Summary
Bacillus subtilis regulates its ability to differentiate into distinct, co-existing cell types in response to extracellular signaling molecules produced either by itself, or present in its environment. The production of molecules by B. subtilis cells, as well as their response to these signals, is not uniform across the population. There is specificity and heterogeneity both within genetically identical populations as well as at the strain- and species-levels. This review will discuss how extracellular signaling compounds influence B. subtilis multicellularity with regard to matrix-producing cannibal differentiation, germination, and swarming behavior, as well as the specificity of the quorum-sensing peptides ComX and CSF. It will also highlight how imaging mass spectrometry can aid in identifying signaling compounds and contribute to our understanding of the functional relationship between such compounds and multicellular behavior.
Introduction
Bacillus subtilis is the model Gram-positive bacterium. It is also an archetype of cellular differentiation, generating two morphologically and transcriptionally distinct cells during sporulation. In the years since the regulatory cascade controlling sporulation was first described, our understanding of B. subtilis’s capacity for generating different cell types has greatly expanded. We now appreciate that B. subtilis – along with many other bacteria – exists as distinct subpopulations of differentiated cells [1,2]. In addition to sporulating cells, there are cell types that produce flagella and are motile; cells that produce an extracellular matrix for robust biofilm formation; cells that secrete peptide toxins to cannibalize their siblings; and cells that synthesize the machinery to take up DNA for competence [2]. These different cell types manifest as a result of the expression of different sets of genes. While phenotypic cellular heterogeneity exists in many contexts, it has been best studied within multicellular communities such as biofilms [1,3,4]. Many years of work by numerous groups have gone into describing the characteristic features of these cell types as well as elucidating the regulatory pathways controlling B. subtilis’s multicellular lifestyle [2,5].
Cellular development in B. subtilis is regulated, at least in part, by heterogeneity within the signaling networks controlling it. Recent work has demonstrated how regulatory feedback loops influence the complex genetic interactions upstream of cell-type-specific genes. The differentiation into certain cell types depends on the input of multiple genetic pathways, the response time of the regulatory proteins themselves, and the inherent stochasticity of transcription [5–9]. Thus the structure of B. subtilis’s genetic networks, as well as noise from biological processes, has an important role in influencing cellular heterogeneity.
In addition to these effects, extracellular signaling molecules can also affect cellular heterogeneity and bacterial multicellularity [10–12]. [Note that we use the term “signal” with no implication of evolutionary adaptation but simply to capture the concept of chemical compounds that alter bacterial physiology.] For example, the ComX and CSF peptides (discussed further below) are canonical “quorum sensing” peptides in B. subtilis, and are well known to affect differentiation [13]. Fewer studies have examined how other types of small molecule natural products that B. subtilis might encounter in the natural world – including those produced by other organisms – alter its differentiation.
The profound understanding of how cellular differentiation is regulated in B. subtilis has positioned us to now investigate how chemical signals influence this bacterium’s physiology. This review will focus on recent results illuminating the role of compounds that trigger the transcriptional developmental cascades leading to population heterogeneity in B. subtilis (see Table 1).
Table 1.
This table summarizes the compounds discussed in this review that influence B. subtilis multicellularity. The data are meant to be representative and not comprehensive. For instance, the producing organisms listed are not the only ones capable of making these natural products, and the bioactivities are only those currently described. The references listed refer to papers discussing the effects of these compounds specifically on B. subtilis.
| Molecule | Producer(s) | Structure | Other bioactivies | Effect on B. subtilis | Ref |
|---|---|---|---|---|---|
| Nystatin | Streptomyces noursei | Macrolide polyene | Antifungal | Matrix-production | [21] |
| Amphotericin | Streptomyces nodosus | Macrolide polyene | Antifungal; Antiprotozoan |
Matrix-production | [21] |
| Gramicidin | Bacillus brevis | Peptide | Gram (+) Antibacterial | Matrix-production | [21] |
| Surfactin | B. subtilis | Lipopeptide | Antibacterial; Antiviral; Antifungal; Hemolytic |
Matrix-production; Tendril avoidance during swarming |
[21], [57] |
| Valinomycin | Streptomyces species | Peptide | Antiviral | Matrix-production | [21] |
| cis-2-decenoic acid | P. aeruginosa | Short-chain fatty acid | Biofilm diserpsal | Biofilm dispersal | [32] |
| D-amino acids |
B. subtilis; Many bacteria |
D-amino acids | Cell wall peptidoglycan; Protein modifications |
Biofilm dispersal | [33] |
| Muropeptides | Many bacteria | Peptidoglycan fragments | Germination stimulation | [37] | |
| Staurosporine | Streptomyces species | Indolocarbazole | Protein kinase inhibition | Germination inhibition | [37] |
| ComX | B. subtilis | Isoprenylated decapeptide |
Competence, sporulation, and surfactin indiuction; Strain-specific |
[30] | |
| CSF |
B. subtilis; Bacillus mojavensis |
Pentapeptide | Competence, sporulation, and surfactin induction; Species-specific |
[54] |
Matrix-producing cannibals
Compounds that increase the subpopulation of matrix-producing cannibals
One well-characterized cell-type of B. subtilis is the matrix-producing cannibal [14]. These cells produce the extracellular matrix components required for robust biofilm formation [15]. In addition, they produce cannibalism toxins along with an immunity system [16,17]. The cannibalism toxins SDP and SKF are peptides that can kill their B. subtilis siblings as well as other bacterial cells and delay entrance of B. subtilis into sporulation [18–20].
The genetic pathway leading to matrix-producing cannibals has many potential inputs, making it a good system in which to search for possible molecules that trigger differentiation [2]. A screen of purified natural products revealed numerous compounds that induced matrix gene expression in B. subtilis [21] (Table 1). The unifying feature of these compounds was their ability to trigger potassium leakage, which was sensed by a sensor histidine kinases, KinC. One of these compounds was the intraspecific signal surfactin, produced by B. subtilis itself. Others, such as gramicidin and nystatin, are produced by other bacteria (Bacillus brevis and Streptomyces noursei, respectively). Thus, B. subtilis alters its differentiation in response to signals produced by a range of phylogenetically diverse bacteria, and such compounds are not limited to small, self-produced peptides. Unpublished work by our group has confirmed the ability of other organisms to produce compounds that affect B. subtilis differentiation.
One compound, multiple bioactivities
The matrix-inducing effect of some of these compounds also clearly illustrates the potential of natural products to exert multiple bioactivities [22–24]. Nystatin has traditionally been considered an anti-fungal [25], yet it was seen to stimulate matrix gene expression in a subpopulation of B. subtilis cells [21]. Similarly, gramicidin is characterized as an antibiotic, but at sub-lethal concentrations it induces biofilm formation in B. subtilis [21]. Such multifaceted bioactivities are being accepted as the norm and not the exception [22,23,26]. This is because while some natural products act via a specific receptor to affect transcription (see the ComX story below), many others exert their physiological effects via general mechanisms. That these compounds do not bind to a co-evolved receptor does not make their ability to modulate bacterial behavior any less potent. However, it does make understanding the relevance of such cellular interactions more complicated. In order to grasp the functional role of compounds in mediating native microbial interactions we will need to probe both microbes and molecules in situ. This will require the application and combination of techniques such as advanced microscopy, fluorescence in situ hybridization, and imaging mass spectrometry (described below), along with other techniques to explore the ecophysiology of microbes [27–29].
Paracrine signaling
Surfactin is produced by B. subtilis and elicits differentiation into matrix-producing cannibals [21]. It is tempting to then assume that surfactin producers would subsequently become matrix producers, since the concentration of surfactin should be high near the cells secreting it. However, this is not the case: matrix-producers and surfactin-producers are independent cell types that stably co-exist [30]. It is unclear why only some cells respond to surfactin, or why surfactin producers are immune e to the signal they produce. These results demonstrate heterogeneity with regard to production of and response to differentiation signals within populations of genetically identical B. subtilis cells. This unidirectional paracrine signaling, where one cell type produces a signal to which another cell type responds, allows the compartmentalization of cellular differentiation and permits cell-type status to be maintained over numerous generations [30].
Compounds that decrease biofilm formation
Both intra- and interspecific signaling paradigms are present in the recent identification of biofilm-inhibiting compounds [31]. The unsaturated fatty acid cis-2-decenoic acid was purified from Pseudomonas aeruginosa as a compound that dispersed the biofilms of many bacteria, including B. subtilis [32]. The mechanism by which this compound is sensed and acts in B. subtilis is unknown. Another recently identified biofilm disassembly agent is a cocktail of D-amino acids produced by B. subtilis during late stages of biofilm growth, which causes amyloid-like protein fibers present in the extracellular matrix to detach from the cells, allowing the cells to escape from the grasp of the matrix [33]. These compounds also act on other bacteria, preventing biofilm formation in Staphylococcus aureus and P. aeruginosa [33]. These results warn us against being intellectually biased towards chemically “interesting” molecules: while chemically simple, the biological effect of these simple metabolites is intriguing and profound.
Germination
A characteristic feature of Bacillus species is their ability to sporulate and form dormant, stress resistant endospores. Equally essential to this developmental process is the ability to germinate and emerge from this quiescent state. Multiple compounds have been identified that can stimulate or inhibit germination [34]. In the laboratory, germination is stimulated by simple nutrients such as amino acids, sugars, and nucleosides, although often requiring higher levels than seem probable in natural environments [34,35]. There are also non-nutrient germinants (dodecylamine and atmospheric pressure), and inhibitors of germination (diphenylamine and certain alcohols) [34,36]. While these signals are not likely physiologically relevant, studies of their effects have provided meaningful information about the mechanism of germination, highlighting the advantage of exploring of a diverse range of developmental signaling molecules, even those not likely relevant in the natural world.
Germination signals that are bacterially produced have also been identified. In particular, muropeptides, peptidoglycan fragments from bacterial cell walls, are sensed as germination signals in B. subtilis by PrcK, a membrane Ser/Thr kinase [37]. PrcK specifically responds to muropeptides from related bacteria, and actively growing cells’ muropeptides are more potent germinants than those from lysed cells [37,38]. Thus, B. subtilis does not indiscriminately respond to muropeptides but does so preferentially to those that indicate organisms such as themselves are actively growing in the vicinity. B. subtilis also releases a muralytic enzyme, YocH, that digests peptidoglycan in the environment [38]. The resulting muropeptides are sensed by PrkC; this leads both to germination and the upregulation of YocH in a positive regulatory feedback loop [38]. Finally, muropeptide-stimulated germination can be inhibited by staurosporine, a natural product of Streptomyces species that B. subtilis may interact with in soil [37].
CSF and ComX signaling peptides
The extracellular signaling peptides ComX and CSF (Competence and Sporulation Factor, also PhrC) have been known for many years to alter B. subtilis gene expression in response to cell density [39,40]. These compounds are both processed and secreted peptides (ComX is isoprenylated), and both affect the phosphorylation state of ComA, although they do so differently [41–43]. ComA is the response regulator of the ComP/ComA two-component signal transduction system, and regulates the transcription of genes required for surfactin production and differentiation into competent cells, among other traits [44,45]. The signaling specificity of these two signaling peptides also differs, as discussed below.
ComX, an intraspecific signal
ComX was first identified almost two decades ago as a signaling pheromone isolated from culture supernatant at high cell density [45]. However, the dynamics of ComX production and the specificity of its ComA activation were not explored until recently [30]. ComX is produced by most cells in a B. subtilis population [30]. While there is strain-to-strain variation in terms of cells’ response to ComX, in the less-domesticated lab strain 3610, only a small subpopulation of cells responds to the ComX pheromone to activate the surfactin promoter [30].
If ComX is ubiquitous throughout the extracellular milieu, why do only some cells respond to it? This effect is at least partially mediated by cellular differentiation. The extracellular matrix itself prevents ComX from activating ComP; thus matrix-producing cells do not become surfactin-producing cells [30]. In this way B. subtilis enforces a chronology in its developmental program: once matrix-producers differentiate, it reduces the chance of making more surfactin producers, since those cells are now immune to the ComX signal [30,46]. This is not the only developmental timing mechanism B. subtilis employs: KinD is a checkpoint protein that delays progression to sporulation until matrix has been produced [46]. These systems appear to have evolved to allow B. subtilis to regulate the development of its cell type subpopulations in ways that are reminiscent of how metazoan multicellular organisms regulate their cellular development [47].
Pheromone signaling in natural environments
The gene encoding ComX is found in an operon that contains two other genes, comQ and comP, whose products are both involved in sensing ComX. The exquisite sensitivity of the ComQXP signaling system is not only in the heterogeneous response to ComX within genetically identical populations of B. subtilis cells, but there is also diversity between B. subtilis strains. ComQXP is a strain-specific pheromone system related to those of Staphylococcus and Streptococcus strains [48–51]. In B. subtilis, four different pherotype groups have so far been identified; cells communicate effectively within but not between groups based on their ComX and ComP sequence specificity [50,51]. This heterogeneity was originally identified using strains isolated from geographically distant areas. Stefanic and Mandic-Mulec examined how this polymorphic diversity was spatially distributed in wild isolates from soil microenvironments [52]. They found multiple pherotypes co-existing within 2.5 mm3 soil samples, suggesting that even at relatively small scales there is diversity of this signaling system in the wild [52]. Hopefully this study will prompt additional explorations into the cellular heterogeneity that exists in B. subtilis within microbial communities at even smaller scales more commensurate with natural bacterial microcolonies [53].
CSF, a species-specific signaling molecule
In contrast to ComX, CSF appears to be conserved as a species-specific signaling molecule in B. subtilis and closely related strains such as B. mojavensis [54]. Pottahil and coworkers examined the rapC-phrC operon from six different strains of B. subtilis and B. mojavensis. This operon encodes CSF (phrC) and its receptor (rapC). The sequences of these genes and the promoter region were highly conserved between strains, indicating that these CSF peptides were effectively identical; this was supported by an explicit test of surfactin transcription in response to these strains’ conditioned medium. (Because the reporter strain was a different ComX pherotype than the tested strains there was minimal risk of surfactin activation via ComX.) These data demonstrate that CSF acts as a communication signal across multiple related strains, even those that are unable to sense each other using ComX [54]. This work illustrates the lack of strain-specific variability of the CSF peptide. It remains to be seen what breadth of organisms are capable of responding to this signaling compound.
Swarming
B. subtilis is capable of swarming, a form of motility on moist surfaces that requires flagella and surfactin [55]. It seems unlikely that swarming is a distinct developmental state, yet it is clearly a multicellular behavior [55]. Although there has been confusion in the literature, the genetic underpinnings of this behavior were recently clarified [56]. On certain media, swarming B. subtilis cells form stereotypical tendrils. These tendrils avoid touching the tendrils of adjacent, genetically identical colonies, a behavior that could encourage dispersed bacterial exploration in native habitats [57]. This avoidance was dependent on surfactin [57]. Understanding how matrix- and surfactin-producing cells are distributed throughout swarming tendrils would complement existing data about where surfactin itself is localized in these features (discussed below) [58].
New methods in compound localization and identification
Producing and responding to small molecule natural products is essential to the establishment and propagation of different cells types by B. subtilis. This knowledge has emerged from our ability to monitor the transcriptional behavior of cells at the single cell and population levels. The ability to spatially visualize the distribution of the molecules themselves, meanwhile, remains challenging. Various forms of imaging mass spectrometry of microbial samples provide a mechanism to directly visualize the distribution of molecular ions in biological samples [28,59,60]. These approaches involve collecting mass spectrometry data at raster points across single cells, bacterial colonies, or interspecies interaction zones.
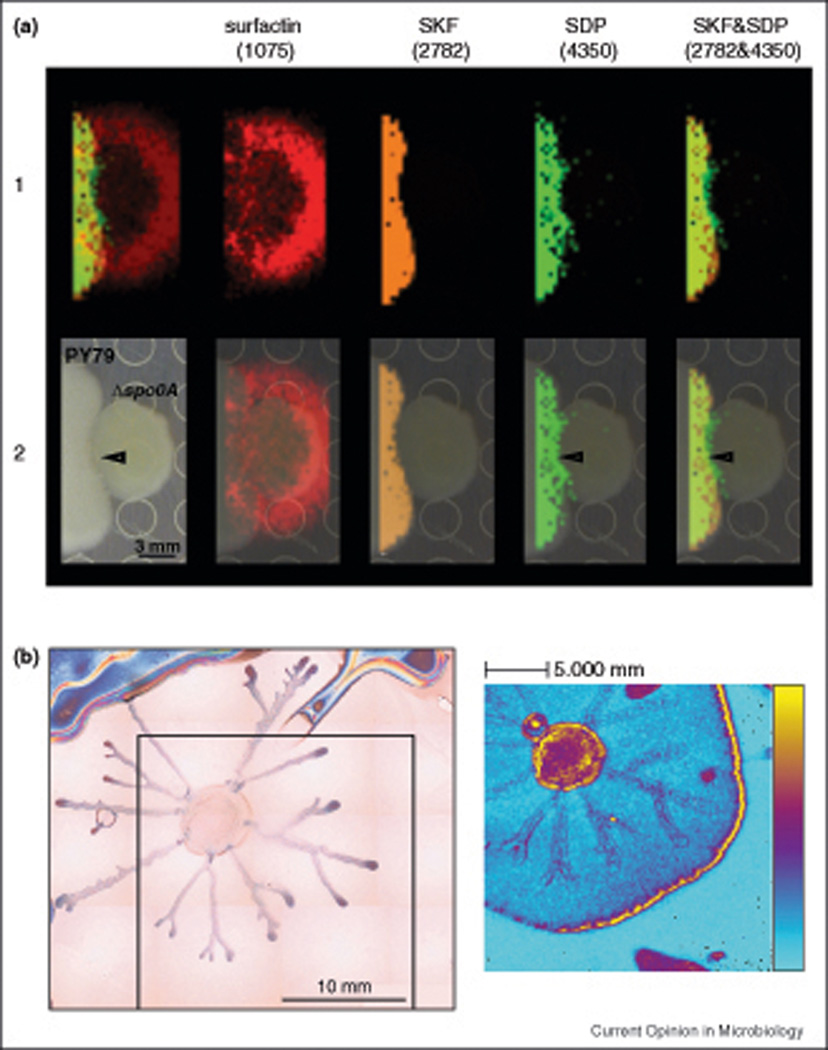
One way these techniques can be used is in the structural characterization of unknown signals mediating multicellular behavior. The cannibalism toxins of B. subtilis were known to exist for more than a decade, but the molecules themselves remained uncharacterized [16]. Based on their ability to preferentially kill particular cell types, imaging mass spectrometry was used to localize candidate compounds and led to the elucidation of their structure [19] (Figure 1).
Figure 1. Imaging mass spectrometry applied to B. subtilis.
Imaging mass spectrometry involves collecting mass spectra in a grid pattern across a surface. This allows the spatial distribution of metabolites to be visualized by displaying the relative abundance of individual ion masses in false-color across the corresponding optical image. A) Thin-layer agar MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) imaging mass spectrometry data from the interaction between two B. subtilis colonies: on the left a wild type strain (PY79), and on the right a spo0A mutant, which is unable to produce the cannibalism toxins or immunity to them. Row 1 shows the ion distributions of surfactin, SKF and SDP; their masses are shown in parentheses at the top. Row 2 is the superimposition of the imaging mass spectrometry data with the colony image. This interaction led to killing of the spo0A mutant at the interface of these colonies (arrowhead in row 2), where the cannibalism factors were hypothesized to act. By examining the molecular ions produced only by PY79 that were distributed across this region, the cannibalism factors (SKF and SDP) were identified. Reprinted with permission from Reference 19: PNAS, 2010, 107(37):16286–16290. B) Swarming B. subtilis colonies were transferred onto silicon wafers and submitted to TOF-SIMS (time-of-flight secondary ion mass spectrometry) imaging to visualize the distribution of surfactin structural variants across the surface. On the left is the microscope image of the swarming colony. On the right is the TOF-SIMS image of the sum of surfactin ions across the imaged area. The color scale corresponds to zero (blue) to the maximal counts of surfactin per pixel (yellow). Different distributions of surfactin acyl chains were observed in different areas of the swarming coony. Reprinted with permission from Reference 58: Proteomics, 2008, 8:3682–3691.
Not surprisingly, different chemical micro-environments have been detected within macroorganisms such as cyanobacteria and sponges as well as within bacterial colonies and swarming tendrils of B. subtilis [58,59,61]. In the case of the latter, mass spectrometry was used to examine the distribution of surfactin within swarming tendrils [58] (Figure 1). Surfactin contains a fatty acyl tail that can vary in length; these structural variants were distributed in a specific manner across the swarming tendril [24,58]. The functional relevance of these structural variants of surfactin are unknown, as is their potential relationship to multicellular behavior.
Such molecular distributions are particularly interesting in light of the known spatiotemporal features of B. subtilis multicellular biofilm communities [62,63]. Combining an understanding of heterogeneous cellular distributions with mass spectrometry data about which compounds are present will allow us to correlate particular cell types with the signaling cues that they are producing and to which they are responding. These sorts of comprehensive examinations may allow us to generate hypotheses about how heterogeneity is generated and what its functional role may be in natural environments.
Conclusions
Taken together, the results summarized here demonstrate how B. subtilis regulates its population heterogeneity in response to extracellular signaling compounds. By looking closely at the effects of self-produced intra-specific signaling molecules on individual cells, we now know that signal production and response are not uniform across cellular populations. B. subtilis possesses different signaling compounds that exhibit specificity that operates at the sibling-, strain-, and species-level.
The ability of B. subtilis to differentiate into various cell types is highly conserved, suggesting this capacity confers a fitness advantage. It is unknown, however, whether cell-to-cell signaling occurs in natural environments, and if so, what the functional consequences of cellular differentiation might be there. Investigations into the in situ functions of these bacterial phenotypes are distinctly challenging. Nevertheless, we must continue to expand our understanding of how interspecies signaling molecules affect B. subtilis multicellularity. Because many compounds exhibit multiple bioactivities, it will be essential to determine not just which chemically mediated outcomes are possible, but which are relevant. Understanding the interplay between signaling molecules and multicellularity will require the simultaneous examination of the spatiotemporal distributions of both differentiated cell types and the molecules inducing their development. Much remains to be learned about the relationship between extracellular signaling molecules and bacterial multicellularity, not only in B. subtilis, but in bacteria in general.
Acknowledgements
The authors thank Hera Vlamakis for her critical reading of the manuscript. Work carried out in our laboratory on this subject is funded by a grant from the NIH (GM58213 to RK).
Contributor Information
Elizabeth Anne Shank, Email: Elizabeth_shank@hms.harvard.edu.
Roberto Kolter, Email: rkolter@hms.harvard.edu.
References
- 1.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 2.Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev. 2009;33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar C, Vlamakis H, Losick R, Kolter R. Thinking about Bacillus subtilis as a multicellular organism. Curr Opin Microbiol. 2007;10:638–643. doi: 10.1016/j.mib.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 6.Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 7.Veening JW, Igoshin OA, Eijlander RT, Nijland R, Hamoen LW, Kuipers OP. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol Syst Biol. 2008;4:184. doi: 10.1038/msb.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong IG, Veening JW, Kuipers OP. Heterochronic phosphorelay gene expression as a source of heterogeneity in Bacillus subtilis spore formation. Journal of Bacteriology. 2010;192:2053–2067. doi: 10.1128/JB.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chastanet A, Vitkup D, Yuan GC, Norman TM, Liu JS, Losick RM. Broadly heterogeneous activation of the master regulator for sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 2010;107:8486–8491. doi: 10.1073/pnas.1002499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straight PD, Kolter R. Interspecies chemical communication in bacterial development. Annu Rev Microbiol. 2009;63:99–118. doi: 10.1146/annurev.micro.091208.073248. [DOI] [PubMed] [Google Scholar]
- 12.Lopez D, Kolter R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev. 2010;34:134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 13.Pottathil M, Lazazzera BA. The extracellular Phr peptide-Rap phosphatase signaling circuit of Bacillus subtilis. Front Biosci. 2003;8:32–45. doi: 10.2741/913. [DOI] [PubMed] [Google Scholar]
- 14. Lopez D, Vlamakis H, Losick R, Kolter R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol Microbiol. 2009;74:609–618. doi: 10.1111/j.1365-2958.2009.06882.x. ● This paper provides evidence that two B. subtilis cell types (matrix-producers and cannibals) are overlapping subpopulations. This physiological overlap means that cannibalism promotes the maintenance of matrix-production in the population. This work demonstrates the inter-dependence that likely exists between many bacterial cell subpopulations.
- 15.Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Pastor JE, Hobbs EC, Losick R. Cannibalism by sporulating bacteria. Science. 2003;301:510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- 17.Ellermeier CD, Hobbs EC, Gonzalez-Pastor JE, Losick R. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell. 2006;124:549–559. doi: 10.1016/j.cell.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Nandy S, Bapat P, Venkatesh K. Sporulating bacteria prefers predation to cannibalism in mixed cultures. FEBS Lett. 2007;581:151–156. doi: 10.1016/j.febslet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 19. Liu WT, Yang YL, Xu Y, Lamsa A, Haste NM, Yang JY, Ng J, Gonzalez D, Ellermeier CD, Straight PD, et al. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc Natl Acad Sci U S A. 2010;107:16286–16290. doi: 10.1073/pnas.1008368107. ● This study used imaging mass spectrometry to identify the SKF and SDP cannibalism peptides of B. subtilis which led to their structural elucidation. This work demonstrates how imaging mass spectrometry can be used not only to spatially localize molecules within bacterial colonies but also lead to the identification of new compounds
- 20.González-Pastor JE. Cannibalism: a social behavior in sporulating Bacillus subtilis. FEMS Microbiol Rev. 2011;35:415–424. doi: 10.1111/j.1574-6976.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 21. Lopez D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A. 2009;106:280–285. doi: 10.1073/pnas.0810940106. ● In this paper, numerous functionally related molecules, all of which cause potassium leakage in the cell, are shown to lead to the development of matrix-producing cannibals. One of these compounds, surfactin, is produced by B. subtilis itself as a quorum-sensing-like molecule, while others are produced by diverse bacterial species. Thus B. subtilis develops multicellularity in response to multiple microbially produced signals.
- 22.Yim G, Wang HH, Davies J. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fajardo A, Martinez JL. Antibiotics as signals that trigger specific bacterial responses. Curr Opin Microbiol. 2008;11:161–167. doi: 10.1016/j.mib.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev. 2010;34:1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 25.Waksman SA, Lechevalier HA, Schaffner CP. Candicidin and other polyenic antifungal antibiotics. Bull World Health Organ. 1965;33:219–226. [PMC free article] [PubMed] [Google Scholar]
- 26.Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci U S A. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neu TR, Manz B, Volke F, Dynes JJ, Hitchcock AP, Lawrence JR. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol. 2010;72:1–21. doi: 10.1111/j.1574-6941.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 28.Watrous JD, Dorrestein PC. Imaging mass spectrometry in microbiology. Nat Rev Microbiol. 2011;9:683–694. doi: 10.1038/nrmicro2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner M, Nielsen PH, Loy A, Nielsen JL, Daims H. Linking microbial community structure with function: fluorescence in situ hybridization-microautoradiography and isotope arrays. Curr Opin Biotechnol. 2006;17:83–91. doi: 10.1016/j.copbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 30. Lopez D, Vlamakis H, Losick R, Kolter R. Paracrine signaling in a bacterium. Genes Dev. 2009;23:1631–1638. doi: 10.1101/gad.1813709. ●● This paper demonstrates that unidirectional paracrine signaling occurs within genetically identical bacterial populations in the case of both ComX- and surfactin-signaling. Such heterogeneity between the cells producing and responding to extracellular signals allows different cell types to be stably maintained over time. This cellular compartmentalization is potentially a common feature of all bacterial multicellularity.
- 31.Abee T, Kovacs AT, Kuipers OP, van der Veen S. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011;22:172–179. doi: 10.1016/j.copbio.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Davies DG, Marques CN. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2008 doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. ●● This paper describes the role of D-amino acids in triggering and preventing biofilm formation in B. subtilis by causing the extracellular matrix to be released from the cell wall. D-amino acids also prevent biofilm formation in Staphylococcus aureus and Pseudomonas aeruginosa suggesting that they may be a general mechanism of biofilm disassembly. These results highlight the fact that biologically important molecules are not necessarily structurally complex.
- 34.Setlow P. Spore germination. Curr Opin Microbiol. 2003;6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Dworkin J, Shah IM. Exit from dormancy in microbial organisms. Nat Rev Microbiol. 2010;8:890–896. doi: 10.1038/nrmicro2453. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda-Yasaki Y, Namiki-Kanie S, Hachisuka Y. Inhibition of Bacillus subtilis spore germination by various hydrophobic compounds: demonstration of hydrophobic character of the L-alanine receptor site. J Bacteriol. 1978;136:484–490. doi: 10.1128/jb.136.2.484-490.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah IM, Laaberki MH, Popham DL, Dworkin J. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell. 2008;135:486–496. doi: 10.1016/j.cell.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah IM, Dworkin J. Induction and regulation of a secreted peptidoglycan hydrolase by a membrane Ser/Thr kinase that detects muropeptides. Mol Microbiol. 2010;75:1232–1243. doi: 10.1111/j.1365-2958.2010.07046.x. [DOI] [PubMed] [Google Scholar]
- 39.Grossman AD. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 40.Lazazzera BA, Grossman AD. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 41.Solomon JM, Magnuson R, Srivastava A, Grossman AD. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 42.Solomon JM, Lazazzera BA, Grossman AD. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 43.Dubnau D, Turgay K. The regulation of competence in Bacillus subtilis and its relation to stress response. In: Storz G, Hengge-Aronis R, editors. Bacterial Stress Responses. Washington, DC: American Society for Microbiology; 2000. pp. 249–260. [Google Scholar]
- 44.Nakano MM, Xia LA, Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991;173:5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 46.Aguilar C, Vlamakis H, Guzman A, Losick R, Kolter R. KinD Is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. MBio. 2010;1 doi: 10.1128/mBio.00035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakoby M, Schnittger A. Cell cycle and differentiation. Curr Opin Plant Biol. 2004;7:661–669. doi: 10.1016/j.pbi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 49.Tortosa P, Dubnau D. Competence for transformation: a matter of taste. Curr Opin Microbiol. 1999;2:588–592. doi: 10.1016/s1369-5274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 50.Tran LS, Nagai T, Itoh Y. Divergent structure of the ComQXPA quorum-sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol Microbiol. 2000;37:1159–1171. doi: 10.1046/j.1365-2958.2000.02069.x. [DOI] [PubMed] [Google Scholar]
- 51.Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol Microbiol. 2002;44:1561–1573. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 52. Stefanic P, Mandic-Mulec I. Social interactions and distribution of Bacillus subtilis pherotypes at microscale. J Bacteriol. 2009;191:1756–1764. doi: 10.1128/JB.01290-08. ● This paper looks at how different pherotypes of the ComX signal transduction pathway are spatially distributed among wild isolates of B. subtilis at the centimeter to millimeter scale, establishing that functional diversity was present in the soil. This is one of the few studies to examine the spatial distribution of bacterial signaling systems in situ
- 53.Grundmann GL. Spatial scales of soil bacterial diversity--the size of a clone. FEMS Microbiol Ecol. 2004;48:119–127. doi: 10.1016/j.femsec.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Pottathil M, Jung A, Lazazzera BA. CSF, a species-specific extracellular signaling peptide for communication among strains of Bacillus subtilis and Bacillus mojavensis. J Bacteriol. 2008;190:4095–4099. doi: 10.1128/JB.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patrick JE, Kearns DB. Laboratory strains of Bacillus subtilis do not exhibit swarming motility. J Bacteriol. 2009;191:7129–7133. doi: 10.1128/JB.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James BL, Kret J, Patrick JE, Kearns DB, Fall R. Growing Bacillus subtilis tendrils sense and avoid each other. FEMS Microbiol Lett. 2009;298:12–19. doi: 10.1111/j.1574-6968.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- 58.Debois D, Hamze K, Guerineau V, Le Caer JP, Holland IB, Lopes P, Ouazzani J, Seror SJ, Brunelle A, Laprevote O. In situ localisation and quantification of surfactins in a Bacillus subtilis swarming community by imaging mass spectrometry. Proteomics. 2008;8:3682–3691. doi: 10.1002/pmic.200701025. [DOI] [PubMed] [Google Scholar]
- 59.Yang YL, Xu Y, Straight P, Dorrestein PC. Translating metabolic exchange with imaging mass spectrometry. Nat Chem Biol. 2009;5:885–887. doi: 10.1038/nchembio.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roach PJ, Laskin J, Laskin A. Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 2010;135:2233–2236. doi: 10.1039/c0an00312c. [DOI] [PubMed] [Google Scholar]
- 61.Esquenazi E, Coates C, Simmons L, Gonzalez D, Gerwick WH, Dorrestein PC. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol Biosyst. 2008;4:562–570. doi: 10.1039/b720018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vlamakis H, Aguilar C, Losick R, Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLoon AL, Kolodkin-Gal I, Rubinstein SM, Kolter R, Losick R. Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis. J Bacteriol. 2010 doi: 10.1128/JB.01186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]