Abstract
We report, for the first time, serologic evidence of Rickettsia felis and R. aeschlimannii infections acquired in Tunisia from 1998 to 2003. We found that most patients with antibodies against both R. conorii and R. typhi had serologic evidence of R. felis infection.
Keywords: Rickettsia felis, serology, Tunisia, dispatch
Rickettsioses are arthropod-borne zoonoses with geographic distributions determined by the ecology of their vectors. The genus Rickettsia is divided into 2 groups, the spotted fever group (SFG) and the typhus group (TG), mainly based on their intracellular positions, optimal growth temperatures, gas chromatographic proportion (%), DNA content, clinical features, epidemiologic aspects, and antigenic characteristics. Recently, a new species of Rickettsia that infected humans, R. felis, was reported (1), and the whole genome of this species has recently been sequenced (2). The pathogenic role of R. felis in humans was demonstrated first in Texas (1), with subsequent reports of this "fleaborne spotted fever" confirmed in patients from Europe and South/Central America (3–5) by polymerase chain reaction (PCR), serologic tests, or both. In Tunisia, North Africa, the epidemiology of rickettsial diseases has not been documented and only 1 study concerning these diseases has been conducted. The study, conducted in 1995, confirmed that R. conorii and TG rickettsia were endemic in Tunisia, with estimated antibody prevalences of 9% and 3.6%, respectively (6). The aim of our study was to investigate the diversity of rickettsioses in Tunisia through the use of serologic assays.
The Study
Serum samples obtained from patients suspected to have clinical rickettsial infection (fever associated with an eschar or cutaneous rash) were collected from 1998 to 2003 at the Laboratory of Microbiology CHU Habib Bourguiba, Sfax, Tunisia. Acute- and convalescent-phase serum samples, when available, were stored at –80°C until they were tested in a multiple-antigen immunofluorescence assay (IFA) at the Unité des Rickettsies Marseille (7). Ten SFG rickettsial antigens were used: R. conorii conorii strain 7, R. africae strain ESF-5, R. sibirica mongolitimonae strain HA-91T, Rickettsia aeschlimannii strain MC16T, R. massiliae strain Mtu1T, R. helvetica strain C9P9, R. slovaca strain 13-B, R. conorii israelensis strain ISTTCDC1, R. felis strain URRWXCal2 ATTC VR-1525, and R. typhi strain Wilmington. Antigens were produced at the Unité des Rickettsies Marseille as previously reported (8). Immunoglobulin G (IgG) antibody titers of 1:128, seroconversion in paired serum specimens, or IgM antibody titers of 1:32 against any species were considered evidence of recent Rickettsia infection (7).
Identification at the species level was determined by Western blot (WB) and cross-adsorption assays in accordance with procedures described elsewhere (7,8). R. conorii, R. aeschlimannii, R. felis, and R. typhi isolates were suspended briefly in sterile distilled water and adjusted to 2 mg protein/mL. Twenty microliters of the preparation was electrophoresed at 100 V for 2 h through a separating gel containing 12% polyacrylamide by means of a Mini-Protean II cell apparatus (Bio-Rad, Marnes la Coquette, France). A mixture of prestained molecular mass standards (Kaleidoscope; Bio-Rad) was used to estimate the molecular masses of the separated antigens. Resolved antigens were transferred onto a 0.45-μm pore nitrocellulose membrane (Bio-Rad) that was electrophoresed for 1 h at 4°C and 100 V. The blots were blocked overnight at 4°C with 5% nonfat milk powder in Tris-buffered saline (TBS) and were washed with distilled water. Serum specimens (diluted at a ratio of 1:200 in TBS with 3% nonfat milk powder) were applied to the blots for 1 h at room temperature. After three 10-min washes in TBS with 3% nonfat milk powder, the blots were incubated for 1 h with peroxidase-conjugated goat antihuman IgG (Southern Biotechnology Associates, Birmingham, AL, USA) diluted at a ratio of 1:750 in TBS with 3% nonfat milk powder. The blots were washed 3 times in TBS, and bound conjugate was shown by incubation in a solution of 0.015% 4-chloro-1-naphthol (Sigma, Lyon, France) and 0.015% hydrogen peroxide in TBS with 16.7% methanol for 15 min. WB analysis was done both before and after cross-adsorption in accordance with procedures described elsewhere (8). For patients with serologic evidence of R. felis infection, epidemiologic and clinical data were collected from medical records.
From 1998 to 2003, 753 serum samples were collected from 638 patients in Tunisia and sent to Marseille for analysis. Paired serum specimens were available for 115 patients. Serologic evidence of recent Rickettsia infection was found in 86 patients. Serum samples from 63 of these patients exhibited wide cross-reactive antibodies between SFG rickettsia and either R. felis (45 cases) or R. typhi (18 cases) (Figure); 19 serum samples had cross-reactive antibodies between R. felis and R. typhi. Finally, 3 samples had cross-reactive antibodies for SFG only (except R. felis), and 1 serum sample was positive for R. typhi only. WB with cross-adsorption analysis was performed for 21 serum samples, yielding species-level identification for 19 cases as follows: 3 R. conorii, 2 R. aeschlimannii, 6 R. typhi, and 8 R. felis.
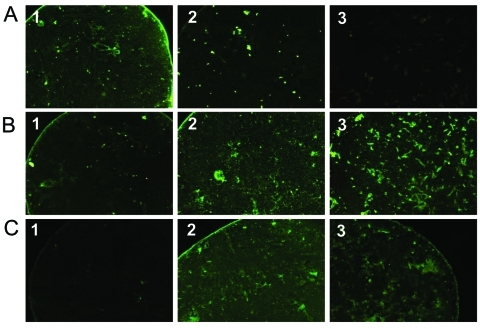
Figure.
Pictures of immunofluorescence assay performed on serum specimens with proven Rickettsia conorii (A), R. felis (B), or R. typhi (C) infection showing cross-reactive antibodies. Antigens tested were R. conorii (column 1), R. felis (column 2), and R. typhi (column 3). The serum with R. conorii infection reacts with R. conorii and R. felis antigens but not with R. typhi (A). Conversely, the serum with R. typhi infection reacts with R. typhi and R. felis but not with R. conorii (C). Finally, the serum with R. felis infection reacts with R. felis, R. conorii, and R. typhi.
Clinical data were available for only 1 patient with R. aeschlimannii infection. This patient had fever with meningitis without inoculation eschar or cutaneous rash. Serologic and clinical characteristics were available for 8 patients with R. felis infection (Table); most of the patients lived in urban areas, and 1 came from Libya. All 8 patients had fever and a maculopapular rash, and none reported a history of flea or tick bite or had an eschar. Two patients had peripheral adenopathy on admission: cervical and inguinal in the first patient and axillary and inguinal in the second patient.
Table. Epidemiologic, clinical, and serologic data for patients with fleaborne spotted fever*.
| No. | Serologic titers (IgG/IgM)* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rickettsia conorii | R. felis | R. typhi | Group | Locality | Animal contact | Fever | Eschar | Cutaneous rash | Other signs | |
| 2,120 | 1:128/1:64 | 1:128/1:16 | 0/0 | 2 | Sfax | – | 40° | No | MP | No |
| 2,274 | 1:256/1:256 | 1:512/1:512 | 0/0 | 2 | – | – | – | – | – | – |
| 2,275 | 1:256/1:256 | 1:512/1:512 | 0/0 | 2 | Moknine | Sheep/cows | 40° | No | No | No |
| 2,147 | 1:128/1:128 | 1:1,024/1:1,024 | 1:128/1,024 | 3 | Sousse | Dogs/birds | 40° | No | MP | Interstitial pneumopathy |
| 2,245 | 1:256/1:64 | 1:1,024/1:512 | 1:128/1:512 | 3 | Sfax | Cats/sheep | 40° | No | MP | adenopathy |
| 2,608 | 1:512/1:64 | 1:1,024/1:256 | 1:1,024/0 | 3 | Sfax | No | Yes | No | MP | adenopathy |
| 2,547 | 1:32/1:32 | 1:512/1:256 | 1:256/1:128 | 3 | Libya | No | Yes | No | MP | |
| 2,421 | 1:128/1:32 | 1:512/1:128 | 1:256/0 | 3 | Sfax | No | Yes | No | MP | |
*Ig, immunoglobulin; MP, maculopapular.
Conclusions
In this study, IFA and WB identified R. felis infection in patients from Tunisia. Serologic cross-reactions are common among Rickettsia species in both the SFG and TG. A difference in specific IgG or IgM antibody titers has been useful for distinguishing murine typhus from epidemic typhus (8). More sophisticated serologic methods are needed to identify the causative agent at the species level (9). WB performed on 7 of 18 serum samples with cross-reactions between SFG, R. felis, and R. typhi confirmed the diagnosis of R. felis in 5 patients and TG rickettsia in 1 patient; the diagnosis remained undetermined in 1 patient. Recently, the whole genome of R. felis has been sequenced and demonstrated genetic similarity with R. typhi but showed some genes missing from the R. conorii genome (2). Thus, R. felis may be the major cause of cross-reactions between R. typhi and R. conorii or other tickborne spotted fever agents. Cross-reactions between the 2 groups of Rickettsia have been puzzling because this activity is not reported in experimentally infected guinea pigs and mice (10). In fact, we speculate that many of the reactions with both R. typhi and R. conorii are caused by R. felis infection. This hypothesis is supported by our findings of cross-reactivity in serum specimens from 5 of 7 patients with confirmed R. felis infection. Indeed, when antigens are not available, this cross-reactivity should be a good screening method for R. felis infection. Alternatively, all serum specimens exhibiting cross-reactivity between R. typhi and R. felis only were considered to be TG rickettsia infection after WB.
To the best of our knowledge, this is the first report of patients with R. felis and R. aeschlimannii infections in Tunisia. In Morocco, similar results have been reported (11). Several cases of SFG rickettsioses have been reported from North Africa, including 1 patient with R. aeschlimannii infection from Morocco (12) and 1 patient with R. sibirica mongolitimonae infection from Algeria (13). These results are not surprising since vectors of R. felis and R. aeschlimannii are present in North Africa (14). Indeed, R. aeschlimannii has been isolated from Hyalomma marginatus ticks collected from camels in Morocco (15), and R. felis–infected fleas in Algeria have been recently reported (14). Since R. felis has a worldwide distribution and infestation with these fleas is very common, R. felis and fleaborne spotted fever may occur worldwide.
Only a few human cases of R. felis infection diagnosed by serologic tests or PCR have been reported: 1 case from the United States (Texas) (1), 3 from Mexico (3), 2 from France, 2 from Brazil (4), and 2 from Germany (5). In the Texas case (1), the patient had clinical features similar to those associated with murine typhus. However, patients with R. felis and central nervous system and pulmonary involvement have been reported from Mexico (3). In our study, 1 of the patients with R. felis infection had pulmonary involvement and 2 had adenopathy. Although none had an eschar or a history of flea bite, 3 patients had contact with animals.
Our findings indicate the need for further studies to determine the distribution of R. felis and the prevalence of this agent and associated infection. These results suggest that fleaborne spotted fever, as well as other SFG rickettsioses, are common in Tunisia.
Acknowledgment
We thank Paul Newton for English corrections.
Biography
Dr Znazen is a medical doctor in the Laboratoire de Microbiologie, Sfax, Tunisia, and is currently studying at the Unité des Rickettsies, Marseilles, France. She is involved in laboratory diagnosis of infectious diseases including human rickettsioses.
Footnotes
Suggested citation for this article: Znazen A, Rolain J-M, Hammami N, Hammami A, Jemaa MB, Raoult D. Rickettsia felis infection, Tunisia. Emerg Infect Dis [serial on the Internet]. 2006 Jan [date cited]. http://dx.doi.org/10.3201/eid1201.050876
References
- 1.Schriefer ME, Sacci JB Jr, Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. 10.1371/journal.pbio.0030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavala-Velasquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–80. 10.1016/S0140-6736(00)02735-5 [DOI] [PubMed] [Google Scholar]
- 4.Raoult D, La Scola B, Enea M, Fournier PE, Roux V, Fenollar F, et al. A flea-associated Rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7:73–81. 10.3201/eid0701.010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter J, Fournier PE, Petridou J, Häussinger D, Raoult D. Rickettsia felis infection acquired in Europe and documented by polymerase chain reaction. Emerg Infect Dis. 2002;8:207–8. 10.3201/eid0802.010293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letaief AO, Yacoub S, Tissot-Dupont H, Le Cam C, Ghachem L, Letaief J, et al. Seroepidemiological survey of rickettsial infections among blood donors in central Tunisia. Trans R Soc Trop Med Hyg. 1995;89:266–8. 10.1016/0035-9203(95)90531-6 [DOI] [PubMed] [Google Scholar]
- 7.Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, Vene S, et al. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis. 2005;40:82–8. 10.1086/426440 [DOI] [PubMed] [Google Scholar]
- 8.La Scola B, Rydkina L, Ndihokubwayo JB, Vene S, Raoult D. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin Diagn Lab Immunol. 2000;7:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to the diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hechemy KE, Raoult D, Eisemann C, Han Y, Fox JA. Detection of antibodies to Rickettsia conorii with a latex agglutination test in patients with Mediterranean spotted fever. J Infect Dis. 1986;153:132–5. 10.1093/infdis/153.1.132 [DOI] [PubMed] [Google Scholar]
- 11.Meskini M, Beati L, Benslimane A, Raoult D. Seroepidemiology of rickettsial infections in Morocco. Eur J Epidemiol. 1995;11:655–60. 10.1007/BF01720299 [DOI] [PubMed] [Google Scholar]
- 12.Raoult D, Fournier PE, Abboud P, Caron F. First documented human Rickettsia aeschlimannii infection. Emerg Infect Dis. 2002;8:748–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier PE, Gouriet F, Brouqui P, Lucht F, Raoult D. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin Infect Dis. 2005;40:1435–44. 10.1086/429625 [DOI] [PubMed] [Google Scholar]
- 14.Bitam I, Parola P, De La Cruz KD, Matsumoto K, Baziz B, Rolain JM, et al. First molecular detection of Rickettsia felis in fleas from Algeria. Am J Trop Med Hyg. 2005. In press. [PubMed] [Google Scholar]
- 15.Beati L, Meskini M, Thiers B, Raoult D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int J Syst Bacteriol. 1997;47:548–54. 10.1099/00207713-47-2-548 [DOI] [PubMed] [Google Scholar]