Abstract
Background
Hippocampal volume reduction and declarative memory deficits are reported in humans and animals exposed to exogenous corticosteroids. The amygdala is another brain structure involved in the stress response that has important interactions with the hypothalamicpituitary-adrenal axis. To our knowledge, no studies in animals or humans have examined the impact of exogenous corticosteroid administration on the amygdala. We assessed amygdala volume in patients receiving chronic prescription corticosteroid therapy and control subjects with similar medical histories not receiving corticosteroids.
Methods
Fifteen patients on long-term prednisone therapy and 13 control subjects of similar age, gender, ethnicity, education, height, and medical history were assessed with magnetic resonance imaging. Amygdala volume was manually traced and compared between groups using a two-way analysis of variance (ANOVA). Correlations between amygdala volume, age, and corticosteroid dose/duration were assessed using Pearson’s correlation coefficient.
Results
Compared with control subjects, corticosteroid-treated patients had significantly smaller amygdala volumes. Right amygdala volume correlated significantly with age in control subjects and with duration of corticosteroid therapy in patients.
Conclusions
Patients receiving chronic corticosteroid therapy had smaller amygdala volumes than control subjects that correlated with duration of corticosteroid therapy. These findings suggest that corticosteroid exposure may be associated with changes in the amygdala as well as hippocampus.
Keywords: Amygdala, corticosteroid, magnetic resonance imaging, prednisone
Animal studies demonstrate reversible and irreversible hippocampal changes following corticosteroid exposure (1). Hippocampal atrophy is reported in Cushing’s ease (2). We previously reported on patients with asthma or rheumatic illnesses and control subjects with similar demographic characteristics and medical histories but minimal lifetime corticosteroid use (3). Compared control subjects, the corticosteroid-treated group had poorer declarative memory performance, smaller hippocampal volumes, lower levels of temporal lobe N-acetylaspartate (NAA, a putative marker of neuronal viability), and greater depressive symptom severity.
The amygdala is a brain structure involved in emotional pathways, particularly fear (4), that play an important stress response (5). Corticosteroids appear to act amygdala to regulate emotional learning and memory. In rats, corticosteroid-induced enhancement of fear conditioning is blocked by the administration of atenolol in the amygdala (6). In humans, higher cortisol levels while viewing emotionally arousing pictures were associated with greater amygdalar activation on functional magnetic resonance imaging (MRI) (7).
The amygdala has facilitatory effects on the hypothalamic-pituitary-adrenal (HPA) axis (8) that are sensitive to negative feedback from corticosteroids (9). Corticosterone increases corticotropin-releasing hormone (CRH) messenger RNA (mRNA) in the amygdala (10). Electrical stimulation of the amygdala increases corticosterone release (11), while electrical stimulation of the hippocampus decreases corticosterone release (12). Simalarly, amygdala lesions decrease corticosterone release (13) but hippocampal lesions increase corticosterone release (14), although neither hippocampal nor amygdalar lesions appear to alter HPA axis responsiveness in rhesus macaques (15). Animal models of stress are associated with an increase in dendritic growth and dendritic spine formation in the amygdala, in contrast to the inhibitory effects of stress on dendritic formation in the hippocampus (16). To our knowledge, the effects of exogenous corticosteroid administration on the amygdala have not been explored in animal models.
In humans, cortisol levels are related to the degree of amygdala activation during functional imaging (17). Merke et al. (18) reported that children with adrenal hyperplasia, and in many cases postnatal corticosteroid therapy, had significantly smaller amygdala volumes than matched control subjects. Merke et al. (19) also reported smaller amygdala volumes in children Cushing’s disease than in matched control subjects. To our knowledge, no studies have examined amygdala volume in adults exposed to corticosteroid excess. In this report, we examine amygdala volume in patients with asthma or rheumatic diseases receiving chronic corticosteroid therapy and in control subjects with similar medical histories but with minimal lifetime corticosteroid exposure.
Methods and Materials
A total of 15 right-handed outpatients with asthma or rheumatic diseases between 18 and 65 years of age receiving chronic prednisone therapy (≥10 mg/day for ≥6 months) and 13 right-handed control subjects with asthma or rheumatic diseases but minimal lifetime corticosteroid exposure (none in the prior 6 months, no course longer than 4 weeks, and <6 months lifetime total) were used in this analysis. Exclusion criteria included major psychiatric illness unrelated to corticosteroid use, substance abuse/dependence, illnesses with significant neurological manifestations, or contraindications to MRI. All participants signed a University of Texas (UT) Southwestern Institutional Review Board (IRB)-approved consent form. In addition to MRI scans, participants were assessed with the Hamilton Rating Scale for Depression (HRSD) (20), Young Mania Rating Scale (YMRS) (21), Brief Psychiatric Rating Scale (BPRS) (22), Rey Verbal Learning Test (RAVLT) (23), Stroop Color Word Test (24), and Trail Making Test A and B (25). Detailed information about the study procedures; patient characteristic; mood, cognitive, and other imaging findings (hippocampal volume, temporal lobe spectroscopy) can be found in the previous report by Brown et al. (3).
Magnetic resonance imaging data were obtained using a GE Signa 1.5T MR scanner and PROBE PRESS sequence (GE Medical Systems, Milwaukee, Wisconsin). Magnetic resonance (MR) images were obtained in the paracoronal plane, orthogonal to the long axis of the hippocampus. T2-weighted fluid attenuated inversion recovery (FLAIR) (repetition time [TR] 10,000 msec/echo time [TE] 146 msec/inversion time [TI] 2200 msec, 256 × 224, 5 mm, 1 number of excitations [NEX], 200 field of view [FOV]) images were obtained for screening for disease processes. For volumetric analysis, parasagittal images were obtained using a three-dimensional (3-D) volume T1-weighted gradient echo sequence with 100 to 120 slice locations (TR/TE = 10/4 msec, 35° flip angle, FOV 200, in-plane matrix 256 × 224, 3 NEX) with an effective slice thickness of approximately 1.0 mm.
Amygdala volume measurement was performed with MED-X software (Sensor Systems, Inc., Sterling, Virginia) on a LINUX workstation (Red Hat Inc., Raleigh, North Carolina) in a blinded fashion by a staff member (D.J.W.) with experience in amygdala volume measurement. The 3-D volume MR acquisition was reformatted from 1.0 mm sagittal slices to 1.7 mm coronal slices using software supplied on the MR scanner. Amygdalae were traced manually from reformatted coronal slices (Figure 1). Volume was automatically calculated, summing the areas traced. Boundaries of amygdala were defined in Watson et al. (26) and summarized as follows: the anterior end of the amygdala was measured from the closure of the lateral sulcus to form the endorhinal sulcus. The entorhinal cortex inferior to the tentorial indentation was excluded from amygdaloid measurement. If the tentorial indentation was poorly defined or not visible, a line was drawn in direct continuation with the inferior and medial border of the amygdala within the substance of the temporal lobe. The inferior and lateral borders of the amygdala were formed by the inferior horn of the lateral ventricle or white matter. The superior border of the amygdala was defined by drawing a straight line laterally from the endorhinal sulcus to the fundus of the inferior portion of the circular sulcus of the insula. More posteriorly, the superior border of the amygdala was defined by drawing a straight line laterally from the superolateral aspect of the optic tract to the fundus of the inferior portion of the circular sulcus of the insula.
Figure 1.
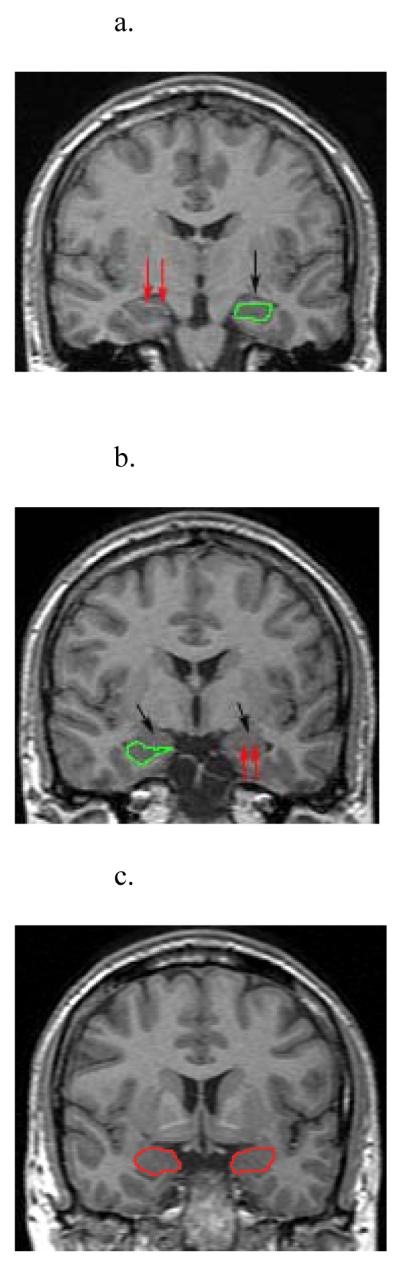
MRI slices from a study participant:. (1a) Hippocampal boundaries are shown in green. Red arrows indicate the alveus, the superior boundary of the hippocampus. Black arrow is shows the emergence of the amygdala. (1b) Hippocampus is again seen in green, with black arrows showing the amygdala, which abruptly increases in size, and abuts the hippocampus from above. (1c) Amygdala boundaries are traced in red.
Statistical Analyses
Independent t-tests for continuous measures or chi-square tests for discrete measures were used to examine participant characteristics. Actual amygdala volume (not adjusted for total brain volume) was analyzed using a two-way analysis of variance (ANOVA) examining the main factors of group membership and side of amygdala, using age as a covariate. Significance was set at p ≤ .05 for the analyses. Correlations between age, corticosteroid dose and time on corticosteroids, psychiatric symptoms, cognitive assessments, hippocampal volume, spectroscopic findings, and amygdala volume were explored using Pearson’s correlation coefficient. We also conducted a post hoc ANOVA, also controlling for age, in corticosteroid-treated patients with and without a history of a mood disorder secondary to corticosteroids.
Results
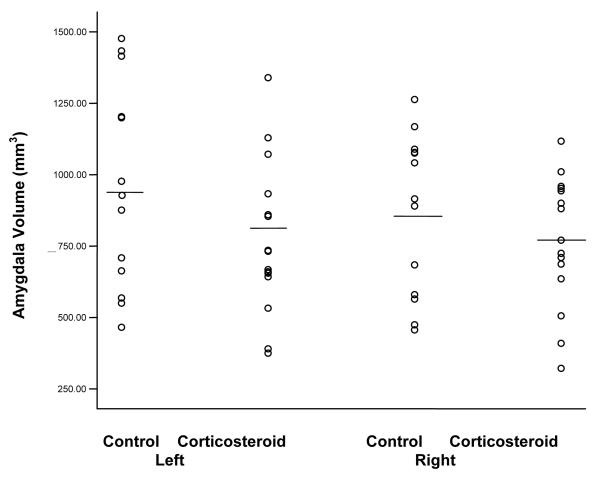
Groups did not differ significantly (p > .05) on age, ethnicity, height, gender, educational level, or nature of medical illness (Table 1). Concomitant medications were common but similar in the two groups and in many cases included bronchodilators in the patients with asthma, analgesics in the patients with rheumatic diseases, and in some cases immunosuppressants. Detailed information on concomitant medications can be found in Brown et al. (3). Amygdala volume of corticosteroid-treated patients (n = 15) was approximately 20% smaller on the left (771.8 ± 266.9 mm3 vs. 960.4 ± 358.2 mm3) and 11% smaller on the right (768.5 ± 229.5 mm3 vs. 867.8 ± 281.1 mm3) than control subjects (n = 13), resulting in a significant between-group effect for volume (F = 4.49, df = 1,52, p = .039) (Figure 2). A significant between-side effect of amygdala volume observed (F = .44, df = 1,52, p = .512).
Table 1.
Characteristics of Corticosteroid-Treated Patients and Control Subjects
| Characteristic | Corticosteroid-Treated Patients (n = 15) |
Control Subjects (n = 13) |
|---|---|---|
| Age (years) | 47.3 ± 11.3 (range 27–63) | 48.5 ± 10.8 (range 30–64) |
| Gender | 14 women, 1 man | 12 women, 1 man |
| Height (cm) | 164.6 ± 9.5 | 165.0 ± 7.2 |
| Education (years) | 13.1 ± 1.6 | 13.6 ± 2.4 |
| Medical Illness | ||
| Asthma | 2 (13%) | 0 (0%) |
| Rheumatic disease | 11 (73%) | 11 (85%) |
| Both | 2 (13%) | 2 (15%) |
| Current Prednisone Dose (mg/day) | 15.1 ± 10.6 (range 7.5–40)a | NA |
| Length of Current Prednisone Therapy (months) | 89.4 ± 112.2 (range 7–360) | NA |
NA, not applicable.
Minimum dose for enrollment was 10 mg/day but one participant was assessed following a recent dose reduction to 7.5 mg/day after receiving 10 to 20 mg/day for 6 years.
Figure 2.
Left and right amygdala volumes in corticosteroid-treated patients and controls.
A total of 9 of 15 corticosteroid-treated participants had a current or past prednisone-induced mood disorder on structured clinical interview. Although amygdala volume was numerically large on the right (795.2 ± 244.2 mm3 vs. 738.0 ± 226.5 mm3) and left (838.5 ± 288.0 mm3 vs. 695.6 ± 288.8 mm3) in those with mood disorders secondary to corticosteroids, a significant between-group effect for volume was not observed (F = .807, df = 1, p = .387).
In control subjects, left and right amygdala volume correlated with each other (r = .864, p < .001). Age correlated significantly with right (r=−.573, p = .040) but not left (r=−.451, p = .122) amygdala volume. Young Mania Rating Scale scores showed a trend toward a significant correlation with right (r=−.511, p = .075) not but not left (r = −.437, p = .135) amygdala volume. Hamilton Rating Scale for Depression and BPRS scores did not correlate significantly with amygdala volume (r=−.453 to .270, p = .116 to .372). Rey Auditory Verbal Learning Test total scores showed a trend toward a significant correlation with left (r = −.508, p = .077) but not right (r=−.464, p = .110) amygdala volume. Hippocampal volume, NAA ratios, RAVLT delayed recall, Stroop Color Word Test, and Trails Making Test A and B did not correlate significantly with left or right amygdala volume (r=−.411 to .340, p = .163 to .941).
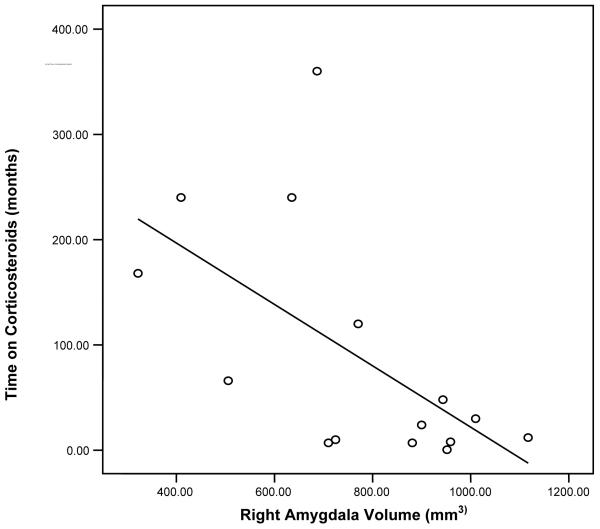
In the corticosteroid-treated patients, left and right amygdala volume correlated with each other (r = .587, p = .021). A significant correlation was also observed between length of corticosteroid therapy and right (r=−.597, p = .019) but not left (r = −.412, p = .127) amygdala volume (Figure 3). Current corticosteroid dose did not correlate significantly with right (r = −.077, p = .786) or left (r = .132, p = .639) amygdala volume. Age did not correlate significantly with length of corticosteroid therapy (r = −.196, p = .484). Right hippocampal volume correlated with right amygdala volume (r = .554, p = .032), but left hippocampal volume did not correlate significantly with left amygdala volume (r = .311, p = .260). Age, NAA ratios, and RAVLT, Stroop Color Word Test, Trails Making Test A and B, HRSD, YMRS, and BPRS scores did not correlate significantly with amygdala volume (r=−.395 to .132, p = .145 to .999) in the corticosteroid-treated group.
Figure 3.
Graph of right amygdala volume and time on corticosteroids (r=-0.597, p=0.019).
Discussion
We previously reported that patients receiving chronic corticosteroid therapy had smaller hippocampal volumes and NAA ratios, poorer performance on some cognitive domains, and greater depressive symptom severity than control subjects (3). In the current report, we found significantly smaller amygdala volumes in these same patients. The between-group difference in amygdala volumes (20% on the left and 11% on the right) was even greater than for hippocampal volume (8% on the left and 9% on the right).
In animals, stress is associated with an increase rather than decrease in amygdala dendrite formation (16). However, reports in children with Cushing’s disease (19) and in those receiving corticosteroid replacement for adrenal hyperplasia (18) suggest atrophy of the amygdala. To our knowledge, no prior reports have explored amygdala volume in either humans or animals exposed to exogenous corticosteroids. Our findings are consistent with the findings in children exposed to an excess of endogenous corticosteroids.
Reasons for the apparent difference in response of the human and animal amygdala to corticosteroids are not entirely clear. In contrast to the stress paradigms used in the animal studies, in the human studies, corticosteroids were elevated unrelated to emotional stress. Thus, chronic activation of glucocorticoid receptors on the amygdala, in the absence of emotional stress, may result in atrophy. Chronic stress, as in the animal studies, may result in amygdala hypertrophy through fear or anxiety. The mechanism by which amygdala volume is reduced with corticosteroid exposure is not clear. Hippocampal changes during stress or corticosteroid exposure in animals appear to be related to glutamate release (27,28). Although similar processes have not reported in the amygdala, modulation of sensory inputs (29) and extinction learning (30) appear to be regulated by interactions between corticosteroids and glutamate. The amygdalar atrophy could be related to water loss within the amygdala. Reductions in unsuppressed water peaks in the white matter have been reported with proton spectroscopy of patients with cerebral edema given corticosteroids (31). The smaller amygdala volume in the corticosteroid-treated patients is not clearly related to higher levels of depressive symptoms in this group. Hamilton Rating Scale for Depression scores did not correlate significantly with amygdala volume, and patients with a history of corticosteroid-induced mood disorders had nonsignificantly larger, not smaller, amygdala volumes than patients who received prednisone but did not report a history of mood disorders. Amygdala volume findings in depressed patients are mixed. Perhaps most pertinent to the current findings was a report by Frodl et al. (32) that amygdala volume was enlarged, relative to control subjects, in patients with first-episode depression but not with recurrent depression. The authors suggested that initial increase in amygdala volume may be due to increased metabolism and blood flow, but recurrent depression is associated with stress-related increases in glutamate. The report by Sheline et al. (33) of decreased amygdala core volume also on patients with recurrent depression. These findings and the association between length of corticosteroid therapy and amygdala volume are potentially consistent with progressive effects of corticosteroids on the amygdala.
The significant negative correlation between length of corticosteroid therapy, but not current corticosteroid dose, and right amygdala volume is potentially consistent with a process whereby corticosteroids over time result in amygdala atrophy. This interpretation of the data is strengthened by the lack of relationship between age and amygdala volume or age and length of corticosteroid therapy. This significant relationship between duration of corticosteroid therapy and amygdala volume is only observed on the right side. However, a moderate correlation coefficient (r > .4) is also observed on the left, suggesting that examination of a larger patient sample might reveal significant correlations in both amygdalae. Thus, the relationship between length of corticosteroid exposure and amygdala volumes does not appear to be due to older age in patients with longer corticosteroid exposure.
Patients were on other medications as described in Brown et al. (3). However, other medications were similar in each group. The only consistent medication difference between groups was corticosteroids. Severity of medical illness was not assessed in the groups. Participants receiving chronic corticosteroid therapy may have had more severe asthma and rheumatic illness than control subjects. However, to our knowledge, no data suggest that asthma or rheumatic diseases, or their severity, are associated with amygdala atrophy.
In summary, we found significantly smaller amygdala volumes in patients receiving long-term oral corticosteroid therapy than in similar control subjects not taking corticosteroids. In these patients, right amygdala volume was negatively related to length of corticosteroid therapy. The findings are consistent with research suggesting amygdala atrophy in children with Cushing’s disease and suggest that the amygdala, as well as hippocampus, is sensitive to corticosteroid effects.
Acknowledgments
Supported by National Institutes of Health Grant No. MH 01725 and the National Alliance for Research on Schizophrenia and Depression (NARSAD).
Dr. Brown reports receiving grant and research funding from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the Stanley Medical Research Institute, Forest Labs, Glaxo-Smith Kline, Astra-Zeneca, and UCB Pharma. He further discloses consulting and advisor fees from Forest Labs. Dr. Woolston declares receiving research funding from the Gulf War Research Project.
Footnotes
Dr. Frol declares having no potential conflict of interest related directly or indirectly to this work.
References
- 1.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 2.Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 3.Brown ES, Woolston DJ, Frol A, Bobadilla L, Khan DA, Hanczyc M, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 5.Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Feldman S, Conforti N, Itzik A, Weidenfeld J. The role of limbic structures in the modulation of ACTH responses following adrenalectomy. Ann N Y Acad Sci. 1995;771:73–81. doi: 10.1111/j.1749-6632.1995.tb44671.x. [DOI] [PubMed] [Google Scholar]
- 9.Weidenfeld J, Itzik A, Feldman S. Effect of glucocorticoids on the adrenocortical axis responses to electrical stimulation of the amygdala and the ventral noradrenergic bundle. Brain Res. 1997;754:187–194. doi: 10.1016/s0006-8993(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 10.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 11.Feldman S, Weidenfeld J. Glucocorticoid receptor antagonists in the hippocampus modify the negative feedback following neural stimuli. Brain Res. 1999;821:33–37. doi: 10.1016/s0006-8993(99)01054-9. [DOI] [PubMed] [Google Scholar]
- 12.Feldman S, Weidenfeld J. Electrical stimulation of the dorsal hippocampus caused a long lasting inhibition of ACTH and adrenocortical responses to photic stimuli in freely moving rats. Brain Res. 2001;911:22–26. doi: 10.1016/s0006-8993(01)02538-0. [DOI] [PubMed] [Google Scholar]
- 13.Feldman S, Conforti N, Weidenfeld J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci Biobehav Rev. 1995;19:235–240. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goursaud AP, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: Potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- 19.Merke DP, Giedd JN, Keil MF, Mehlinger SL, Wiggs EA, Holzer S, et al. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J Clin Endocrinol Metab. 2005;90:2531–2236. doi: 10.1210/jc.2004-2488. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young R, Biggs J, Meyer D. A rating scale for mania: Reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 22.Overall J, Gorham D. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 23.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40:785–787. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Spreen O, Strauss E. A Compendium of Neuropsychological Tests. 2nd ed Oxford University Press; New York: 1998. pp. 213–218. [Google Scholar]
- 25.Army Individual Test Battery . Manual of Directions and Scoring. War Department, Adjutant General’s Office; Washington, DC: 1944. [Google Scholar]
- 26.Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 28.Kole MH, Swan L, Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- 29.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: Dependency on corticosterone. J Neurosci. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang YL, Chao PK, Ro LS, Wo YY, Lu KT. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32:1042–1051. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
- 31.Chumas P, Condon B, Oluoch-Olunya D, Griffiths S, Hadley D, Teasdale G. Early changes in peritumorous oedema and contralateral white matter after dexamethasone: A study using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1997;62:590–595. doi: 10.1136/jnnp.62.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 33.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]