Abstract
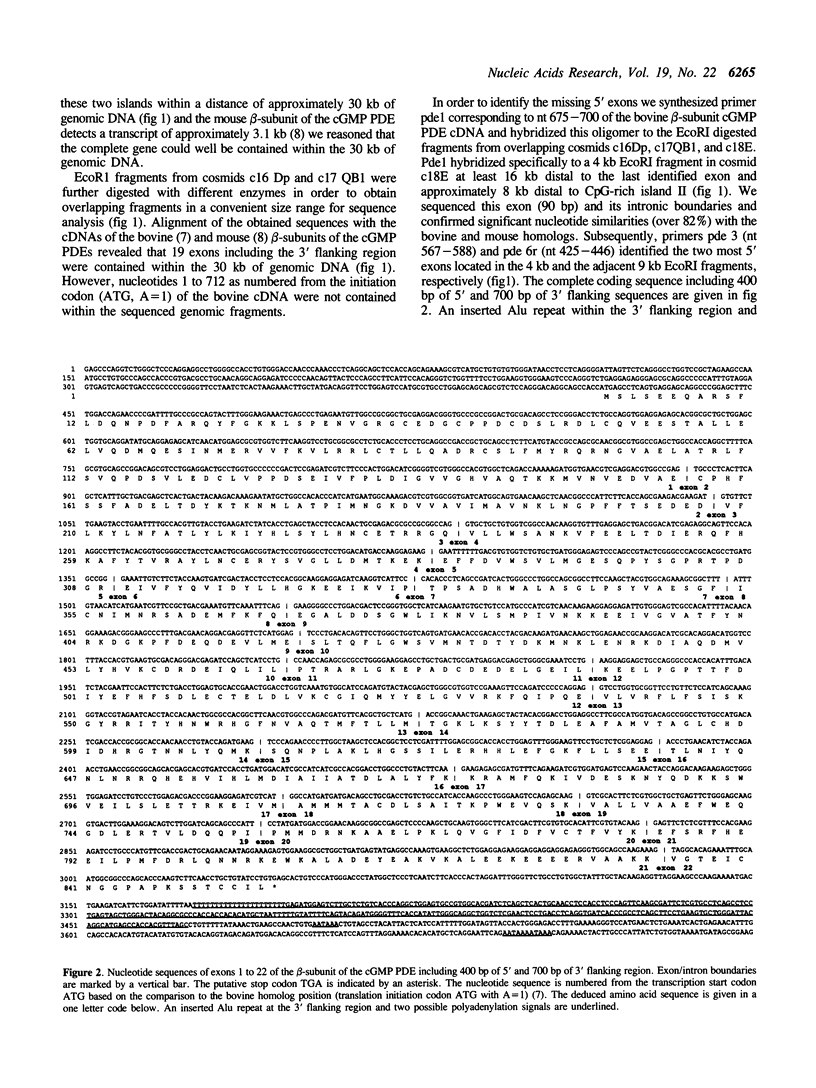
As part of the search for the Huntington disease (HD) gene we have cloned and sequenced 34 kb of genomic DNA containing the full-length gene for the beta-subunit of the human cGMP phosphodiesterase (beta-cGMP PDE). This gene is localized to 4p16.3 about 700 kb proximal to the 4p telomere and represents the most telomeric gene characterized on 4p to date. We show that this gene is comprised of 22 exons spanning approximately 43 kb of genomic DNA. We also provide 400 bp immediately 5' to the putative initiator methionine and 700 bp of 3' flanking sequences. Northern blot analysis of several human tissues revealed a highly abundant 3.5 kb transcript and a minor signal of 4.5 kb in retinal tissue. Alignment of the deduced amino acid sequence to the previously identified beta-subunits of the cGMP PDEs of mouse and cow demonstrates highly significant similarities and, therefore, confirms the identity of the cloned gene. A defect in the beta-subunit of the cGMP PDE gene has been shown recently to be the cause for the retinal degeneration in the rd mouse. The cloning of the human homolog and the knowledge of its genomic organization with exon/intron boundaries will allow rapid assessment of the role of this gene in the causation of human retinopathies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bates G. P., MacDonald M. E., Baxendale S., Sedlacek Z., Youngman S., Romano D., Whaley W. L., Allitto B. A., Poustka A., Gusella J. F. A yeast artificial chromosome telomere clone spanning a possible location of the Huntington disease gene. Am J Hum Genet. 1990 Apr;46(4):762–775. [PMC free article] [PubMed] [Google Scholar]
- Boughman J. A., Conneally P. M., Nance W. E. Population genetic studies of retinitis pigmentosa. Am J Hum Genet. 1980 Mar;32(2):223–235. [PMC free article] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Bunker C. H., Berson E. L., Bromley W. C., Hayes R. P., Roderick T. H. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984 Mar;97(3):357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- Bućan M., Zimmer M., Whaley W. L., Poustka A., Youngman S., Allitto B. A., Ormondroyd E., Smith B., Pohl T. M., MacDonald M. Physical maps of 4p16.3, the area expected to contain the Huntington disease mutation. Genomics. 1990 Jan;6(1):1–15. doi: 10.1016/0888-7543(90)90442-w. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Beier N., Walsh K. A., Beavo J. A. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Prusti R. K., LeTrong H., Sonnenburg W. K., Mullaney P. J., Walsh K. A., Beavo J. A. Identification of a noncatalytic cGMP-binding domain conserved in both the cGMP-stimulated and photoreceptor cyclic nucleotide phosphodiesterases. Proc Natl Acad Sci U S A. 1990 Jan;87(1):288–292. doi: 10.1073/pnas.87.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. V., Martin G. R., Nadeau J. H., Haines J. L., Bucan M., Kozak C. A., MacDonald M. E., Lockyer J. L., Ledley F. D., Woo S. L. Synteny on mouse chromosome 5 of homologs for human DNA loci linked to the Huntington disease gene. Genomics. 1989 Apr;4(3):419–426. doi: 10.1016/0888-7543(89)90349-2. [DOI] [PubMed] [Google Scholar]
- Cremers F. P., van de Pol D. J., van Kerkhoff L. P., Wieringa B., Ropers H. H. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990 Oct 18;347(6294):674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- Danciger M., Bowes C., Kozak C. A., LaVail M. M., Farber D. B. Fine mapping of a putative rd cDNA and its co-segregation with rd expression. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1427–1432. [PubMed] [Google Scholar]
- Danciger M., Tuteja N., Kozak C. A., Farber D. B. The gene for the gamma-subunit of retinal cGMP-phosphodiesterase is on mouse chromosome 11. Exp Eye Res. 1989 Feb;48(2):303–308. doi: 10.1016/s0014-4835(89)80079-x. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett N. A., Cheng J. F., Smith C. L., Cantor C. R. The Huntington disease locus is most likely within 325 kilobases of the chromosome 4p telomere. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10011–10014. doi: 10.1073/pnas.86.24.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Hahn L. B., Cowley G. S., Olsson J. E., Reichel E., Sandberg M. A., Berson E. L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990 Nov 8;323(19):1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res. 1976;2(3):139–148. [PubMed] [Google Scholar]
- Li T. S., Volpp K., Applebury M. L. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1990 Jan;87(1):293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin V. M., Khramtsov N. V., Vasilevskaya I. A., Atabekova N. V., Muradov K. G., Gubanov V. V., Li T., Johnston J. P., Volpp K. J., Applebury M. L. Beta-subunit of bovine rod photoreceptor cGMP phosphodiesterase. Comparison with the phosphodiesterase family. J Biol Chem. 1990 Aug 5;265(22):12955–12959. [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Looney J., Steel G., Suchanek M., Dowling C., Der Kaloustian V., Kaiser-Kupfer M., Valle D. An initiator codon mutation in ornithine-delta-aminotransferase causing gyrate atrophy of the choroid and retina. J Clin Invest. 1988 Feb;81(2):630–633. doi: 10.1172/JCI113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOELL W. K. Differentiation, metabolic organization, and viability of the visual cell. AMA Arch Ophthalmol. 1958 Oct;60(4 Pt 2):702–733. doi: 10.1001/archopht.1958.00940080722016. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Kumarev V. P., Gubanov V. V., Khramtsov N. V., Akhmedov N. B., Zagranichny V. E., Muradov K. G. Cyclic GMP phosphodiesterase from cattle retina. Amino acid sequence of the gamma-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1986 Aug 18;204(2):288–292. doi: 10.1016/0014-5793(86)80830-4. [DOI] [PubMed] [Google Scholar]
- Penotti F. E. Human pre-mRNA splicing signals. J Theor Biol. 1991 Jun 7;150(3):385–420. doi: 10.1016/s0022-5193(05)80436-9. [DOI] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. The molecular genetics of retinal photoreceptor proteins involved in cGMP metabolism. Prog Clin Biol Res. 1991;362:33–66. [PubMed] [Google Scholar]
- Pittler S. J., Baehr W., Wasmuth J. J., McConnell D. G., Champagne M. S., vanTuinen P., Ledbetter D., Davis R. L. Molecular characterization of human and bovine rod photoreceptor cGMP phosphodiesterase alpha-subunit and chromosomal localization of the human gene. Genomics. 1990 Feb;6(2):272–283. doi: 10.1016/0888-7543(90)90567-e. [DOI] [PubMed] [Google Scholar]
- Pritchard C. A., Casher D., Uglum E., Cox D. R., Myers R. M. Isolation and field-inversion gel electrophoresis analysis of DNA markers located close to the Huntington disease gene. Genomics. 1989 Apr;4(3):408–418. doi: 10.1016/0888-7543(89)90348-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Stroop S. D., Charbonneau H., Beavo J. A. Direct photolabeling of the cGMP-stimulated cyclic nucleotide phosphodiesterase. J Biol Chem. 1989 Aug 15;264(23):13718–13725. [PubMed] [Google Scholar]
- Tuteja N., Farber D. B. Gamma-subunit of mouse retinal cyclic-GMP phosphodiesterase: cDNA and corresponding amino acid sequence. FEBS Lett. 1988 May 9;232(1):182–186. doi: 10.1016/0014-5793(88)80413-7. [DOI] [PubMed] [Google Scholar]




