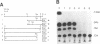Abstract
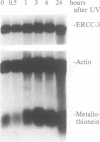
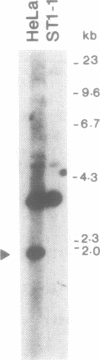
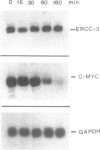
The human XPBC/ERCC-3 was cloned by virtue of its ability to correct the excision repair defect of UV-sensitive rodent mutants of complementation group 3. The gene appeared to be in addition implicated in the human, cancer prone repair disorder xeroderma pigmentosum group B, which is also associated with Cockayne's syndrome. Here we present the genomic architecture of the gene and its expression. The XPBC/ERCC-3 gene consists of at least 14 exons spread over approximately 45 kb. Notably, the donor splice site of the third exon contains a GC instead of the canonical GT dinucleotide. The promoter region, first exon and intron comprise a CpG island with several putative GC boxes. The promoter was confined to a region of 260 bp upstream of the presumed cap site and acts bidirectionally. Like the promoter of another excision repair gene, ERCC-1, it lacks classical promoter elements such as CAAT and TATA boxes, but it shares with ERCC-1 a hitherto unknown 12 nucleotide sequence element, preceding a polypyrimidine track. Despite the presence of (AU)-rich elements in the 3'-untranslated region, which are thought to be associated with short mRNA half-life actinomycin-D experiments indicate that the mRNA is very stable (t 1/2 greater than 3h). Southern blot analysis revealed the presence of XPBC/ERCC-3 cross-hybridizing fragments elsewhere in the genome, which may belong to a related gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. E., Bone N. M., Johnson R. T. Molecular cloning and characterization of a mammalian excision repair gene that partially restores UV resistance to xeroderma pigmentosum complementation group D cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6997–7001. doi: 10.1073/pnas.86.18.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Vogeli G., Yamada Y., Maizel J. V., Jr, Pastan I., de Crombrugghe B. Correlation between splicing sites within an intron and their sequence complementarity with U1 RNA. Cell. 1980 Oct;21(3):689–696. doi: 10.1016/0092-8674(80)90432-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick T. P., Schaff D. A., Bertino A. M., Dush M. K., Tischfield J. A., Stambrook P. J. Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement. Proc Natl Acad Sci U S A. 1987 May;84(10):3349–3353. doi: 10.1073/pnas.84.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D., Greiner C., Lewis K., Ford R., Adair G., Thompson L. Summary of complementation groups of UV-sensitive CHO cell mutants isolated by large-scale screening. Mutagenesis. 1989 Sep;4(5):349–354. doi: 10.1093/mutage/4.5.349. [DOI] [PubMed] [Google Scholar]
- Dery C. V., Herrmann C. H., Mathews M. B. Response of individual adenovirus promoters to the products of the E1A gene. Oncogene. 1987;2(1):15–23. [PubMed] [Google Scholar]
- Dodgson J. B., Engel J. D. The nucleotide sequence of the adult chicken alpha-globin genes. J Biol Chem. 1983 Apr 10;258(7):4623–4629. [PubMed] [Google Scholar]
- Dynan W. S., Saffer J. D., Lee W. S., Tjian R. Transcription factor Sp1 recognizes promoter sequences from the monkey genome that are simian virus 40 promoter. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4915–4919. doi: 10.1073/pnas.82.15.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Sazer S., Tjian R., Schimke R. T. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986 Jan 16;319(6050):246–248. doi: 10.1038/319246a0. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Erbil C., Niessing J. The primary structure of the duck alpha D-globin gene: an unusual 5' splice junction sequence. EMBO J. 1983;2(8):1339–1343. doi: 10.1002/j.1460-2075.1983.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Orkin S. H., Hamer D. H. Abnormal RNA splicing causes one form of alpha thalassemia. Cell. 1982 Jul;29(3):895–902. doi: 10.1016/0092-8674(82)90451-2. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Zmudzka B., Hollander M. C., Wilson S. H. Induction of beta-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol Cell Biol. 1989 Feb;9(2):851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987 Jul 20;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Kadonaga J. T., Barrera-Saldaña H., Takahashi K., Chambon P., Tjian R. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science. 1985 Nov 1;230(4725):511–517. doi: 10.1126/science.2996137. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Thomas G. H., Elgin S. C. Drosophila nuclear proteins bind to regions of alternating C and T residues in gene promoters. Science. 1989 Sep 29;245(4925):1487–1490. doi: 10.1126/science.2781290. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Yeung A. T. The UvrABC endonuclease of Escherichia coli. Photochem Photobiol. 1990 Jun;51(6):749–755. [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Hoeijmakers J. H., Odijk H., Westerveld A. Differences between rodent and human cell lines in the amount of integrated DNA after transfection. Exp Cell Res. 1987 Mar;169(1):111–119. doi: 10.1016/0014-4827(87)90230-8. [DOI] [PubMed] [Google Scholar]
- Htun H., Dahlberg J. E. Single strands, triple strands, and kinks in H-DNA. Science. 1988 Sep 30;241(4874):1791–1796. doi: 10.1126/science.3175620. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Elliott G. C., Squires S., Joysey V. C. Lack of complementation between xeroderma pigmentosum complementation groups D and H. Hum Genet. 1989 Feb;81(3):203–210. doi: 10.1007/BF00278989. [DOI] [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S., Bird A. P. Use of restriction enzymes to detect potential gene sequences in mammalian DNA. 1987 May 28-Jun 3Nature. 327(6120):336–338. doi: 10.1038/327336a0. [DOI] [PubMed] [Google Scholar]
- Mai S., Stein B., van den Berg S., Kaina B., Lücke-Huhle C., Ponta H., Rahmsdorf H. J., Kraemer M., Gebel S., Herrlich P. Mechanisms of the ultraviolet light response in mammalian cells. J Cell Sci. 1989 Dec;94(Pt 4):609–615. doi: 10.1242/jcs.94.4.609. [DOI] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- McClanahan T., McEntee K. DNA damage and heat shock dually regulate genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Jan;6(1):90–96. doi: 10.1128/mcb.6.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzina M., Nocentini S., Sarasin A. DNA ligase activity in carcinogen-treated human fibroblasts. Biochimie. 1982 Aug-Sep;64(8-9):743–748. doi: 10.1016/s0300-9084(82)80122-3. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Mudgett J. S., MacInnes M. A. Isolation of the functional human excision repair gene ERCC5 by intercosmid recombination. Genomics. 1990 Dec;8(4):623–633. doi: 10.1016/0888-7543(90)90248-s. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Robbins J. H. No lack of complementation for unscheduled DNA synthesis between xeroderma pigmentosum complementation groups D and H. Hum Genet. 1989 Dec;84(1):99–101. doi: 10.1007/BF00210685. [DOI] [PubMed] [Google Scholar]
- Robinson G. W., Nicolet C. M., Kalainov D., Friedberg E. C. A yeast excision-repair gene is inducible by DNA damaging agents. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1842–1846. doi: 10.1073/pnas.83.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Teitz T., Naiman T., Avissar S. S., Bar S., Okayama H., Canaani D. Complementation of the UV-sensitive phenotype of a xeroderma pigmentosum human cell line by transfection with a cDNA clone library. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8801–8804. doi: 10.1073/pnas.84.24.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Jones N. J., Allen S. A., Carrano A. V. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990 Dec;10(12):6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Troelstra C., Odijk H., de Wit J., Westerveld A., Thompson L. H., Bootsma D., Hoeijmakers J. H. Molecular cloning of the human DNA excision repair gene ERCC-6. Mol Cell Biol. 1990 Nov;10(11):5806–5813. doi: 10.1128/mcb.10.11.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Stefanini M., Giliani S., Hoeijmakers J. H., Bootsma D. Xeroderma pigmentosum complementation group H falls into complementation group D. Mutat Res. 1991 Sep;255(2):201–208. doi: 10.1016/0921-8777(91)90054-s. [DOI] [PubMed] [Google Scholar]
- Vivino A. A., Smith M. D., Minton K. W. A DNA damage-responsive Drosophila melanogaster gene is also induced by heat shock. Mol Cell Biol. 1986 Dec;6(12):4767–4769. doi: 10.1128/mcb.6.12.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Masurel R., Westerveld A., Odijk H., de Wit J., Bootsma D., van der Eb A. J., Hoeijmakers J. H. Molecular cloning and biological characterization of the human excision repair gene ERCC-3. Mol Cell Biol. 1990 Jun;10(6):2570–2581. doi: 10.1128/mcb.10.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Burki H. J. Repair capability and the cellular age response for killing and mutation induction after UV. Mutat Res. 1982 Aug;95(2-3):505–514. doi: 10.1016/0027-5107(82)90281-0. [DOI] [PubMed] [Google Scholar]
- van Duin M., Koken M. H., van den Tol J., ten Dijke P., Odijk H., Westerveld A., Bootsma D., Hoeijmakers J. H. Genomic characterization of the human DNA excision repair gene ERCC-1. Nucleic Acids Res. 1987 Nov 25;15(22):9195–9213. doi: 10.1093/nar/15.22.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]