in the past, so is not only productive but also (if no alarm pheromone is present) safe. While these explicit arguments are most likely not considered consciously by the ants, the behavioral rules with which ants are equipped suggest a complex and subtle tuning of recruitment behavior, based on multiple information sources.
A picture is emerging of great complexity in the rules affecting foraging and recruitment in L. niger. Individual ants are equipped with many rules governing their behavior, and alter their behavior depending on multiple factors including, but no doubt not limited to, trail pheromone presence, home range marking presence, travel direction and experience level, and the interactions between these information sources. This mirrors work uncovering similar sophistication in the communication of honey bees, which have at least four mechanical signals and two pheromones which affect foraging,24-26 and foraging in Pharaohs ants, which have multiple trail pheromones and can even extract information from the geometry of the trail system.8,9,27 Multiple signals and information sources seem to be the rule in natural complex systems such as ant foraging, and we predict that by studying individual foragers over multiple foraging trips more such rules might emerge. Progress is being made in understanding the intricate rule sets ants use when foraging, but we are still far from a complete understanding of the system. Uncovering new behavioral rules may inspire development of next generation ACO logic systems.6 After all, if so much can be built on basic behavioral rules uncovered over half a century ago, the application of current and future findings may provide a great step forward.
Acknowledgments
T.C. was supported by a PhD studentship from BBSRC. G.C. was funded by a postdoctoral fellowship from the Swiss National Science Foundation (SNSF grant no: PA00P3 129134). S.J. was funded by a Sussex University GTP studentship.
Glossary
- List of abbreviations: ACO
Ant Colony Optimisation
- CHCs
Cuticular Hydrocarbons
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18209
References
- 1.Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraula G, Bonabeau E. Self-organization in biological systems: Princeton University Press, 2003. [Google Scholar]
- 2.Dorigo M, Stützle T. Ant Colony Optimization. MIT Press, 2004. [Google Scholar]
- 3.Dorigo M, Di Caro G. Ant colony optimization: a new meta-heuristic; in: Proceedings of the 1999 Congress on Evolutionary Computation: 2. IEEE, 1999: 1477. [Google Scholar]
- 4.Mullen RJ, Monekosso D, Barman S, Remagnino P. A review of ant algorithms. Expert Syst Appl. 2009;36:9608–17. doi: 10.1016/j.eswa.2009.01.020. [DOI] [Google Scholar]
- 5.Beckers R, Deneubourg J, Goss S, Pasteels JM. Collective decision making through food recruitment. Insectes Soc. 1990;37:258–67. doi: 10.1007/BF02224053. [DOI] [Google Scholar]
- 6.Ratnieks FLW. Biomimicry: further insights from ant colonies? in Liò P, Yoneki E, Crowcroft J, Verma DC (eds): Bio-Inspired Computing and Communication. Berlin, Heidelberg: Springer, 2008: 58-66. [Google Scholar]
- 7.Hangartner W. Orientierung von Lasius fuliginosus Latr. An Einer Gabelung der Geruchsspur. Insectes Soc. 1969;16:55–60. doi: 10.1007/BF02224462. [DOI] [Google Scholar]
- 8.Robinson EJH, Green KE, Jenner EA, Holcombe M, Ratnieks FLW. Decay rates of attractive and repellent pheromones in an ant foraging trail network. Insectes Soc. 2008;55:246–51. doi: 10.1007/s00040-008-0994-5. [DOI] [Google Scholar]
- 9.Robinson EJH, Jackson DE, Holcombe M, Ratnieks FLW. Insect communication: `No entry’ signal in ant foraging. Nature. 2005;438:442. doi: 10.1038/438442a. [DOI] [PubMed] [Google Scholar]
- 10.Beckers R, Deneubourg JL, Goss S. Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J Insect Behav. 1993;6:751–9. doi: 10.1007/BF01201674. [DOI] [Google Scholar]
- 11.Mailleux AC, Detrain C, Deneubourg JL. Starvation drives a threshold triggering communication. J Exp Biol. 2006;209:4224–9. doi: 10.1242/jeb.02461. [DOI] [PubMed] [Google Scholar]
- 12.Beckers R, Deneubourg J, Goss S. Trail laying behaviour during food recruitment in the ant Lasius niger (L.) Insectes Soc. 1992;39:59–72. doi: 10.1007/BF01240531. [DOI] [Google Scholar]
- 13.Fourcassie V, Beugnon G. How do red wood ants orient when foraging in a three dimensional system? I. Laboratory experiments. Insectes Soc. 1988;35:92–105. doi: 10.1007/BF02224141. [DOI] [Google Scholar]
- 14.Rosengren R, Fortelius W. Ortstreue in foraging ants of the Formica rufa group — Hierarchy of orienting cues and long-term memory. Insectes Soc. 1986;33:306–37. doi: 10.1007/BF02224248. [DOI] [Google Scholar]
- 15.Grüter C, Czaczkes TJ, Ratnieks FLW. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav Ecol Sociobiol. 2010;64:141–8. [Google Scholar]
- 16.Avebury: Ants, Bees And Wasps - The International Scientific Series Vol. Xl. Kegan Paul: 1906. [Google Scholar]
- 17.Harrison JF, Fewell JH, Stiller TM, Breed MD. Effects of experience on use of orientation cues in the giant tropical ant. Anim Behav. 1989;37:869–71. doi: 10.1016/0003-3472(89)90076-6. [DOI] [Google Scholar]
- 18.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. Synergy between social and private information increases foraging efficiency in ants. Bio Letters 2011; DOI: 10.1098/rsbl.2011.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaoka R, Akino T. Ecological importance of cuticular hydrocarbons secreted from the tarsus of ants; in: Les Insectes Sociaux. Paris, Université Paris-Nord, 1994, p 222. [Google Scholar]
- 20.Lenoir A, Depickère S, Devers S, Christidès JP, Detrain C. Hydrocarbons in the ant Lasius niger. from the cuticle to the nest and home range marking. J Chem Ecol. 2009;35:913–21. doi: 10.1007/s10886-009-9669-6. [DOI] [PubMed] [Google Scholar]
- 21.Devigne C, Detrain C. How does food distance influence foraging in the ant Lasius niger: the importance of home-range marking. Insectes Soc. 2006;53:46–55. doi: 10.1007/s00040-005-0834-9. [DOI] [Google Scholar]
- 22.Devigne C, Detrain C. Collective exploration and area marking in the ant Lasius niger. Insectes Soc. 2002;49:357–62. doi: 10.1007/PL00012659. [DOI] [Google Scholar]
- 23.Devigne C, Renon A, Detrain C. Out of sight but not out of mind: modulation of recruitment according to home range marking in ants. Anim Behav. 2004;67:1023–9. doi: 10.1016/j.anbehav.2003.09.012. [DOI] [Google Scholar]
- 24.Seeley TD. Thoughts on information and integration in honey bee colonies. Apidologie (Celle) 1998;29:67–80. doi: 10.1051/apido:19980104. [DOI] [Google Scholar]
- 25.Pankiw T. Worker honey bee pheromone regulation of foraging ontogeny. Naturwissenschaften. 2004;91:178–81. doi: 10.1007/s00114-004-0506-z. [DOI] [PubMed] [Google Scholar]
- 26.Thom C, Gilley DC, Hooper J, Esch HE. The scent of the waggle dance. PLoS Biol. 2007;5:e228. doi: 10.1371/journal.pbio.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson DE, Holcombe M, Ratnieks FLW. Trail geometry gives polarity to ant foraging networks. Nature. 2004;432:907–9. doi: 10.1038/nature03105. [DOI] [PubMed] [Google Scholar]