Abstract
A multiplexed bead-based immunoassay was developed to simultaneously profile glycosylation patterns of serum proteins to investigate their usefulness as biomarkers for pancreatic cancer. The multiplex assay utilized protein-specific capture antibodies chemically coupled individually to beads labeled with specific amounts of fluorescent dye. Captured proteins were detected based on the extent and specific type of glycosylation as determined by successive binding of fluorescent lectin probes. Advantages to this technique include the fact that antibodies coupled to the beads had minimal nonspecific binding to the lectins ConA/SNA, avoiding the step of chemically blocking the antibody glycans and the bead assays were performed in a 96-well filter plate enabling high-throughput screening applications with improved reproducibility. The assay was tested with ConA and SNA lectins to examine the glycosylation patterns of α-1-β glycoprotein (A1BG) and serum amyloid p (SAP) component for use as potential biomarkers for the detection of pancreatic cancer based on the results from prior biomarker studies. The results showed that the SNA response on the captured A1BG protein could distinguish chronic pancreatitis samples from pancreatic cancer with a p-value of 0.035 and for the SAP protein with SNA, a p-value of 0.026 was found between the signal of normal controls and the pancreatic cancer samples. For the ConA response, a decline in the signal for both proteins in the serum samples was found to distinguish pancreatic cancer from normal controls and renal cell carnoma samples (A1BG, p<0.05; and SAP, p<0.0001).
Keywords: Antibody, Bead-based multiplexed assay, Biomarker, Cancer, Glycoproteins
1 Introduction
Carbohydrate modifications on glycoproteins show a high-structural diversity reflecting inherent functional roles for particular biological environments [1–7]. Serum represents one of the most physiologically relevant glycoproteomes in the human body. The alteration of glycan structure and coverage on several major glycoproteins in serum has been shown to be associated with the progression of cancer [8–12]. Studies comparing the carbohydrate chains of glycoproteins produced in serum from patients with developed malignancy to patients with corresponding chronic disease and normal controls may provide useful information for the diagnosis, prognosis, and the development of therapeutic strategies [13–17].
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States [18]. According to the SEER (Surveillance, Epidimiology and End Results) database, pancreatic adenocarcinoma is associated with a 4% 5-year survival rate. While a majority of these patients do not achieve curability, pancreatic resection is associated with substantially improved outcomes including a fivefold higher 5-year survival rate [19]. Unfortunately, the overwhelming majority of patients do not present with early-stage disease and currently there are no clinically useful strategies for the detection of early pancreatic cancer. Many strategies utilizing both invasive and noninvasive techniques have been employed to detect early pancreatic cancer among patients at high risk of developing this malignancy. Endoscopic ultrasound has been studied for screening purposes but has been found limited in several aspects [20]. CA 19-9 is a serologic biomarker of pancreatic cancer but it is ineffective at detecting early pancreatic cancer [21, 22]. Driven by the advances in proteomics techniques, a number of efforts have been made to find new biomarkers in the serum proteome [23–27]. More recently, a novel mass spectrometry-based approach has exploited the field of glycomics to discover unique differential glycosylation patterns of specific proteins associated with malignant processes [28]. Glycoproteins from patient serum were extracted and separated by a combination of multilectin affinity and reverse-phase chromatography, then spotted on glass slides. Five lectins were utilized to investigate the differential glycosylation pattern of the proteins in each fraction. The study identified potential glycoprotein biomarkers with a lectin response that could significantly distinguish between the disease state and the normal controls.
Microarray immunoassays have been increasingly used for profiling and characterizing captured proteins and their modifications, with the appeal of multiplexing and low sample cost. A new type of antibody-lectin array has been recently developed to detect the glycosylation of specific proteins in a complex mixture [29–31]. A variety of glycan structures of interest can be probed with commercially available lectins. In glycoprotein research, this technique provides the highest throughput and reproducibility in measuring the abundance and glycosylation of multiple proteins, which is essential for developing an assay for validation purposes. This sandwich-based assay allows one to assay a large number of samples based on lectin response to specific glycan structures while eliminating the need for any detailed knowledge of the structure which would require mass spectrometry analysis.
Alternatively, microsphere or bead-based immunoassay techniques [32, 33] can be used which have similar advantages for this study. Bead-based multiplex protein profiling is derived from antibody microarrays, but uses polystyrene beads labeled with amounts of discrete fluorophore instead of a flat surface for antibody immobilization. The fluorophore intensity varies between different bead types and is easily distinguished using a flow cytometer. Additional multiplexing capability is achieved through the use of differently sized beads also distinguishable by flow cytometers.
The advent of the MultiBeads® immunoassay platform provides beads internally labeled with discrete amounts of fluorescent dye producing 12 spectrally distinct bead types. Two sizes of beads are available allowing 24 different bead types to be combined. Similar to an antibody sandwich assay, each antibody-coupled bead captures an antigen that can be detected by a fluorophore-conjugated secondary antibody. A mixture of bead types, each hosting a separate immunoassay can be simultaneously read in a flow cytometer. Each reading contains the measurement of multiple antigens, each differentiated based on the intrinsic fluorescence and size of the host bead. The beads used in a large set of parallel assays are uniformly fabricated while for the alternative microarraybased assays, for example, each block of the microarray is printed individually providing the potential for bead-based immunoassays to outperform microarray-based assays in terms of reproducibility and accuracy. In addition, protein microarrays typically use noncovalent interactions and require drying during the printing process to bind the antibody to the solid phase. Bead-based immunoassays utilize covalent linkage chemistry and the immobilized antibodies are maintained in aqueous buffers. The result is that bead-based assays typically produce improved background due to reduced nonspecific absorption.
To take advantage of the benefit of bead-based immunoassays for glycosylation detection, we converted an antibody-lectin technique developed on the microarray platform for use on the bead platform (experimental scheme shown in Fig. 1). Two types of antibody-coupled beads targeting α-1-β glycoprotein (A1BG) and serum amyloid p component as potential markers from the previous study were prepared and tested to find the optimal conditions [30]. As a proof-of-concept, the potential markers are employed to measure the glycosylation response to SNA (sialylation) and ConA (mannosylation or complex biantennary glycans) for serum samples from different groups of patients including pancreatic cancer, chronic pancreatitis, and normal controls to demonstrate that we can indeed use this bead-based assay to rapidly distinguish cancer from normal using this methodology.
Figure 1.
The diagram of bead-based antibody-lectin multiplex assay. (1) production of two sizes of unique labeled beads; (2) chemical coupling of different antibodies to different types of beads; (3) hybridization of the antibody-conjugated beads with diluted serum in a 96-well filter plate; (4) sequential reaction of antigen capture, glycan-lectin binding, and fluorescence detection; (5) the detection of signal for each type of bead with a flow cytometer; (6) gating signal points to extract data for each analyte.
2 Materials and methods
2.1 Chemicals
AB34 buffer was obtained from Assay Designs or Enzo Life Sciences. NaIO4, Tween-20, PBS powder pouch, Brij-35, and Gly-Cys were purchased from Sigma. 4-(4-N-maleimidophenyl) butyric acid hydrazide hydrochloride (MPBH) was purchased from Thermo Scientific. All biotinylated lectins were purchased from Vector Laboratory. Alexa 555-conjugated streptavidin was purchased from Invitrogen Biotechnology.
2.2 Serum samples
Sera from 20 patients (10 males) with pancreatic cancer were obtained at the time of cancer diagnosis in the Multidisciplinary Pancreatic Tumor Clinic at the University of Michigan Comprehensive Cancer Center. This was performed following the completion of informed consent using IRB-approved guidelines. All patients in the study had histologically confirmed diagnosis of pancreatic adenocarcinoma, stage III or IV. None of the patients were actively undergoing chemotherapy or radiation therapy for pancreatic cancer, or had other malignancies diagnosed or treated within the previous 5 years. Sera were also obtained from 20 patients (12 males) with chronic pancreatitis in the absence of acute flare symptoms who were seen in the Gastroenterology Clinic at University of Michigan Medical Center, and from healthy individuals collected under the auspices of the Early Detection Research Network (EDRN). The mean age of the pancreatic cancer group was 65.2 years (range, 44–83 years) and from the chronic pancreatitis group was 62.6 years (range, 48–83 years).
Inclusion criteria for the study included patients with pancreatic cancer, chronic pancreatitis, or healthy adults with the ability to provide written, informed consent. The samples were permitted to sit at room temperature for a minimum of 30 min (and a maximum of 60 min) to allow the clot to form in the red top tubes (Fisher Scientific, 10 mL glass tubes), and then centrifuged at 1300 × g at 4°C for 20 min. The serum was removed, transferred to polypropylene tubes in 1mL aliquots, and frozen. The frozen samples were stored at −80°C until assayed. All serum samples were labeled with a unique identifier to protect the confidentiality of the patient. None of the samples were thawed more than twice before analysis.
2.3 Mouse mAbs
A1BG mAb was acquired from Novus, whereas amyloid p component mAb antibody was from Abcam. The primary amine groups of the lysines on the mAbs were covalently coupled to 5.4 μm latex beads via disulfide bridges (Assay Designs, Enzo Life Sciences, Ann Arbor, MI).
2.4 Antibody blocking
To prevent the reaction between the glycans on the antibodies and some specific detection lectins, the antibodies coupled to beads were chemically modified following the glycan-blocking protocol described in our previous study [30]. Briefly, the beads were washed with coupling buffer (AB34) and then incubated in 0.2M NaIO4 for 3 h. When the oxidation reaction was finished, as determined by where longer incubation times would not further reduce the binding of the lectin to the antibody glycans (data not shown), the precipitate was removed by washing the beads three times with coupling buffer with 0.1% Tween-20. The oxidized antibody beads were incubated with 1mM 4-(4-N-maleimidophenyl)butyric acid hydrazide hydrochloride and 1mM Cys-Gly dipeptide for 2 h. Finally, the beads were kept in 1mM Cys-Gly in dark at 4°C overnight. The blocked beads were extensively washed to eliminate reagent in the solution before being stored in a refrigerator at 4°C.
2.5 Sample incubation and flow cytometry detection
The beads coupled to A1BG and serum amyloid p (SAP) antibodies were mixed with 20 × diluted serum (diluted with PBS containing 0.1% Tween-20 and 0.1% Brij 35) in Eppendorf tubes and incubated on a shaker set at 300 rpm for 1 h at room temperature. Each tube contained 6000 beads of each type. The beads were then transferred to two identical 96-well filter plates where subsequent incubation and washing were performed. The samples from different disease groups were randomized on the well plate to eliminate bias. The two duplicate plates were processed in parallel. Then, the serum solution was removed and the beads were washed with PBST three times. A vacuum manifold was used to remove reagent and washing buffer. Biotinylated lectins (Vector Laboratory) were diluted to 1 ug/mL and applied to each well. The lectin-glycoprotein reaction was allowed to proceed for 45 min before being complete. The filter plates were rinsed to remove unbound lectins. The solution of 1 ug/mL Alexa 555-conjugated streptavidin (Invitrogen Biotechnology) was added to each well for detection. Finally, the beads were washed with water to remove detergent.
The fluorescent signal was read by a flow cytometer (FACSCalibur). The beads were sorted by the flow cytometer based on size and inherent fluorescent intensities using the 670 nm filter. Three hundred beads of each type were counted. The fluorescent signals of the analytes were measured at 575 nm.
2.6 Statistical analysis
Data analysis was carried out in Weasel version 2.6. Weasel is a flow cytometry data analysis program available for download from the Walter and Eliza Hall Institute of Medical Research. The signals were gated to exclude damaged or cross-linked beads. The medians of the select signal spots at each inherent fluorescent level were taken as a data point into analysis. All the samples were measured twice with duplicate wells. The reproducibility of the experiments was assessed by calculating the coefficient of variation (CV) and Pearson correlation for the pairs of duplicates. To compare the signal of cancer and noncancer samples, a Student’ T-test was applied to the data, where a difference is considered statistically significant when p<0.05.
3 Results and discussion
3.1 Selection of lectins for antibody bead assay
In the antibody array/lectin sandwich assay, the binding of the lectins to glycans on the antibodies results in interference for the detection of captured glycoproteins. A previous study using antibody microarrays for glycoprotein studies utilized a blocking procedure to prevent this interaction [30, 31]. As described in Section 2, the cis-diol groups on the glycans were gently oxidized and converted to aldehyde groups which then reacted with hydrazidemaleimide bifunctional cross-linking reagent capped with a Cys-Gly dipeptide. The fluorescent signal of the lectin bound to the blocked antibodies decreased five to tenfold depending on which lectin was used (data not shown). The derivatization procedure prevented the binding of the lectins to glycans on the antibodies but it resulted in reduced stability of the antibody on the antibody-coupled beads, where the signal of the blocked beads degraded over several days. To determine whether the glycan-blocking was necessary, the underivatized beads were treated with/without 20 × diluted serum to assess their binding to five different lectins. The lectins utilized were ConA, SNA, AAL, MAL II, and PNA as in our previous study on glycoprotein markers in pancreatic cancer [28].
In Table 1A, the signal of the lectin-treated beads (without serum) indicates the level of interaction between an element of the structure and the underivatized bead-coupled antibodies. This is a distinct advantage in that derivatizaton of the antibodies to prevent interaction is not required as most other platforms for ConA and SNA. The signal of ConA and SNA bound to underivatized antibodies is 20-fold lower than the signal of the antibody spots exposed to serum, showing a blocking effect of the interaction between these two lectins and the glycans on the antibodies. This response is optimal for our sandwich assay experiments, especially where SNA is one of the key lectins used in a prior study for recognizing changes in α 2–6 sialylation of the glycan structure in response to a change in disease state. AAL had strong interaction with the underivatized form which means that to use AAL for these experiments derivatization would be required as on the microarray platform. MAL II shows a low level of background signal in response of the lectin to the antibodies and could be used in these experiments, whereas PNA barely binds to the antibodies or the captured proteins.
Table 1.
Fluorescence signal of antibody-lectin experiment using either antibody-conjugated beads or microarray platform.
| Control (incubated with buffer)
|
Incubated with serum
|
|||
|---|---|---|---|---|
| A1BG | SAP | A1BG | SAP | |
| (A) Antibody-conjugated beads without glycan blocking (unit = flow cytometer fluorescent unit) | ||||
| Bead unblocked | ||||
| SNA | 4±0 | 32±22 | 537±41 | 687±2 |
| MAL | 14±1 | 57±21 | 56±3 | 154±20 |
| AAL | 276±5 | 353±9 | 291±7 | 379±11 |
| ConA | 4±0 | 22±6 | 162±18 | 253±33 |
| PNA | 2±0 | 6±0 | 3±0 | 8±1 |
| (B) Antibody microarray without glycan blocking (unit = microarray scanner fluorescent unit) | ||||
| Microarray unblocked | ||||
| SNA | 2193±16 | 6031±153 | 9460±78 | 6162±113 |
| MAL | 4280±252 | 1429±82 | 3938±109 | 3222±60 |
| AAL | 22 312±476 | 24 367±306 | 19 958±784 | 22 562±424 |
| ConA | 22 158±709 | 17 181±2071 | 22 093±556 | 16 674±135 |
| PNA | 813±37 | 859±16 | 1358±110 | 1589±113 |
| (C) Antibody microarray with glycan blocking (unit = microarray scanner fluorescent unit) | ||||
| Microarray blocked | ||||
| SNA | 338±33 | 176±15 | 7976±405 | 4229±226 |
| MAL | 288±5 | 226±18 | 2717±180 | 1532±8 |
| AAL | 406±14 | 202±9 | 10 895±444 | 8446±62 |
| ConA | 721±128 | 611±48 | 7663±197 | 6051±238 |
| PNA | 71±1 | 43±2 | 83±13 | 122±2 |
(A) antibody-conjugated beads were incubated with dilution buffer (left) or diluted serum (right), glycans on the antibodies were not chemically blocked after conjugation, fluorescent signal of the two antibodies were measured by flow cytometer; (B) antibody microarrays were incubated with dilution buffer (left) or diluted serum (right), glycans on the antibodies were not blocked after being printed on microarray; (C) same as (B), but with blocking of antibody glycans.
From our experience, ConA/SNA frequently binds to IgG antibodies raised in animals such as mice, sheep, and rabbits; however, no such binding was observed between the lectin and the IgGs-conjugated to beads. The same antibodies as those conjugated to the beads were printed on a microarray and probed with lectin ConA/SNA. Strong interactions between the lectins and the unblocked antibodies spots were observed, indicating these two antibodies are normally glycosylated and their glycans are accessible for the lectins (Table 1B). Only after chemical blocking of the glycans on the antibodies, as summarized in Table 1C, the response of the antibodies to the lectins decreased to a level ’10% of the regular signal (for ConA, around 10%). The process of coupling the antibodies on the beads also generated an effect of reduced lectin binding on the antibodies similar to the chemical blocking of the glycans. Although the mechanism for this is not understood at this time, taking advantage of this property of the bead-conjugated antibodies allows us to eliminate several time-consuming experimental steps in derivatizing the antibodies. Since the underivatized antibodies on the beads do not bind to ConA and SNA and these two lectins were important in identifying markers of pancreatic cancer in our previous study, these two lectins were selected for a trial set to demonstrate the utility of this method.
3.2 Serum concentration
In these experiments, serum dilution for studying glycosylation was targeted to antibody saturation and minimization of background binding. At antibody saturation, glycosylation can be studied independently of target glycoprotein serum concentration since the amount of on-bead glycoprotein is standardized to the amount of antibody conjugated to the bead. Thus, differences in glycosylation can be measured between samples as lectin binding is directly related to the level of its target glycan structures present on the proteins captured on the antibody array. In this study, the two proteins selected were A1BG and SAP which were chosen as our two best potential markers in response to SNA lectin based on a previous study [30].
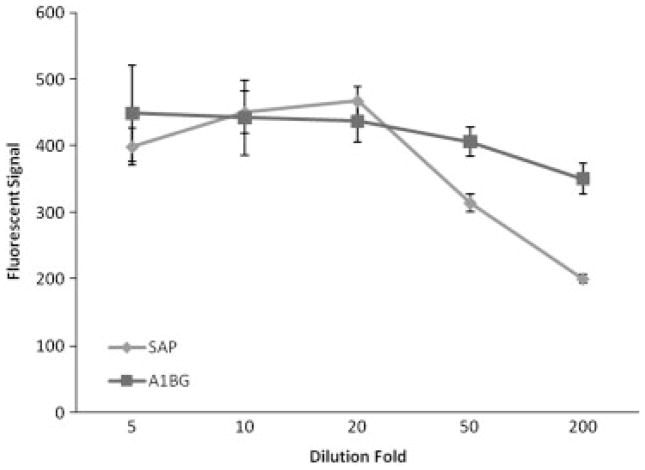
Different dilutions of serum (5, 10, 20, 50, and 200 ×) were tested to determine the optimum concentration of the target glycoproteins. Lectin ConA was used for the detection in this test. Figure 2 shows the intensity of the signal changes for the two antibodies with the dilution fold. A rising trend was noted from the 200× dilution to the 20× dilution for both antibodies. From 20× dilution to 5× dilution, the signal of SAP increased only by 5%. For A1BG, the signal reached a peak at 20× dilution, where a saturation of the target protein on the antibody had occurred. The signal then decreased 10% when the dilution increased to 5×. The decrease of signal was observed along with an increase of outlier data points in the flow cytometry spectrum and a higher standard deviation, likely due to competing nonspecific binding on the antibodies.
Figure 2.
Signal-dilution curve made to obtain the optimum concentration of serum for bead hybridization using lectin ConA. The x-axis is the dilution fold of serum incubated with the beads in each assay ranging from 5 to 200 ×, the y-axis is the resulting fluorescent signal. The value of each spot on the y-axis presents the signal yield of each assay. A1BG, α-1-β glycoprotein; SAP, serum amyloid p component. Unit of the fluorescent signal is the inherent fluorescent unit of the flow cytometer.
The result of the dilution test demonstrated that the antibodies were saturated by their target protein at 20× dilution in the process of hybridization (1 h, room temperature, and gentle shaking). Below 20× dilution would not occupy a majority of all the antibody-binding sites and above 20× dilution could introduce strong nonspecific binding. Thus, 20× was chosen as the optimal dilution fold.
3.3 Serum testing
Lectin ConA and SNA were used in two separate tests with antibody-coupled beads to analyze serum samples from normal control and patients with chronic pancreatitis and pancreatic cancer. In the experiment using ConA as the detection lectin, duplicate pairs of 47 samples including ten normals, ten chronic pancreatitis samples, ten pancreatic cancer samples, ten renal carcinoma samples, and seven esophageal cancer samples were incubated with beads on a single 96-well plate. SNA was used in an experiment to test 20 samples from each of the normal control, chronic pancreatitis and pancreatic cancer groups and duplicate wells were made on two identical plates.
3.3.1 Reproducibility
The reproducibility of the samples incubated on the same plate or two different plates were assessed using the results of ConA and SNA experiments, respectively. For the ConA experiment performed on a single plate, the average CVs of the duplicate pairs for antibody A1BG and SAP were 9.8 and 6.9%, respectively. In the SNA experiment, the average CVs of the duplicate pairs on two different plates for antibody A1BG and SAP were 7.5 and 5.9%, respectively. The intra-assay variability can be decreased by gating out signals of some damaged beads generated from cross-linked beads or nonspecific binding.
3.3.2 Biomarker performance
The proteins A1BG and SAP were found to be pancreatic cancer-related biomarker candidates based on their response to SNA lectin [28, 30]. Their glycosylation levels were found to be elevated in pancreatic cancer serum in this study using a RP HPLC-fraction microarray method [28]. Both of these two glycoproteins produced significant differences when analyzed against lectin SNA and ConA. Their potential to aid diagnosis in pancreatic cancer was further tested utilizing the antibody microarray with lectin SNA in a previous study against 183 samples from various groups [30]. The results determined that A1BG was able to distinguish pancreatic cancer from chronic pancreatitis. On the bead-based platform, a similar experiment was conducted with a different set of samples. The results (Fig. 3) are subjected to a t-test between each pair of sample classes. A p-value of 0.035 was obtained for A1BG between samples from chronic pancreatitis patients and pancreatic cancer patients, whereas the p-value for normal control and pancreatic cancer was 0.096 which is above the significant level. For SAP, the difference between normal control and pancreatic cancer patients is significant (p-value, 0.026). The results demonstrate the importance of the multiplexed biomarker measurement, as neither of the two antibodies can significantly distinguish cancer samples from normal and pancreatitis samples by themselves, while together they create the capacity of differentiating cancer samples from the other two groups (Fig. 3).
Figure 3.
Result of an experiment using ten sera samples from patients with stages III and IV pancreatic adenocarcinoma, chronic pancreatitis, and normal healthy controls. Glycans of the two captured proteins, (A) serum amyloid p component; (B) A1BG were probed with biotinylated SNA and detected by streptavidinylated Alexa555. The bar graph and SEM (error bars) shows the average signal and variation for each group of samples. The group marked with a green star on top can be significantly distinguished from the group marked with a red star. Unit of the fluorescent signal is the inherent fluorescent unit of the flow cytometer.
Two more groups of samples (renal cell carcinoma and esophageal cancer) were added to the experiment in which the glycoproteins were probed with ConA which detects mannosylation or complex biantennary glycans. The average signals and the variation for each group of samples are shown as a bar graph with error bars in Fig. 4. A decline in ConA response on A1BG and SAP was found to distinguish pancreatic adenocarcinoma from normal and renal Cell carcinoma, (p<0.05 and <0.0001, respectively). A decreased ConA response in SAP among patients with pancreatic adenocarcinoma compared with chronic pancreatitis was observed, but this did not reach statistical significance, p<0.11.
Figure 4.
Result of an experiment using ten sera samples from patients with stages III and IV pancreatic adenocarcinoma (PC), chronic pancreatitis (CP), renal cell carcinoma (RC), esophageal carcinoma (EC), and normal healthy controls (NL). Antibody-captured proteins were probed with biotinylated ConA and detected by streptavidinylated Alexa555. The bar graph and SEM (error bars) shows the average signal and variation for each group of samples. The group marked with a green star on top can be significantly distinguished from the group marked with a red star. (A) Serum amyloid p component (SAP-ConA); (B) A1BG-ConA.
4 Concluding remarks
We have developed a method for high-throughput glycoprotein biomarker screening using a novel bead-based antibody-lectin glycoprotein assay. Compared with the microarray platform, the new technique showed improved sensitivity and reproducibility in glycan detection based on the elimination of background noise due to lectin binding to the antibodies for SNA and ConA. Some key steps, such as array printing and slide scanning, that always introduce spatial variation are eliminated when antibody-coupled beads are used to perform the assay in a 96-well plate. Additionally, the glycans on the bead-conjugated antibodies were not reactive to lectin ConA and SNA, and hence the glycan-blocking step can be avoided.
Using the bead-based assay, we discriminated glycosylation patterns among normal and other disease states with two glycoprotein biomarker candidates as a demonstration of the potential of this platform. These two glycoprotein targets were chosen based on the most promising results from the previous study. It should be noted that although we could discriminate the disease and normal groups based on the bead-based platform the results will not necessarily be the same as the microarray platform since the binding of the antibody to the surface and subsequent glycoprotein capture and lectin affinity will be different. Nevertheless, the different groups could be discriminated with significance with the use of two different lectins and could also be discriminated against other disease states included. The lectin-based method allows one to discriminate such disease states based on the changes in glycan structure without the need for detailed structural analysis of the sugar groups based on mass analysis. This study is an initial demonstration of the method but in future study will be multiplexed with additional antibodies on the beads and will use a larger set of serum samples to verify the validity of the biomarkers. The speed and utility of the bead-based method with detection by flow cytometry will be essential, especially as the number of samples increase.
Acknowledgments
This work was supported by the National Cancer Institute under R21CA124441 (D. M. L.), the National Institute of Health under R01GM49500 (D. M. L.), and K23 DK082097(M. A. A.).
Abbreviations
- A1BG
α-1-β glycoprotein
- SAP
serum amyloid p
Footnotes
The authors have declared no conflict of interest.
References
- 1.Kameyama A, Kaneda Y, Yamanaka H, Yoshimine H, Narimatsu H, Shinohara Y. Anal Chem. 2004;76:4537–4542. doi: 10.1021/ac049897z. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B. Mol Biotechnol. 1994;2:243–280. doi: 10.1007/BF02745880. [DOI] [PubMed] [Google Scholar]
- 3.Wada Y, Tajiri M, Yoshida S. Anal Chem. 2004;76:6560–6565. doi: 10.1021/ac049062o. [DOI] [PubMed] [Google Scholar]
- 4.Imre T, Schlosser G, Pocsfalvi G, Siciliano R. J Mass Spectrom. 2005;40:1472–1483. doi: 10.1002/jms.938. [DOI] [PubMed] [Google Scholar]
- 5.Kameyama A, Kikuchi N, Nakaya S, Ito H, Sato T, Shikanai T, Takahashi Y, Takahashi K, Narimatsu H. Anal Chem. 2005;77:4719–4725. doi: 10.1021/ac048350h. [DOI] [PubMed] [Google Scholar]
- 6.Palm AK, Novotny MV. Rapid Commun Mass Spectrom. 2005;19:1730–1738. doi: 10.1002/rcm.1979. [DOI] [PubMed] [Google Scholar]
- 7.Yu YQ, Gilar M, Kaska J, Gebler JC. Rapid Commun Mass Spectrom. 2005;19:2331–2336. doi: 10.1002/rcm.2067. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Simeone DM, Heidt D, Anderson MA, Lubman DM. J Proteome Res. 2006;5:1792–1802. doi: 10.1021/pr060034r. [DOI] [PubMed] [Google Scholar]
- 9.Reis CA, Osorio H, Silva L, Gomes C, David L. J Clin Pathol. 2010;63:322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 10.Plavina T, Wakshull E, Hancock WS, Hincapie MJ. Proteome Res. 2007;2:662–671. doi: 10.1021/pr060413k. [DOI] [PubMed] [Google Scholar]
- 11.Gorelik E, Galili U, Raz A. Cancer Metastasis Rev. 2001;20:245–277. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 13.Drake RR, Schwegler EE, Malik G, Diaz J, Block T, Mehta A, Semmes OJ. Mol Cell Proteomics. 2006;10:1957–1967. doi: 10.1074/mcp.M600176-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Kobata A, Amano J. Immunol Cell Biol. 2005;83:429–439. doi: 10.1111/j.1440-1711.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Balzarini J. Nat Rev Microbiol. 2007;8:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellokumpu S, Sormunen R, Kellokumpu M. FEBS Lett. 2002;516:217–224. doi: 10.1016/s0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- 17.Peracaula R, Tabares G, Royle L, Harvey DJ, Dwek RA, Rudd PM, de Llorens RA. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 19.Sohn TA, Lillemoe KD, Cameron JL, Huang JJ, Pitt HA, Yeo CJJ. Am Coll Surg. 1999;188:658–666. doi: 10.1016/s1072-7515(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 20.Larghi A, Verna EC, Lecca PG, Costamagna G. Clin Cancer Res. 2009;15:1907–1914. doi: 10.1158/1078-0432.CCR-08-1966. [DOI] [PubMed] [Google Scholar]
- 21.Dalgleish AG. Br Med J. 2000;321:380–380. [PubMed] [Google Scholar]
- 22.Nazli O, Bozdag AD, Tansug T, Kir R, Kaymak E. Hepatogastroenterology. 2000;47:1750–1752. [PubMed] [Google Scholar]
- 23.Goggins M. J Clin Oncol. 2005;23:4524–4531. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 24.Grantzdorffer I, Carl–McGrath S, Ebert MP, Rocken C. Pancreas. 2008;36:329–336. doi: 10.1097/MPA.0b013e31815cc452. [DOI] [PubMed] [Google Scholar]
- 25.Vimalachandran D, Costello E. Expert Rev Proteomics. 2004;1:493–501. doi: 10.1586/14789450.1.4.493. [DOI] [PubMed] [Google Scholar]
- 26.Yu KH, Barry CG, Austin D, Busch CM, Sangar V, Rustgi AK, Blair IA. J Proteome Res. 2009;8:1565–1576. doi: 10.1021/pr800904z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faca VM, Song KS, Wang H, Zhang Q, Krasnoselsky AL, Newcomb LF, Plentz RR, Gurumurthy S, Redston MS, Pitteri SJ, Pereira–Faca SR, Ireton RC, Katayama H, Glukhova V, Phanstiel D, Brenner DE, Anderson MA, Misek D, Scholler N, Urban ND, Barnett MJ, Edelstein C, Goodman GE, Thornquist MD, McIntosh MW, DePinho RA, Bardeesy N, Hanash SM. PLoS Med. 2008;5:e123. doi: 10.1371/journal.pmed.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Patwa TH, Qiu WL, Shedden K, Hinderer R, Misek DE, Anderson MA, Simeone DM, Lubman DM. J Proteome Res. 2007;5:1864–1874. doi: 10.1021/pr070062p. [DOI] [PubMed] [Google Scholar]
- 29.Wu YM, Nowack DD, Omenn GS, Haab BB. J Proteome Res. 2009;8:1876–1886. doi: 10.1021/pr8008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Simeone DM, Brenner DE, Anderson MA, Shedden KA, Ruffin MT, Lubman DM. J Proteome Res. 2009;8:483–492. doi: 10.1021/pr8007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SM, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, Haab BB. Nat Methods. 2007;5:437–444. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- 32.Joos TO, Stoll D, Templin MF. Curr Opin Chem Biol. 2002;6:76–80. doi: 10.1016/s1367-5931(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 33.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Clin Immunol. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]