Abstract
Introduction
The present study investigated if T-cells infiltrating the periapical lesion produce RANKL and whether bacteria infecting the root canal can activate T-cells to produce RANKL.
Methods
Using a mouse model of periapical lesion induced by artificial dental pulp exposure, the presence of RANKL-positive T-cells and osteoclasts in the periapical lesion was examined by an immuno-histochemical approach. The bacteria colonizing the exposed root canal were identified by 16S ribosomal RNA (rRNA) sequence analysis. The isolated endodontic bacteria were further immunized to normal mice, and sRANKL production by the T-cells isolated from the immunized mice was evaluated by ex vivo culture system.
Results
RANKL-positive T-cells, along with TARP+ osteoclasts, were identified in periapical bone resorption lesions. The Gram-negative bacterium Pasterurella pnumotropica (P. pnumotropica), which was most frequently detected from root canal of exposed pulp, showed remarkably elevated serum IgG antibody response in pulp-exposed mice compared to control non-treated mice. Immunization of mice with P. pneumotropica induced not only serum IgG antibody but also primed bacteria reactive T-cells that produced sRANKL in response to ex vivo exposure to P. pneumotropica.
Conclusion
T-cells infiltrating the periapical region express RANKL, and the endodontic bacteria colonizing the root canal appear to induce RANKL expression from bacteria-reactive T-cells, suggesting the possible pathogenic engagement of immune response to endodontic bacteria in the context of developing boneresorptive periapical lesions.
Introduction
Periapical periodontitis is caused by bacterial infection of the dental pulp that leads to the development of a pathogenic bone resorption lesion (1, 2), which is characterized by the infiltration of inflammatory cells predominantly composed of macrophages and T cells (1, 3, 4), in conjunction with the production of proinflammatory cytokines (2, 5, 6). The current paradigm of bone resorption supports the hypothesis that receptor activator of nuclear factor k B-ligand (RANKL) directly induces osteoclast differentiation and activation (7), while its soluble decoy-receptor osteoprotegerin (OPG) neutralizes such osteoclastogenic effects by RANKL (8). The level of RANKL mRNA expression in human periapical lesion is significantly higher than healthy periapical tissue (9, 10), indicating that locally produced RANKL also elicits periapical bone resorption. However, the biological mechanism underlying such increase of RANKL in the periapical lesion with attendant pathogenic bone resorption remains unclear.
It is reported that RANKL produced by T-cells, but not monocyte linage cells, appears to be engaged in the bone loss exhibited in marginal periodontitis as a result of an adaptive immune response to the bacterium (11, 12). In a mouse model of periodontal disease, bacteria reactive T-cells are demonstrated as the source of RANKL (13). On the other hand, while the kinetics of RANKL-expressing cells were reported to parallel to that of emergent osteoclasts in the rodent model of periapical lesion (14), the cellular source of RANKL is unclear, especially whether T lymphocytes produce RANKL in the periapical lesion.
Therefore, in the present study, we investigated the localization of RANKL-producing T-cells using a mouse model of periapical lesion induced by artificial dental pulp exposure. The bacteria colonizing in the exposed root canal were identified by 16S ribosomal RNA (rRNA) sequencing. The isolated endodontic bacteria were further immunized to normal mice, and sRANKL production by the T-cells isolated from the immunized mice was evaluated by ex vivo culture system.
Materials & Methods
Animals
C57BL/6j mice (8-week-old males) were kept in the Forsyth Animal Facility with 12h light-dark cycle at constant temperature. The experimental protocols used in this study were approved by the Forsyth IACUC.
Induction of periapical lesion
Periapical lesions were induced by pulp exposure of the mandibular first molar pulps of mice (8–10-week-old males, n=5/group) following the protocol published previously (15). Both sides of mandibular first molars were exposed using a ¼-size dental round bur. Healthy non-treated mice served as baseline control. Blood serum was collected on day-0 and -14.
Histochemical analyses
The mandibles from mice were fixed in 4% paraformaldehyde and decalcified in 10% EDTA solution. These mandibles were embedded in Tissue-Tek OTC compound (Sakura, Torrance, CA), and sections (8 µm in thickness) of specimens were cut in a buccal-lingual direction by using a cryostat. The sections were stained with 0.1% Mayers Hematoxylin and 0.5% Eosin or with TRAP (13), and the nuclei were counter-stained with methyl green.
Immunofluorescent Laser-Scanning confocal microscopy
Frozen sections were reacted with the anti-mouse CD3 ε chain conjugated with fluorescein isothiocyanate (FITC) (2.5 µg/ml) (BD Biosciences, San Jose, CA) or biotinylated-OPG-Fc (10 µg/ml) followed by Texas Red-Avidin (10 µg/ml) (Invitrogen, Carlsbad, CA) (12). The staining pattern was analyzed by using a Leica TCS/SP-2 confocal microscope (Leica, Wetzlar, Germany).
16S rRNA-based identification of mouse endodontic bacteria
At 14 days after pulp exposure, microbial specimens were collected from the root canal space of anesthetized mice placing paper points for 30 s. The bacteria recovered on paper points were cultured on sheep blood agar plates in an anaerobic chamber at 37°C for 2 days. The total genomic DNA was extracted from all cultivable bacterial isolates, using QIAmp DNA mini kit (Qiagen, Valencia, CA), and subjected to 16S rRNA-based bacterial identification (13, 16).
Immunization of mice with bacterial antigen
C57BL/6j mice (8-week-old males, n=5/group) were immunized with or without heat-killed Pasteurella pneumotropica (P. pneumotropica) or Enterococcus saccharolyticus (E. saccharolyticus) (3×108 CFU/mouse, subcutaneous [s.c.] injection) in Freund’s adjuvant following the method previously published (17). Four days after booster injection (s.c.) of bacteria in PBS, lymph nodes and blood serum were isolated. Injection of PBS in a mixture of Freund’s adjuvant constituted a control.
The measurement of serum IgG antibody responses to bacterial antigens
Sera isolated from mice were reacted to heat-killed bacteria (107/ml) coated on ELISA plates, and the reacted serum IgG antibodies were measured by ELISA (17).
The bacterial antigen-specific memory T-cell response
CD11c-positive dendritic cells (DCs) developed by incubation of bone marrow cells of C57BL/6j mice with GM-CSF (20 ng/ml) for 7 days were purified using MACS beads (Miltenyi, Auburn, CA). T-cells (2×105 cells/well) isolated from the lymph nodes of mice immunized with or without P. pneumotropica or E. saccharolyticus were co-cultured in vitro with Mitomycin C (20 µg/ml, Sigma)-treated DCs (2×104 cells/well) in the presence or absence of fixed P. pneumotropica or E. saccharolyticus (107 CFU/well) in RPMI 1640 medium containing 10% FBS for 3 days (17). The proliferation of T-cells was evaluated by the [3H] thymidine incorporation assay (18). The sRANKL production was monitored using an ELISA kit (PeproTech, Rocky Hill, NJ) (17).
Statistical analysis
Differences between the two groups were analyzed with Student’s t-test.
Results
Pulp exposure induces inflammatory tissue disruption in periapical lesions
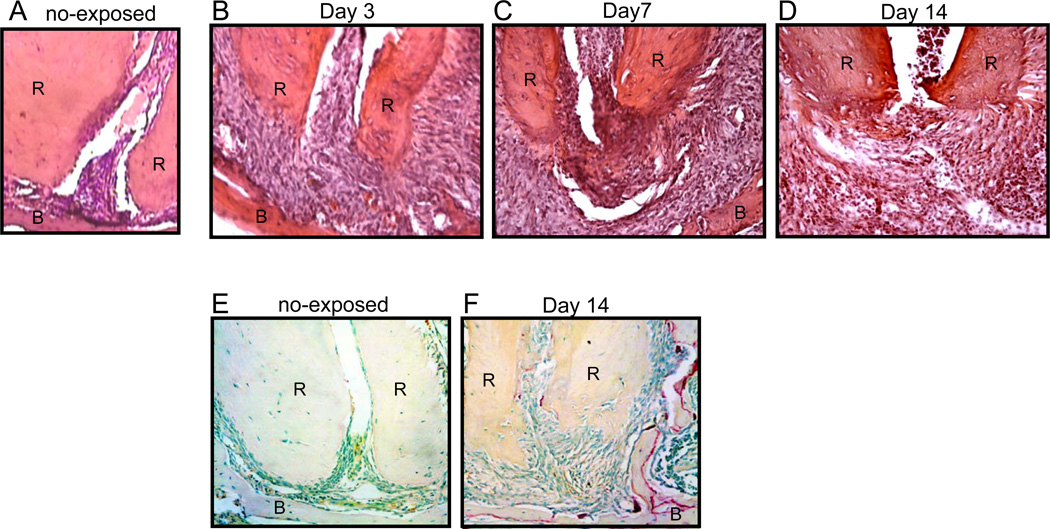
On day-3 after pulp exposure, the periodontal ligament space at the apex of teeth was enlarged (Fig. 1B) compared to control healthy tissue (Fig. 1A). Newly formed blood vessels were also observed in the periapical lesion (Fig. 1B), indicating the induction of primary inflammatory response by the pulp exposure. At day-7, remarkable inflammatory cell infiltration was observed in the periapical lesion (Fig. 1C). Furthermore, massive bone resorption was found at the apical area at day-14 (Fig. 1D) accompanied by the emergence of TRAP+ osteoclasts on the alveolar bone surface of the periapical area (Fig. 1F).
Figure 1.
Pulp exposure induces inflammatory tissue disruption and osteoclastogenesis at the periapical area. Periapical tissues isolated from non-exposed mice (A) or pulp-exposed mice on day-3 (B), day-7 (C), and day-14 (D) were decalcified and stained with H&E. Root (R) and alveolar bone (B) are indicated by the upper case letters. Decalcified periapical tissues of first mandibular molars sampled from (E) control non-treated mice or (F) pulp-exposed mice were stained for TRAP+ osteoclasts. Original magnification: (A) ×400 and (B–F) ×200.
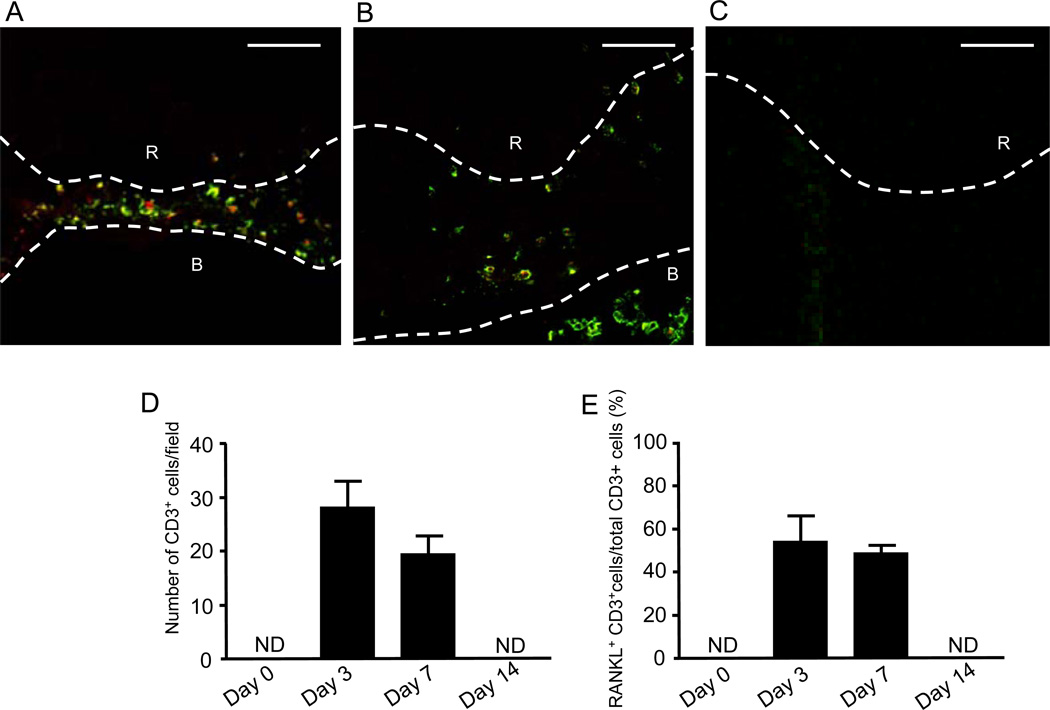
Temporal changes of RANKL+/CD3+ T-cells in the mouse periapical lesions
According to the double-color confocal microscopy that stained RANKL (red) and T lymphocyte-specific CD3 (green) in the periapical tissue, few or no CD3+ T-cells were detected in control healthy periapical area (Fig. 2D), whereas, in the periapical lesion, remarkable infiltration of CD3+ T-cells was observed at both day-3 (Fig. 2A and 2D) and day-7 (Fig. 2B and 2D), followed by the remission of CD3+ T-cell infiltration at day-14 (Fig. 2C and 2D). Importantly, more than 50% of CD3+ cells, which existed in the periapical lesion, expressed RANKL (Fig. 2A, 2B and 2E). Interestingly, on day-7, the RANKL-expressing CD3+ T-cells were also found in medullary cavity of alveolar bone (Fig. 2B). These findings indicated that RANKL-producing T-cells infiltrate into the periapical lesion in response to pulp exposure.
Figure 2.
Chronology of RANKL+/CD3+ T-cells localization pattern in periapical lesions in pulp exposure mouse model. (A–C) The periapical connective tissues at 3 (A), 7 (B) and 14 (C) days after pulp exposure was stained with anti-CD3 mouse antibody-FITC (green) for identification of T-cells and OPG-Fc-biotin/TexasRed-avidin (red) for RANKL. All fluorescent images were captured using a laser scanning confocal microscope. Root (R) and alveolar bone (B) are indicated by the upper case white letters. The white broken line demonstrates the border of root and alveolar bone. Scale bars: 50µm. (D and E) Quantitative assessment of total number of CD3+ T-cells (D) and percentage of RANKL+/CD3+ T-cells in the total CD3+ T-cells (E) are shown in the histograms. The column and bar indicate means ± SD; *, significantly higher than control non-pulp exposure group (day-0) by Student’s t-test (p < 0.05). NA: data not available.
Orally colonizing bacteria induce RANKL-producing, bacteria-specific activated T lymphocytes
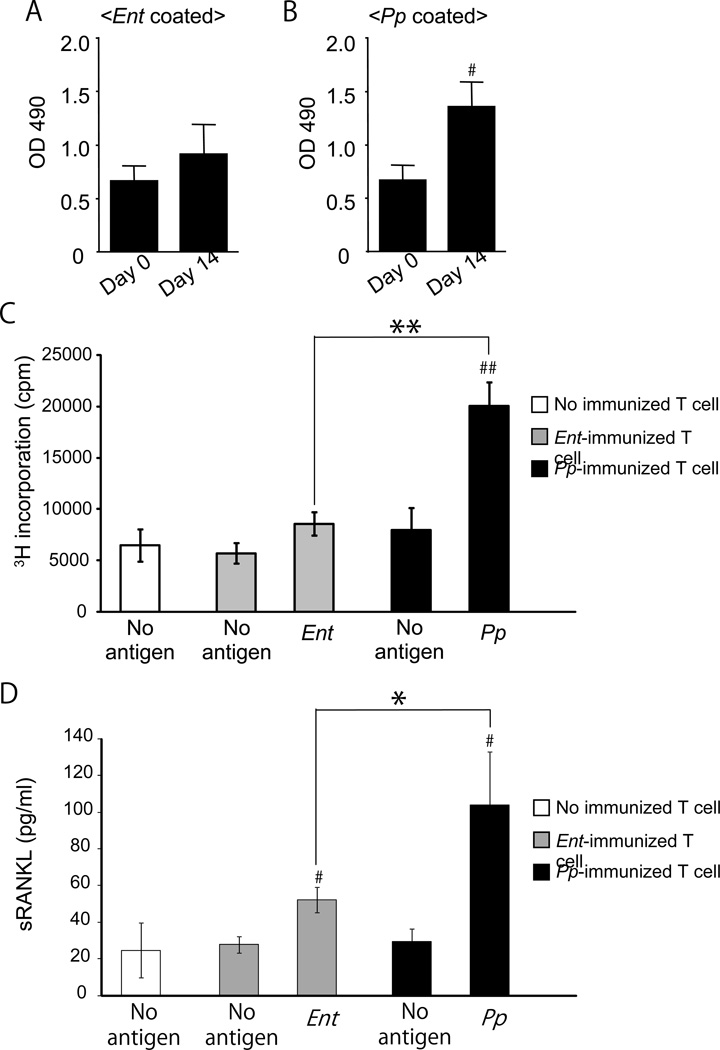
Using 16S rRNA sequencing, only two bacterial strains, E. saccharolyticus and P. pneumotropica, were found in root canals of all five mice receiving pulp exposure. Other bacteria, such as Klebsiella pneumoniae and Staphylococcus sciuri, were detected in a few, but not all, exposed roots. E. saccharolyticus and P. pneumotropica infections in the exposed pulp were further evaluated by the serum IgG response to these two bacteria (Fig. 3A and 3B). Interestingly, serum IgG antibody to P. pneumotropica was significantly elevated at 14 days post-pulp exposure compared to that of control baseline level (Fig. 3B), whereas serum IgG antibody to E. saccharolyticus showed no significant difference, either before or after pulp exposure (Fig. 3A), suggesting that P. pneumotropica invading the root canal was more actively recognized by adaptive immune response than E. saccharolyticus. Furthermore, mice immunized with P. pneumotropica or E. saccharolyticus were examined for their ability to induce T-cell-mediated adaptive immune response. The lymph node T-cells isolated from P. pneumotropica-immunized mice showed remarkable increase of T-cell proliferation in response to in vitro P. pneumotropica-antigen presentation by DCs (Fig. 3C), while E. saccharolyticus-immunized mice showed no induction of such in vitro T-cell response to E. saccharolyticus-antigen presentation (Fig. 3C). Finally, and most importantly, sRANKL production from these T-cells in response to respective bacterial antigen presentation was remarkably higher in P. pneumotropica-reactive T-cells than saccharolyticus-reactive T-cells (Fig. 3D), suggesting that the T-cell response is species specific and the T cells activation represented by their proliferation correlates to the amount of sRANKL produced by these bacterial antigen-reactive T cells.
Figure 3.
Orally colonizing bacteria induce RANKL-producing, bacteria-specific activated T lymphocytes. (A and B) Blood serum was collected on day-0 and day-14 after pulp exposure. (n=5/group). The serum IgG antibody response to E. saccharolyticus (A) or P. pneumotropica (B) was measured using ELISA, as described in Materials and Methods. Raw readout of ELISA measured at OD490 absorption is shown. Data indicate the mean ± SD of five different mice. * Significantly higher than day-0 mice sera IgG by Student’s t-test (P < 0.05). (C and D) The T-cells isolated from lymph nodes of mice immunized with E. saccharolyticus, P. pneumotropica, or control non-immunized mice, were cultured with or without heat-killed E. saccharolyticus or P. pneumotropica in the presence of MMC-treated dendritic cells for 3 days to measure the expressions of sRANKL, using ELISA, or for 4 days to assess the proliferation, using a [3H] thymidine incorporation assay. Proliferation of T lymphocytes (C) and production of sRANKL in culture supernatant are shown in histograms. Data indicate the mean ± SD of three cultures. # P < 0.05, ## P < 0.01, significantly higher than control non-immunized T-cells by Student’s t-test. * P < 0.05, ** P < 0.01, significantly higher than E. saccharolyticus-immunized T-cells with each bacterial antigen by Student’s t-test.
Discussion
In the present study, we revealed that CD3+ T lymphocytes produce RANKL in the periapical lesion with resulting pathogenic bone resorption. The infiltration of RANKL-expressing CD3+ T-cells in the periapical lesion peaked between day-3 and day-7, while such infiltration remitted to baseline level by day-14 after pulp exposure. The inflammatory cell infiltration in the lesion that occurred within the first 3 days after pulp exposure appears to be composed of innate immune cells (Fig. 1B), some of which, probably macrophages, are considered to present bacterial antigen to T cells. The FOXP3+ Treg cells that emerge in the lesion during the later stage (day-7 to day-14)(15) are considered to suppress RANKL expression by CD3+ T cells. Therefore, it is conceivable that CD3+/RANKL+ cells found in this study are adaptive immune T cells, not Treg cells, and that they are engaged in bone resorption by the production of RANKL in a manner similar to that reported in rheumatoid arthritis and periodontal disease (12, 19).
We previously reported that mucosal immune tolerance appeared to be elicited by P. pneumotropica that inhabits the mouse oral cavity (13). More specifically, immune tolerance was represented by low antibody response and minimal T-cell response to P. pneumotropica. However, P. pneumotropica that invaded exposed root canals induced IgG antibody response, suggesting that immune tolerance to P. pneumotropica was breached by the bacterial challenge from the root canal or periradicular tissue. However, the other endodontic bacterium found in this study, E. saccharolyticus, which was also isolated from exposed pulp, did not show such remarkable immune responses. Although, the nature and mechanism underlying the adaptive immune recognition and immune tolerance to oral bacteria remains to be elucidated, the different immune responses detected between E. saccharolyticus and P. pneumotropica, suggested that immune responses induced against endodotic bacteria are regulated in a manner specific to bacterial species..
We did not find human endodontic pathogens, such as Fusobacterium, Porphyromonas, Prevotella, or Streptococcus strains in the mouse (20–22). Nonetheless, the adaptive immune response to the endodontic bacterium P. pneumotropica was shown to result in RANKL production from bacteria-reactive T cells, which, in turn, contributed to osteoclast-mediated bone resorption. This line of evidence strongly suggests the pathogenic roles of the T-cell mediated immune responses elicited against endodontic bacteria and clearly supports the necessity of removing endodontic bacteria and bacterial components from an affected tooth during endodontic treatment.
Conclusion
Specific bacterial invasion into root canals activates adaptive immune response which results in the induction of RANKL-producing, bacteria-specific memory T-cells, and possibly contributing to osteoclast-mediated bone resorption in the periapical lesion.
Acknowledgement
This work was supported by grants DE-03420, DE-18499 and DE-19917 from the National Institute of Dental and Craniofacial Research.
References
- 1.Marton IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000;15(3):139–150. doi: 10.1034/j.1399-302x.2000.150301.x. [DOI] [PubMed] [Google Scholar]
- 2.Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9(4):498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 3.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: a quantitative immunohistochemical study. J Endod. 1996;22(6):311–316. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 4.Stashenko P, Yu SM. T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dent Res. 1989;68(5):830–834. doi: 10.1177/00220345890680051601. [DOI] [PubMed] [Google Scholar]
- 5.Ataoglu T, Ungor M, Serpek B, Haliloglu S, Ataoglu H, Ari H. Interleukin-1beta and tumour necrosis factor-alpha levels in periapical exudates. Int Endod J. 2002;35(2):181–185. doi: 10.1046/j.1365-2591.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 6.Radics T, Kiss C, Tar I, Marton IJ. Interleukin-6 and granulocyte-macrophage colony-stimulating factor in apical periodontitis: correlation with clinical and histologic findings of the involved teeth. Oral Microbiol Immunol. 2003;18(1):9–13. doi: 10.1034/j.1399-302x.2003.180102.x. [DOI] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 8.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima N, Suzuki N, Yang G, Ohi C, Okuhara S, Nakano-Kawanishi H, et al. Kinetics of RANKL, RANK and OPG expressions in experimentally induced rat periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(5):707–711. doi: 10.1016/j.tripleo.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Vernal R, Dezerega A, Dutzan N, Chaparro A, Leon R, Chandia S, et al. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006;12(3):283–289. doi: 10.1111/j.1601-0825.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 11.Kajiya M, Giro G, Taubman MA, Han X, Mayer MP, Kawai T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J Oral Microbiol. 2:5532. doi: 10.3402/jom.v2i0.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169(3):987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Paster BJ, Komatsuzawa H, Ernst CW, Goncalves RB, Sasaki H, et al. Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-kappaB ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol Immunol. 2007;22(3):208–215. doi: 10.1111/j.1399-302X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Peng B. Immunolocalization of receptor activator of NF kappa B ligand in rat periapical lesions. J Endod. 2005;31(8):574–577. doi: 10.1097/01.don.0000153839.74546.66. [DOI] [PubMed] [Google Scholar]
- 15.Alshwaimi E, Purcell P, Kawai T, Sasaki H, Oukka M, Campos-Neto A, et al. Regulatory T cells in mouse periapical lesions. J Endod. 2009;35(9):1229–1233. doi: 10.1016/j.joen.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner A, Maiden MF, Paster BJ, Dewhirst FE. The impact of 16S ribosomal RNA-based phylogeny on the taxonomy of oral bacteria. Periodontol 2000. 1994;5:26–51. doi: 10.1111/j.1600-0757.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 17.Rezende TM, Vieira LQ, Sobrinho AP, Oliveira RR, Taubman MA, Kawai T. The influence of mineral trioxide aggregate on adaptive immune responses to endodontic pathogens in mice. J Endod. 2008;34(9):1066–1071. doi: 10.1016/j.joen.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam KT, et al. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386(2):316–321. doi: 10.1016/j.bbrc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 20.Farber PA, Seltzer S. Endodontic microbiology. I. Etiology. J Endod. 1988;14(7):363–371. doi: 10.1016/S0099-2399(88)80200-0. [DOI] [PubMed] [Google Scholar]
- 21.Sundqvist G, Johansson E, Sjogren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15(1):13–19. doi: 10.1016/S0099-2399(89)80092-5. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Fukushima H, Yamamoto K, Ogawa K, Toda T, Sagawa H. Correlation between clinical symptoms and microorganisms isolated from root canals of teeth with periapical pathosis. J Endod. 1987;13(1):24–28. doi: 10.1016/S0099-2399(87)80088-2. [DOI] [PubMed] [Google Scholar]