Abstract
The study of P transposable element repression in Drosophila melanogaster led to the discovery of the trans-silencing effect (TSE), a homology-dependent repression mechanism by which a P-transgene inserted in subtelomeric heterochromatin (Telomeric Associated Sequences) represses in trans, in the female germline, a homologous P-lacZ transgene inserted in euchromatin. TSE shows variegation in ovaries and displays a maternal effect as well as epigenetic transmission through meiosis. In addition, TSE is highly sensitive to mutations affecting heterochromatin components (including HP1) and the Piwi-interacting RNA silencing pathway (piRNA), a homology-dependent silencing mechanism that functions in the germline. TSE appears thus to involve the piRNA-based silencing proposed to play a major role in P repression. Under this hypothesis, TSE may also be established when homology between the telomeric and target loci involves sequences other than P elements, including sequences exogenous to the D. melanogaster genome. We have tested whether TSE can be induced via lacZ sequence homology. We generated a piggyBac-otu-lacZ transgene in which lacZ is under the control of the germline ovarian tumor promoter, resulting in strong expression in nurse cells and the oocyte. We show that all piggyBac-otu-lacZ transgene insertions are strongly repressed by maternally inherited telomeric P-lacZ transgenes. This repression shows variegation between egg chambers when it is incomplete and presents a maternal effect, two of the signatures of TSE. Finally, this repression is sensitive to mutations affecting aubergine, a key player of the piRNA pathway. These data show that TSE can occur when silencer and target loci share solely a sequence exogenous to the D. melanogaster genome. This functionally supports the hypothesis that TSE represents a general repression mechanism which can be co-opted by new transposable elements to regulate their activity after a transfer to the D. melanogaster genome.
Keywords: RNA silencing, epigenetics, germline, transposable elements, Drosophila
Transposable elements (TEs) are present in all organisms, and their activity can both induce severe deleterious effects by disrupting gene activity and create genetic novelties possibly useful from an evolutionary point of view (Wicker et al. 2007). Various mechanisms exist for repressing TE mobility, including autorepression by proteins encoded by TEs themselves and host defense mechanisms via DNA methylation, heterochromatin formation, and small RNA silencing (Cam et al. 2008; Girard and Hannon 2008; Slotkin and Martienssen 2007). In a given organism, these mechanisms can vary depending on the cellular context. For example in Drosophila melanogaster, TEs are regulated by different RNA silencing pathways in somatic and germline tissues (Dufourt et al. 2011; Hartig and Forstemann 2011; Li et al. 2009; Malone et al. 2009). In species that have been recently invaded by a particular family of TEs, it is possible to recover strains with or without these TEs. These strains are useful to study the mechanisms of repression since TEs containing strains can be crossed to control strains (devoid of the TEs) to genetically isolate and identify regulatory TE copies. D. melanogaster has been invaded in the last century by three families of TEs: the I factor, the hobo element, and the P element (Anxolabehere et al. 1988; Blackman et al. 1987; Blackman 1989; Chambeyron and Bucheton 2005; Engels 1989; Finnegan 1989; Rio 2002). The first one transposes via a RNA intermediate (Class I element), and the two others transpose via a DNA intermediate (Class II elements). These TEs can induce hybrid dysgenesis, a syndrome of genetic abnormalities (e.g., high mutation rate, chromosomal breakages, sterility) which occurs in the germline of progeny produced by crossing females lacking these elements and males carrying theses elements (Blackman et al. 1987; Kidwell et al. 1977; Picard et al. 1978).
The P element presents a maternally inherited repression termed “P cytotype” (Engels 1979). P cytotype shows epigenetic transmission through meiosis because memory of this maternal effect can be detected for more than five generations (Coen et al. 1994; Engels 1979). Genetic investigations to identify P copies responsible for the establishment of P cytotype allowed the discovery that P elements inserted at the telomere of the X chromosome have very strong repressive capacities (Ronsseray et al. 1991, 1996; Stuart et al. 2002) that show the complex rules of epigenetic transmission over generations typical of the P cytotype (Coen et al. 1994; Niemi et al. 2004; Ronsseray et al. 1991). These elements are inserted in subtelomeric heterochromatin (Ronsseray et al. 1996; Stuart et al. 2002), i.e., tandemly repeated noncoding sequences called “Telomeric Associated Sequences” (TAS) (Karpen and Spradling 1992). Repression elicited by these elements requires a certain length of common sequence between regulatory elements inserted in TAS and repressed elements located in euchromatin (Marin et al. 2000). It is sensitive to mutations affecting heterochromatin protein 1 (Ronsseray et al. 1996), a major component of heterochromatin, and to aubergine (Reiss et al. 2004; Simmons et al. 2007), a gene playing a major role in the small RNA-silencing pathway termed Piwi-interacting RNA (piRNA) silencing (Brennecke et al. 2007). Furthermore, P element−derived piRNAs have been found in ovaries of P strain females, which can be correlated to maternal effect of P cytotype (Brennecke et al. 2008).
The discovery of a transgenic system mimicking the P cytotype properties provided an important opportunity to analyze phenotypic, genetic, and molecular properties of P element repression established by telomeric P elements. A P-lacZ transgene carrying the Escherichia coli β-galactosidase gene in frame with sequence encoding the N terminal domain of the P element transposase that was inserted in the TAS of the X chromosome was shown to repress ovarian expression of second P-lacZ located on another chromosome: this phenomenon was termed trans-silencing effect (TSE) (Roche and Rio 1998; Ronsseray et al. 2003). TSE has become a key tool to study the underlying mechanism of P cytotype, allowing visualization of the distribution of repression in ovaries and even within ovarioles using simple histochemical X-gal staining (Ronsseray et al. 2003). Further studies showed that TSE (1) can be also established by P-transgenes inserted in the TAS of autosomal telomeres (Josse et al. 2008; Roche and Rio 1998); (2) is restricted to the germline (Josse et al. 2008); (3) shows variegation in ovaries when repression is incomplete (Josse et al. 2007); (4) has a maternal effect whose memory can persist for more than five generations (Josse et al. 2007); (5) involves both a chromosomally and a cytoplasmically transmitted factor (Josse et al. 2007); (6) is sensitive to mutants affecting HP1 and the piRNA pathway (Josse et al. 2007; Todeschini et al. 2010); and (7) is linked to maternal transmission of small RNAs derived from the telomeric transgenes (Todeschini et al. 2010), which were recently characterized as piRNAs (Muerdter et al. 2012).
TSE variegation results in a bimodal stochastic distribution of egg chamber staining, some showing very strong lacZ repression while others showing null repression. Intermediate staining is rarely observed. Inside a given egg chamber, the 15 nurse cells show, in most of the cases, identical on or off staining. It must be emphasized that TSE functions only in germline cells, the tissue in which P transposition is restricted (Laski et al. 1986), and does not function in ovarian somatic follicle cells (Josse et al. 2008). TSE therefore likely involves a germline-specific piRNA repression pathway. Because the piRNA-silencing pathway has been shown to affect a large number of different families of TEs, one of the remaining questions is whether TSE is specific to P element sequences or, on the contrary, whether TSE can be obtained between a telomeric and an euchromatic locus sharing sequence homology other than P element sequences.
We have functionally tested the latter possibility by constructing a transgene in which the lacZ sequence is carried by a TE different from the P element, that is, piggyBac (Cary et al. 1989; Fraser et al. 1995), which shares no sequence similarity with the P element. LacZ expression was placed under the control of a female germline promoter (ovarian tumor). D. melanogaster embryos were transformed by the piggyBac-otu-lacZ transgene, allowing recovery of several insertions strongly expressed in nurse cells and the oocyte, the two populations of germline cells of ovaries. In this article, we report that telomeric P-transgenes carrying lacZ strongly repress, in the germline, expression of a lacZ gene carried by piggyBac transgene insertions. This repression presents the genetic and phenotypic properties of TSE and is sensitive to aubergine mutants. Trans-silencing can therefore be established via solely lacZ homology. This shows that TSE can be established for sequences exogenous to the D. melanogaster genome. In addition, this strongly reinforces the hypothesis that TSE represents a typical homology-dependent piRNA repression mechanism in the germline and that its complex trans-generational epigenetic properties therefore reflect those of a piRNA pathway functioning in germline cells of ovaries.
MATERIALS AND METHODS
Establishment of the transgenic lines
The piggyBac-otu-lacZ transgene plasmid was generated by extracting two fragments, one containing the white gene and the second containing the lacZ gene under the control of the ovarian tumor (otu) gene promoter, from the PCO plasmids described in Boivin et al. 2003. These two fragments were cloned between the HindIII and EcoRI sites of pXL-BacII (Li et al. 2001; 2005). The transgene is 9815 bp long and is shown in Figure 1A. Transgenic lines were obtained by microinjection in the w1118 strain (devoid of P elements) performed by the BestGene company. New insertions were further produced by remobilization of a primary insertion using the jumpstarter element encoding piggyBac transposase (Horn et al. 2003).
Figure 1 .
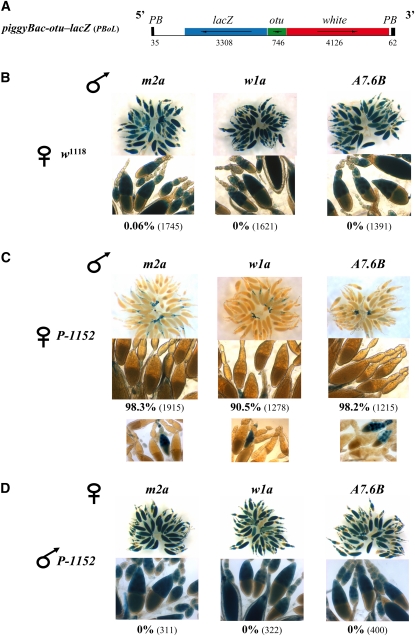
A telomeric P-lacZ strongly represses a PBoL transgene in the germline with a maternal effect. (A) Schematic representation of a piggyBac-otu-lacZ transgene named “PBoL”. LacZ expression is under the control of the otu promoter, and the white gene serves as a transformation marker. The length (bp) is indicated for the fragments constituting the transgene. piggyBac transformation vector sequences present at extremities of the transgene are in black: black boxes correspond to terminal regions of piggyBac (PB) and black lines to sequences required for germline transformation in Drosophila. (B-D) Three PBoL insertions (m2a, w1a, and A7.6B) were analyzed. Overnight lacZ staining of ovaries of G1 females produced by crossing males and females of the strains indicated above and to the left of the images are shown. The images were taken at lower (top) and greater (bottom) magnifications. In each case, the percentage of repressed egg chambers (at ovarian stages 9−10; see Materials and Methods) is given together with the total number of egg chambers counted between parenthesis. (B) The three PBoL insertions are strongly expressed in the germline (nurse cells and oocytes) due to the otu promoter. (C) These insertions are strongly repressed by a maternally inherited telomeric P-lacZ transgene (P-1152). Below the percentages, examples of variegating lacZ repression are shown. (D) PBoL insertions are not repressed by a paternally inherited P-1152 locus.
Characterization of the transgenic lines
The transgenic lines were named “PBoL,” for PiggyBac-based transgenes containing the lacZ gene under control of the otu gene promoter. They carry the mini-white gene as transformation marker. Three PBoL-carrying lines were analyzed: m2a, w1a, and A7.6B.
The m2a and w1a insertions are located on chromosome 2, and the A7.6B insertion is located on chromosome 3. The m2a and A7.6B insertions are homozygous viable, whereas w1a is lethal and maintained over a balancer chromosome (Cy).
Precise localization of PBoL insertions was performed using inverse polymerase chain reaction (PCR) and the following oligonucleotides as primers: i2PCR3′Pb- (GTTCCTTGTGTAGATGCATCTC), i2PCR5′Pb+ (GTCATTTTGACTCACGCGGTCG), i2PCR5′Pb− (CGACCGCGTGAGTCAAAATGAC), iPCR5′Pb+ (ACTGAGATGTCCTAAATGCACAGC), iPCR3′Pb− (GGATTTCACTGGAACTAGAATTCG), and iPCR5′Pb+ (ACTGAGATGTCCTAAATGCACAGC).
All lines apparently carry a single PBoL transgene insertion. The m2a insertion is located between the mir-8 and the Ugt37c1 genes at cytological site 53D. The w1a insertion is located in the Arc-p20 gene at cytological site 26B. The A7.6B insertion is located in the Alhambra gene at cytological site 84B.
P-element−derived transgenes and Drosophila lines
P-lacZ fusion enhancer trap transgenes:
P-1152, P-1155 are enhancer-trap transgenes and contain an in-frame translational fusion of the E. coli lacZ gene to the second exon of the P-transposase gene. They carry rosy+ as a transformation marker (P{lArB} transgene) (O’Kane and Gehring 1987). P-1152 (FBti0005700) comes from the stock previously known as #11152 in the Bloomington Stock Center and was mapped to the telomere of the X chromosome (site 1A); this stock carries two P-lacZ insertions in the same TAS unit and in the same orientation (Josse et al. 2007). P-1155 (FBti0005691) comes from the stock previously known as #11155 of the Bloomington Stock Center. It contains a single P-lacZ insertion in TAS at the 3R chromosome arm telomere (site 100F). P-1152 and P-1155 are homozygous viable and fertile. P-1152 shows no lacZ expression in the ovary, whereas P-1155 shows weak and nonuniform lacZ staining in follicle cells but no staining in the germline. The T-1 line carries a cluster of P-lacZ-white elements (P{lacW} transgene) located at cytological site 50C on the second chromosome (Dorer and Henikoff 1994, 1997). The cluster contains seven transgene copies, including a defective copy, all inserted in direct orientation. In addition, the T-1 line has complex chromosomal rearrangements, including translocations between the second and the third chromosomes due to X-ray treatment. After overnight staining, weak lacZ expression is detected in follicle cells of T-1 ovaries, presumably because of a position effect at 50C, but no staining is observed in the germline. P-1152, P-1155, and T-1 have a strong capacity to induce TSE which is maternally inherited (Josse et al. 2008; Roche and Rio 1998; Ronsseray et al. 2001).
Three strong mutant alleles of aubergine induced by EMS were used. All of them are homozygous female sterile. aubQC42 (Schupbach and Wieschaus 1991) comes from the Bloomington Stock Center (stock #4968) and has not been characterized at the molecular level. aubHN2 (Schupbach and Wieschaus 1991) has an amino acid substitution. aubN11 (Wilson et al. 1996) has a 154-bp deletion, resulting in a frameshift that is predicted to add 16 novel amino acids after residue 740 (Harris and MacDonald 2001).
All stocks used carrying transgenic insertions have a M genetic background (devoid of P transposable elements), as do the multimarked balancer stocks and those carrying aubergine mutations. The Cantony and w1118 lines were used as control lines, and these are completely devoid of any P element or P element−derived transgene (M lines) and of any piggyBac-derived transgene.
Experimental conditions
All crosses were performed at 25° and involved three to five couples in most of the cases. Ovary lacZ expression assays were performed using X-gal overnight staining as described in Josse et al. 2007, except experiments involving aubergine mutants for which 24-hr staining was conducted because weaker lacZ expression required these conditions to facilitate scoring of TSE.
Quantification of TSE
When TSE is incomplete, variegation is observed because “on/off” lacZ expression occurs between egg chambers (Josse et al. 2007). TSE was quantified by determining the percentage of egg chambers with no expression among ovarian stages 9-10 because lacZ expression of PBoL insertions was intense and reproducible at these stages.
RESULTS
Production of PBoL transgenic lines
A transgene was designed to test whether “non P-element” homology between a telomeric transgene and a target euchromatic transgene allows trans-silencing to take place. More precisely, we asked whether a telomeric P-lacZ transgene can repress, in trans, a transgene carrying the lacZ sequence in a TE other than the P element. The piggyBac-based transgenic system was used. The Trichoplusia ni piggyBac element is absent from the Drosophila melanogaster genome and shows no significant sequence similarity with the P element, as tested by BLAST analysis (data not shown). Because TSE is restricted to the female germline, expression of the lacZ gene in piggyBac was placed under the control of the germline-specific promoter of the otu (Figure 1A). After transformation of embryos and remobilization, six transgenic lines were recovered (named PBoL). All insertions but one showed β-galactosidase expression restricted to germline cells of the ovary. However, lacZ expression levels varied from one PBoL insertion to another, likely because of position effects. The three lines showing the strongest lacZ expression were selected for further analysis. Details about these lines are given in Materials and Methods. These lines are called m2a and w1a (chromosome 2) and A7.6B (chromosome 3). For all PBoL insertions, lacZ expression in ovaries was assayed in two different genetic backgrounds (Cantony and w1118) to take into account possible background effects on transgene expression. No significant difference was observed between the two backgrounds (data not shown). The m2a, w1a, and A7.6B insertions produced strong lacZ expression in nurse cells, especially at late stages of oogenesis and in mature oocytes (Figure 1B). Scoring egg chambers at stages 9-10 allowed detection of lacZ expression in all (but one) egg chamber among more than 4700 egg chambers assayed for the three PBoL insertions tested (Figure 1B).
LacZ homology between a telomeric and an euchromatic locus allows trans-silencing to take place in germline cells of the ovary
P-lacZ insertions located in subtelomeric heterochromatin (TAS) of the X chromosome induce strong repression of any P-lacZ transgene inserted in euchromatin expressed in the female germline (Josse et al. 2008; Roche and Rio 1998; Ronsseray et al. 2001). In addition, incomplete repression results in variegation for X-gal staining from one egg chamber to another (Josse et al. 2007). This repression shows a strong maternal effect because strong repression is observed only when the telomeric locus is maternally inherited (Josse et al. 2008; Ronsseray et al. 2001). For example, crossing P-1152 females with males carrying an euchromatic P-lacZ transgene resulted in G1 females showing 80% to 95% of egg chambers with repressed lacZ expression, whereas the reciprocal cross resulted in only 15% to 30% repression in G1 females (Josse et al. 2007, 2008). When P-1152 females were crossed with males carrying any one of the three PBoL insertions tested, G1 females showed strong lacZ silencing in all cases (Figure 1C: 98% for m2a and A7.6B ; 90% for w1a). In addition, in each case, incomplete repression resulted in variegating lacZ expression characterized by on/off egg chamber lacZ expression (Figure 1C). Finally, the reciprocal cross was performed, and no repression was detected with any of the three PBoL insertions tested (Figure 1D). Therefore, lacZ homology allows trans-silencing to take place in the female germline and repression shows phenotypic and genetic properties of TSE.
PBoL repression by autosomal silencers
Previous studies of TSE allowed the identification of several silencers located on autosomes (Josse et al. 2008; Roche and Rio 1998). First, P-lacZ transgenes inserted in subtelomeric heterochromatin of chromosomes 2 and 3 were found to be able to establish strong repression of a P-lacZ target transgene (Josse et al. 2008). This repression is also maternally inherited and shows variegation: for example, the P-1155 telomeric P-lacZ transgene, located in the TAS of the 3R chromosomal arm, was shown to repress a P-lacZ transgene located in euchromatin of chromosome 3 (named P-Co1). This repression is however weaker (TSE = 65%) than that induced by X chromosome telomeric insertion P-1152 [TSE = 88% (Josse et al. 2008)]. Second, complete trans-silencing of P-lacZ was found to be induced by the T-1 line (Ronsseray et al. 2001), which carries a cluster of P-lacZ transgenes (Dorer and Henikoff 1994, 1997) inducing local heterochromatin formation (Fanti et al. 1998) and which has complex chromosomal rearrangements induced by X-rays. Again trans-silencing was maternally inherited (Ronsseray et al. 2001).
The capacity of these two silencer loci to repress PBoL insertions was tested. The P-1155 telomeric transgene induced repression of the three PBoL insertions tested (Table 1). P-1155-mediated repression is weaker than that obtained for P-1152 (Figure 1) with m2a (56% vs. 98%) and w1a (81% vs. 90%), whereas strong repression was observed for both P-1155 and P-1152 with A7.6B (93% and 98%). Table 1 also shows that T-1 induced complete silencing of the three PBoL tested. Note that in this case, repression can result from both lacZ and white homology. Autosomal P-lacZ silencers can thus strongly repress PBoL transgenes. In addition, a maternal effect was found for both the P-1155 and T-1 autosomal silencers because no repression was observed in the progeny of reciprocal crosses (Table 1).
Table 1 . PBoL transgenes are repressed by autosomal TSE silencers.
| Line name | m2a | w1a | A7.6B |
|---|---|---|---|
| Cantony | |||
| ♀ \♂ | 0% (1344) | 0% (1077) | 0% (1457) |
| ♂\♀ | 0% (420) | 0% (429) | 0% (368) |
| P-1155 | |||
| ♀\ ♂ | 56.3% (448) | 81.2% (399) | 93.2% (382) |
| ♂\♀ | 0% (409) | 0.3% (344) | 0% (305) |
| T-1 | |||
| ♀ \♂ | 100% (769) | 100% (465) | 100% (544) |
| ♂\♀ | 0% (332) | 0% (303) | 0% (353) |
Reciprocal crosses were performed between individuals indicated in column 1 and in line 1. TSE was measured in G1 females. In each case, the first line (♀\ ♂) gives the percentage of TSE (total number of egg chambers scored in parenthesis) in progeny produced by crossing females indicated in column 1 with males indicated in line 1. The second line (♂ \♀) gives the percentage of TSE observed in progeny of the reciprocal cross (total number of egg chambers scored in parenthesis). P-1155 carries a P-lacZ-rosy transgene, similar to P-1152, located at the telomere of the 3R chromosomal arm. The T-1 line carries a tandem array of seven P-lacZ-white transgenes located in the middle of the 2R chromosomal arm. The Cantony reference line is devoid of any P-transgene or P-element. PBol, PiggyBac-based transgenes containing the lacZ gene under control of the otu gene promoter; TSE, trans-silencing effect.
Further, we tested whether single transgenes located in euchromatin and heterochromatin (pericentromeric heterochromatin and fourth chromosome) can repress PBoL insertions. Indeed, previously such transgenes were shown to be unable to repress a P-lacZ transgene expressed in the female germline (Josse et al. 2008). Similarly, of five euchromatic P-lacZ insertions, and three pericentromeric chromosome insertions tested, located on chromosomes X, 2 and 3, none repressed m2a, w1a or A7.6B (Table S1). In addition, a P-lacZ transgene located on the heterochromatic fourth chromosome, previously shown to be unable to repress a P-lacZ transgene (Josse et al. 2008), did not repress the PBoL insertions. Therefore, P-lacZ and PBoL target transgenes respond in the same way (repressed or not repressed) to all silencer/nonsilencer loci tested.
A telomeric P-lacZ locus can repress two PBoL transgenes inserted on different chromosomes
A single telomeric P-1152 locus was previously shown to be able to strongly repress two P-lacZ targets located at allelic or nonallelic positions (Josse et al. 2008). We tested whether a single P-1152 locus can similarly repress two PBoL insertions. Females having maternally inherited P-1152 and carrying two PBoL insertions presented more than 80% of repressed egg chambers (Table 2). This result was obtained for flies homozygous for the m2a insertion or for flies carrying two nonallelic PBoL insertions located on the same or on different chromosomes (m2a and w1a located on chromosome 2 and A7.6B located on chromosome 3). Therefore, a single P-1152 locus can repress simultaneously two PBoL insertions located at allelic or nonallelic positions. In addition, we found that a single maternally inherited P-1152 locus established strong lacZ repression in females carrying both a hemizygous P-lacZ insertion located on chromosome 3 [BQ16 (Josse et al. 2007)] and a hemizygous PBoL insertion (m2a; 93.8% of TSE, n = 241).
Table 2 . A telomeric P-lacZ locus can repress two PBoL transgenes inserted at allelic or nonallelic positions.
| Row | Parental Cross | Genotype of G1 Females Analyzed | % TSE | n |
|---|---|---|---|---|
| 1 | ♀ m2a x ♂ m2a | + / + ; m2a / m2a ; + / + | 0.0 | 381 |
| 2 | ♀ m2a x ♂ w1a | + / + ; m2a / w1a ; + / + | 0.0 | 844 |
| 3 | ♀ m2a x ♂ A7.6B | + / + ; m2a / + ; + / A7.6B | 0.0 | 316 |
| 4 | ♀ P-1152 ; m2a x ♂ P-1152 ; m2a | P-1152 / P-1152 ; m2a / m2a ; + / + | 95.8 | 406 |
| 5 | ♀ P-1152 ; m2a x ♂ w1118 | P-1152 / + ; m2a / + ; + / + | 84.2 | 310 |
| 6 | ♀ P-1152 ; m2a x ♂ m2a | P-1152 / + ; m2a / m2a ; + / + | 87.3 | 512 |
| 7 | ♀ P-1152 ; m2a x ♂ w1a | P-1152 / + ; m2a / w1a ; + / + | 90.2 | 877 |
| 8 | ♀ P-1152 ; m2a x ♂ A7.6B | P-1152 / + ; m2a / + ; + / A7.6B | 97.4 | 381 |
The parental crosses shown in column 2 were performed in order to generate G1 females whose genotype is given in column 3. In each case, G0 females carrying P-1152 were homozygous for this locus. LacZ staining of G1 female ovaries was performed and TSE was measured. Columns 4 and 5 give the TSE percentage and the total number of egg chambers counted, respectively.
LacZ homology−dependent silencing is sensitive to mutations affecting the piRNA pathway gene aubergine
TSE has been shown to be highly sensitive to mutations affecting the piRNA silencing pathway (Josse et al. 2007; Todeschini et al. 2010). Repression of a PBoL target transgene by a P-lacZ telomeric locus was tested in aubergine mutant contexts (Table 3). aubergine is one of the main actors involved in the piRNA amplification mechanism termed “ping-pong” which functions in the germline (Brennecke et al. 2007; Li et al. 2009; Malone et al. 2009). TSE between P-lacZ transgenes was found previously to be null in aubergine heteroallelic mutant contexts (Josse et al. 2007). Similarly, P-1152 repression of the PBoL A7.6B insertion was almost completely (2.6%) or completely abolished in the two aubergine heteroallelic mutant contexts tested (Table 3). Therefore lacZ homology-based trans-silencing is dependent on aubergine.
Table 3 . PBoL trans-silencing is sensitive to aubergine mutations.
| ♀ | P-1152aubQC42 + | ♀ | P-1152aubHN2 + | |
|---|---|---|---|---|
| P-1152 Cy + | P-1152 Cy + | |||
| P-1152aub* + | P-1152aub* + | |||
| + Cy A7.6B | + Cy A7.6B | |||
| ♂ + aubN11A7.6B | 74.8% (1483) | 60.2% (1255) | ||
| ¬ Cy A7.6B | P-1152aubQC42 + | P-1152aubHN2 + | ||
| + aubN11 A7.6B | + aubN11 A7.6B | |||
| 2.3% (1049) | 0.0% (524) |
Crosses between females (first line) and males (first column) of the indicated genotypes were performed. LacZ staining was carried out on ovaries of G1 females whose genotype is shown inside the table. The percentage of TSE is given for each progeny with the total number of egg chambers scored indicated in parenthesis. aub* indicates either the maternal or paternal aub mutant allele, which cannot be discriminated. TSE, trans-silencing effect.
DISCUSSION
The genome of natural populations of Drosophila melanogaster has been invaded by three TE families during the last century: I, hobo, and P (Blackman 1989; Engels 1989; Finnegan 1989). For all three, a repression mechanism was established, and for two of them, P and I, repression has been shown to involve a maternal effect and complex epigenetic transmission over generations (Bucheton et al. 1976; Engels 1979; Picard et al. 1978). P and I repression involves regulatory copies of these TEs located on all chromosomes (Engels 1979; Picard et al. 1978), but some master regulatory sites corresponding to copies inserted in heterochromatin have been identified (Jensen et al. 2002; Picard 1978; Ronsseray et al. 1991). Both P and I repression mechanisms are sensitive to mutations affecting heterochromatin formation and RNA silencing (Bucheton et al. 2002; Chambeyron et al. 2008; Klenov et al. 2007; Reiss et al. 2004; Ronsseray et al. 1996). For P element repression (P cytotype), the existence of a maternally transmitted cytoplasmic component (pre-P cytotype), coupled with chromosomally inherited P copies, was shown to be necessary to establish strong repression in the zygote (Niemi et al. 2004; Ronsseray et al. 1993). However, the maternally inherited component is not an autoreproducible component (Ronsseray et al. 1993; Sved 1987). Furthermore, upon discovery of the piRNA silencing pathway (Brennecke et al. 2007; Gunawardane et al. 2007) sequence analysis of piRNAs suggested that a high proportion of TEs are repressed in the gonads by this homology-dependent silencing mechanism. In particular, deep-sequencing of ovarian small RNAs allowed detection of piRNAs derived from I and P elements whose maternal transmission is correlated with repression of hybrid dysgenesis induced by massive transposition of these TEs (Brennecke et al. 2008). In the case of P, these maternally transmitted small RNAs very likely correspond to the pre-P cytotype.
All these data suggest a model in which the genome harbors several “traps” for invasive mobile DNA sequences which constitutively produce piRNAs and allow repression by a homology-dependent silencing mechanism. Some families (gypsy, ZAM and Idefix) are regulated by a heterochromatic locus called flam-COM located close to the centromere of the X chromosome (Desset et al. 2003; Mevel-Ninio et al. 2007; Pelisson et al. 1994; Prud’homme et al. 1995). This repression takes place in the somatic follicle cells of the ovary and, therefore, is mediated by a functionally different piRNA pathway (Desset et al. 2008; Malone et al. 2009; Pelisson et al. 2002). It is noteworthy that this regulation presents different genetic properties because gypsy repression, for example, does not exhibit a maternal effect nor trans-generational epigenetic transmission (A. Pélisson, personal communication). For the I factor, repression appears to involve homology-dependent silencing in the germline (Jensen et al. 1999, 2002; Malinsky et al. 2000; Robin et al. 2003) and major repressive loci appear to be located in pericentromeric heterochromatin (Jensen et al. 2002) and in the intercalary heterochromatin 42AB locus which contains numerous fragments of TEs (Brennecke et al. 2008). For the P element, Telomeric-Associated Sequences appear to be a major trap. Indeed, numerous P strains deriving from natural populations having various geographical origins have been found to carry P elements located at the telomere of the X chromosome (Ajioka and Eanes 1989; Ronsseray et al. 1989). Telomeric P elements inserted in TAS deriving from seven natural populations have been further isolated in a genomic background devoid of other P copies and were shown to have repressive capacities (Marin et al. 2000; Ronsseray et al. 1996, 1998; Stuart et al. 2002). The combination of a telomeric defective P element with various target P-transgenes showed that repression induced by the telomeric P-element is dependent on P-element homology between the telomeric and target loci (Marin et al. 2000; Roche and Rio 1998).
To validate and generalize this model, it remained, however, important to determine via a functional assay whether trans-silencing can be established via sequence homology other than that derived from the P element. In the present article, we show that the lacZ gene located inside a piggyBac-derived transgene is strongly repressed by a maternally inherited telomeric P-lacZ transgene, this repression exhibiting variegation, which is a typical phenotype of TSE. The parallel between P-lacZ and PBoL repression by a telomeric P-lacZ also includes the capacity to be repressed by autosomal silencers and sensitivity to aubergine mutations. Therefore, this functional assay indicates that repression by sequences inserted in TAS is not a P-element restricted property but rather a more general repression system that may function for other TEs inserted in TAS, including those recently introduced in the genome. Thus, TSE not only allows us to address the nature of P cytotype but also corresponds to a sensitive or appropriate tool to investigate phenotypic and genetic properties of a piRNA silencing pathway functioning in nurse cells.
Taking into account the epigenetic trans-generational effects of TSE and its variegating phenotype (Josse et al. 2007), predictive assumptions can be proposed for properties of a germline-specific piRNA pathway. For example, variegation between egg chambers inside ovaries resembles position effect variegation in the eye observed for genes located close to heterochromatin (Girton and Johansen 2008), and this suggests that target repression by piRNAs may involve heterochromatin formation. In addition, long-term memory of the maternal effect observed over generations with TSE (Josse et al. 2007) indicates that piRNA-based repression functioning in the germline undergoes amplification that can transcend a single fly generation to reach its maximum level. Note that P is not the only TE displaying long-term inheritance of its repressive properties because I factor regulation also shows such trans-generational effects (Bucheton 1979; Jensen et al. 1999; Picard et al. 1978). It will be interesting to investigate whether such a long-term trans-meiosis epigenetic inheritance exists for other piRNA producing loci in the genome.
Supplementary Material
ACKNOWLEDGMENTS
We thank Malcolm J. Fraser for providing piggyBac plasmids used to generate transgenes and Ernst A. Wimmer for providing piggyBac jumpstarter lines. We thank Paul Macdonald for providing aubergine mutant lines and Doug Dorer and Steve Henikoff for providing the T-1 line. We thank Alain Pélisson for personal communications. We thank the Bloomington Stock Center (http://flystocks.bio.indiana.edu//) and FlyBase (http://flybase.org/). We thank Benjamin Philippini, Corinne Zaffran-Maurel, and Katia Kaci for experimental help. We thank Valérie Ribeiro, Corinne Pappatico, and Paula Graça for technical assistance. We thank Anne-Marie Pret and Neel Randsholt for their help in the preparation of the manuscript. This work was supported by the Centre National de la Recherche Scientifique (Laboratoire Biologie du Développement–UMR 7622 and Institut Jacques Monod–UMR7592), by the Université Pierre & Marie Curie (Paris 6) and the Université Denis Diderot (Paris 7), by the programme ACI–BCMS, and by the Association pour la Recherche sur le Cancer. A.V was supported by a graduate student fellowship from the French government (Ministère de l’Enseignement Supérieur et de la Recherche) and by the Fondation pour la Recherche Médicale. A.L.T was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale.
Footnotes
Communicating editor: B. J. Andrews
LITERATURE CITED
- Ajioka J. W., Eanes W. F., 1989. The accumulation of P-elements on the tip of the X chromosome in populations of Drosophila melanogaster. Genet. Res. 53: 1–6 [DOI] [PubMed] [Google Scholar]
- Anxolabehere D., Kidwell M. G., Periquet G., 1988. Molecular characteristics of diverse populations are consistent with the hypothesis of a recent invasion of Drosophila melanogaster by mobile P elements. Mol. Biol. Evol. 5: 252–269 [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Grimaila R., Koehler M. M., Gelbart W. M., 1987. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell 49: 497–505 [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Gelbart W. M., 1989. The transposable hobo of Drosophila melanogaster, pp. 523–529 in Mobile DNA, edited by Berg D. E., Howe M. M. American Society for Microbiology, Washington, DC [Google Scholar]
- Boivin A., Gally C., Netter S., Anxolabehere D., Ronsseray S., 2003. Telomeric associated sequences of Drosophila recruit polycomb-group proteins in vivo and can induce pairing-sensitive repression. Genetics 164: 195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Aravin A. A., Stark A., Dus M., Kellis M., et al. , 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103 [DOI] [PubMed] [Google Scholar]
- Brennecke J., Malone C. D., Aravin A. A., Sachidanandam R., Stark A., et al. , 2008. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science 322: 1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton A., 1979. Non-Mendelian female sterility in Drosophila melanogaster: influence of aging and thermic treatments. III. Cumulative effects induced by these factors. Genetics 93: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton A., Lavige J. M., Picard G., L’Heritier P., 1976. Non-mendelian female sterility in Drosophila melanogaster: quantitative variations in the efficiency of inducer and reactive strains. Heredity 36: 305–314 [DOI] [PubMed] [Google Scholar]
- Bucheton A., Busseau I., Teninges D., 2002. I Elements in Drosophila melanogaster, pp. 523–525 in Mobile DNA II, edited by Craig N. L., Craigie R., Gellert , et al., American Society for Microbiology, Washington, DC [Google Scholar]
- Cam H. P., Noma K., Ebina H., Levin H. L., Grewal S. I., 2008. Host genome surveillance for retrotransposons by transposon-derived proteins. Nature 451: 431–436 [DOI] [PubMed] [Google Scholar]
- Cary L. C., Goebel M., Corsaro B. G., Wang H. G., Rosen E., et al. , 1989. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 172: 156–169 [DOI] [PubMed] [Google Scholar]
- Chambeyron S., Bucheton A., 2005. I elements in Drosophila: in vivo retrotransposition and regulation. Cytogenet. Genome Res. 110: 215–222 [DOI] [PubMed] [Google Scholar]
- Chambeyron S., Popkova A., Payen-Groschene G., Brun C., Laouini D., et al. , 2008. piRNA-mediated nuclear accumulation of retrotransposon transcripts in the Drosophila female germline. Proc. Natl. Acad. Sci. USA 105: 14964–14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D., Lemaitre B., Delattre M., Quesneville H., Ronsseray S., et al. , 1994. Drosophila P element: transposition, regulation and evolution. Genetica 93: 61–78 [DOI] [PubMed] [Google Scholar]
- Desset S., Meignin C., Dastugue B., Vaury C., 2003. COM, a heterochromatic locus governing the control of independent endogenous retroviruses from Drosophila melanogaster. Genetics 164: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desset S., Buchon N., Meignin C., Coiffet M., Vaury C., 2008. In Drosophila melanogaster the COM locus directs the somatic silencing of two retrotransposons through both piwi-dependent and -independent pathways. PLoS ONE 3: e1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer D. R., Henikoff S., 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993–1002 [DOI] [PubMed] [Google Scholar]
- Dorer D. R., Henikoff S., 1997. Transgene repeat arrays interact with distant heterochromatin and cause silencing in cis and trans. Genetics 147: 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourt J., Brasset E., Desset S., Pouchin P., Vaury C., 2011. Polycomb group-dependent, heterochromatin protein 1-independent, chromatin structures silence retrotransposons in somatic tissues outside ovaries. DNA Res. 18: 451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., 1979. Hybrid dysgenesis in Drosophila melanogaster: rules of inheritance of female sterility. Genet. Res. 33: 219–236 [DOI] [PubMed] [Google Scholar]
- Engels W. R., 1989. P elements in Drosophila, pp. 437–484 in Mobile DNA, edited by D. E. Berg and M. M. Howe. American Society for Microbiology, Washington, DC [Google Scholar]
- Fanti L., Dorer D. R., Berloco M., Henikoff S., Pimpinelli S., 1998. Heterochromatin protein 1 binds transgene arrays. Chromosoma 107: 286–292 [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., 1989. The I factor and I-R hybrid dysgenesis in Drosophila melanogaster, pp. 503–518, in Mobile DNA, edited by D. E. Berg and M. M. Howe. American Society for Microbiology, Washington, DC [DOI] [PubMed] [Google Scholar]
- Fraser M. J., Cary L., Boonvisudhi K., Wang H. G., 1995. Assay for movement of Lepidopteran transposon IFP2 in insect cells using a baculovirus genome as a target DNA. Virology 211: 397–407 [DOI] [PubMed] [Google Scholar]
- Girard A., Hannon G. J., 2008. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 18: 136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girton J. R., Johansen K. M., 2008. Chromatin structure and the regulation of gene expression: the lessons of PEV in Drosophila. Adv. Genet. 61: 1–43 [DOI] [PubMed] [Google Scholar]
- Gunawardane L. S., Saito K., Nishida K. M., Miyoshi K., Kawamura Y., et al. , 2007. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315: 1587–1590 [DOI] [PubMed] [Google Scholar]
- Harris A. N., Macdonald P. M., 2001. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development 128: 2823–2832 [DOI] [PubMed] [Google Scholar]
- Hartig J. V., Forstemann K., 2011. Loqs-PD and R2D2 define independent pathways for RISC generation in Drosophila. Nucleic Acids Res. 39: 3836–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C., Offen N., Nystedt S., Hacker U., Wimmer E. A., 2003. piggyBac-based insertional mutagenesis and enhancer detection as a tool for functional insect genomics. Genetics 163: 647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S., Gassama M. P., Heidmann T., 1999. Taming of transposable elements by homology-dependent gene silencing. Nat. Genet. 21: 209–212 [DOI] [PubMed] [Google Scholar]
- Jensen S., Gassama M. P., Dramard X., Heidmann T., 2002. Regulation of I-transposon activity in Drosophila: evidence for cosuppression of nonhomologous transgenes and possible role of ancestral I-related pericentromeric elements. Genetics 162: 1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse T., Teysset L., Todeschini A. L., Sidor C. M., Anxolabehere D., et al. , 2007. Telomeric trans-silencing: an epigenetic repression combining RNA silencing and heterochromatin formation. PLoS Genet. 3: 1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse T., Maurel-Zaffran C., de Vanssay A., Teysset L., Todeschini A. L., et al. , 2008. Telomeric trans-silencing in Drosophila melanogaster: tissue specificity, development and functional interactions between non-homologous telomeres. PLoS ONE 3: e3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen G. H., Spradling A. C., 1992. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics 132: 737–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A., 1977. Hybrid dysgenesis in Drosophila melanogaster: a syndrome of aberrant traits including mutation, sterility, and male recombination. Genetics 86: 813–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenov M. S., Lavrov S. A., Stolyarenko A. D., Ryazansky S. S., Aravin A. A., et al. , 2007. Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res. 35: 5430–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M., 1986. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell 44: 7–19 [DOI] [PubMed] [Google Scholar]
- Li X., Lobo N., Bauser C. A., Fraser M. J., Jr, 2001. The minimum internal and external sequence requirements for transposition of the eukaryotic transformation vector piggyBac. Mol. Genet. Genomics 266: 190–198 [DOI] [PubMed] [Google Scholar]
- Li X., Harrell R. A., Handler A. M., Beam T., Hennessy K., et al. , 2005. piggyBac internal sequences are necessary for efficient transformation of target genomes. Insect Mol. Biol. 14: 17–30 [DOI] [PubMed] [Google Scholar]
- Li C., Vagin V. V., Lee S., Xu J., Ma S., et al. , 2009. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky S., Bucheton A., Busseau I., 2000. New insights on homology-dependent silencing of I factor activity by transgenes containing ORF1 in Drosophila melanogaster. Genetics 156: 1147–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C. D., Brennecke J., Dus M., Stark A., McCombie W. R., et al. , 2009. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137: 522–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin L., Lehmann M., Nouaud D., Izaabel H., Anxolabehere D., et al. , 2000. P-Element repression in Drosophila melanogaster by a naturally occurring defective telomeric P copy. Genetics 155: 18411854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel-Ninio M., Pelisson A., Kinder J., Campos A. R., Bucheton A., 2007. The flamenco locus controls the gypsy and ZAM retroviruses and is required for Drosophila oogenesis. Genetics 175: 1615–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muerdter F., Olovnikov I., Molaro A., Rozhkov N. V., Czech B., et al. , 2012. Production of artificial piRNAs in flies and mice. RNA 18: 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi J. B., Raymond J. D., Patrek R., Simmons M. J., 2004. Establishment and maintenance of the P cytotype associated with telomeric P elements in Drosophila melanogaster. Genetics 166: 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane C. J., Gehring W. J., 1987. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA 84: 9123–9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisson A., Song S. U., Prud’homme N., Smith P. A., Bucheton A., et al. , 1994. Gypsy transposition correlates with the production of a retroviral envelope-like protein under the tissue-specific control of the Drosophila flamenco gene. EMBO J. 13: 4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisson A., Mejlumian L., Robert V., Terzian C., Bucheton A., 2002. Drosophila germline invasion by the endogenous retrovirus gypsy: involvement of the viral env gene. Insect Biochem. Mol. Biol. 32: 1249–1256 [DOI] [PubMed] [Google Scholar]
- Picard G., 1978. Non mendelian female sterility in Drosophila melanogaster: sterility in the daughter progeny of SF and RSF females. Biol. Cell. 31: 235–244 [Google Scholar]
- Picard G., Bregliano J. C., Bucheton A., Lavige J. M., Pelisson A., et al. , 1978. Non-mendelian female sterility and hybrid dysgenesis in Drosophila melanogaster. Genet. Res. 32: 275–287 [DOI] [PubMed] [Google Scholar]
- Prud’homme N., Gans M., Masson M., Terzian C., Bucheton A., 1995. Flamenco, a gene controlling the gypsy retrovirus of Drosophila melanogaster. Genetics 139: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D., Josse T., Anxolabehere D., Ronsseray S., 2004. aubergine mutations in Drosophila melanogaster impair P cytotype determination by telomeric P elements inserted in heterochromatin. Mol. Genet. Genomics 272: 336–343 [DOI] [PubMed] [Google Scholar]
- Rio D. C., 2002. in P transposable elements in Drosophila melanogaster, pp. 484–518 in Mobile DNA II, edited by Craig N. L., Craigie R., Gellert et al. , American Society for Microbiology, Washington, DC [Google Scholar]
- Robin S., Chambeyron S., Bucheton A., Busseau I., 2003. Gene silencing triggered by non-LTR retrotransposons in the female germline of Drosophila melanogaster. Genetics 164: 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S. E., Rio D. C., 1998. Trans-silencing by P elements inserted in subtelomeric heterochromatin involves the Drosophila Polycomb group gene, Enhancer of zeste. Genetics 149: 1839–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Lehmann M., Anxolabehere D., 1989. Copy number and distribution of P and I mobile elements in Drosophila melanogaster populations. Chromosoma 98: 207–214 [DOI] [PubMed] [Google Scholar]
- Ronsseray S., Lehmann M., Anxolabehere D., 1991. The maternally inherited regulation of P elements in Drosophila melanogaster can be elicited by two P copies at cytological site 1A on the X chromosome. Genetics 129: 501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Lemaitre B., Coen D., 1993. Maternal inheritance of P cytotype in Drosophila melanogaster: a “pre-P cytotype” is strictly extra-chromosomally transmitted. Mol. Gen. Genet. 241: 115–123 [DOI] [PubMed] [Google Scholar]
- Ronsseray S., Lehmann M., Nouaud D., Anxolabehere D., 1996. The regulatory properties of autonomous subtelomeric P elements are sensitive to a Suppressor of variegation in Drosophila melanogaster. Genetics 143: 1663–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Marin L., Lehmann M., Anxolabehere D., 1998. Repression of hybrid dysgenesis in Drosophila melanogaster by combinations of telomeric P-element reporters and naturally occurring P elements. Genetics 149: 1857–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Boivin A., Anxolabehere D., 2001. P-Element repression in Drosophila melanogaster by variegating clusters of P-lacZ-white transgenes. Genetics 159: 1631–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S., Josse T., Boivin A., Anxolabehere D., 2003. Telomeric transgenes and trans-silencing in Drosophila. Genetica 117: 327–335 [DOI] [PubMed] [Google Scholar]
- Schupbach T., Wieschaus E., 1991. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics 129: 1119–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. J., Ryzek D. F., Lamour C., Goodman J. W., Kummer N. E., et al. , 2007. Cytotype regulation by telomeric P elements in Drosophila melanogaster: evidence for involvement of an RNA interference gene. Genetics 176: 1945–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin R. K., Martienssen R., 2007. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 8: 272–285 [DOI] [PubMed] [Google Scholar]
- Stuart J. R., Haley K. J., Swedzinski D., Lockner S., Kocian P. E., et al. , 2002. Telomeric P elements associated with cytotype regulation of the P transposon family in Drosophila melanogaster. Genetics 162: 1641–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved J. A., 1987. Hybrid dysgenesis in Drosophila melanogaster: evidence from sterility and southern hybridization tests that P cytotype is not maintained in the absence of chromosomal P factors. Genetics 115: 121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todeschini A. L., Teysset L., Delmarre V., Ronsseray S., 2010. The epigenetic trans-silencing effect in Drosophila involves maternally-transmitted small RNAs whose production depends on the piRNA pathway and HP1. PLoS ONE 5: e11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T., Sabot F., Hua-Van A., Bennetzen J. L., Capy P., et al. , 2007. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 8: 973–982 [DOI] [PubMed] [Google Scholar]
- Wilson J. E., Connell J. E., Schlenker J. D., Macdonald P. M., 1996. Novel genetic screen for genes involved in posterior body patterning in Drosophila. Dev. Genet. 19: 199–209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.