Abstract
A linkage map of 40 linkage groups (LGs) was developed for brook charr, Salvelinus fontinalis, using an F2 interstrain hybrid progeny (n = 171) and 256 coding gene SNP developed specifically for brook charr and validated from a large (>1000) subset of putative SNP, as well as 81 microsatellite markers. To identify quantitative trait loci (QTL) related to reproduction functions, these fish were also phenotyped at six physiological traits, including spermatozoid head diameter, sperm concentration, plasma testosterone, plasma 11-keto-testosterone, egg diameter, and plasma 17β-estradiol. Five significant QTL were detected over four LGs for egg diameter and plasma 17β-estradiol concentration in females, and sperm concentration as well as spermatozoid head diameter in males. In females, two different QTLs located on LG 11 and LG 34 were associated with the egg number, whereas one QTL was associated with plasma 17β-estradiol concentration (LG 8). Their total percent variance explained (PVE) was 26.7% and 27.6%, respectively. In males, two QTL were also detected for the sperm concentration, and their PVE were estimated at 18.58% and 14.95%, respectively. The low QTL number, associated with the high PVE, suggests that the variance in these reproductive physiological traits was either under the control of one major gene or a small number of genes. The QTL associated with sperm concentration, plasma 17β-estradiol, and egg diameter appeared to be under a dominance effect, whereas the two others were under a negative additive effect. These results show that genes underlying the phenotypic variance of these traits are under different modes of action (additive vs. dominance) and may be used to predict an increase or a decrease in their phenotypic values in subsequent generations of selective breeding. Moreover, this newly developed panel of mapped SNP located in coding gene regions will be useful for screening wild populations, especially in the context of investigating the genetic impact of massive stocking of domestic brook charr to support the angling industry throughout eastern North America.
Keywords: linkage mapping, QTL detection, single nucleotide polymorphisms (SNP), reproduction, Salvelinus fontinalis
Linkages maps have numerous applications in evolutionary and comparative genomics (Gharbi et al. 2006, Jaari et al. 2009). Namely, one of the main applications of linkage maps relies on the mapping of quantitative trait loci (QTL), which allows localizing genomic regions responsible for the variations in continuous phenotypic traits of interest. To this end, the number and the variety of molecular markers developed for linkage-mapping have been associated with technological advances, namely new marker development such as microsatellites and single nucleotide polymorphisms (SNPs). Also, compared with previous methods based on cloning and Sanger sequencing, next-generation sequencing technologies now allow the more rapid development of in silico SNP markers (Hudson 2008). Yet, SNP marker development and in silico validation remain particularly challenging in polyploid species as the result of recent whole-genome duplication events, such as in salmonids (Seeb et al. 2011).
Salmonids are of considerable economic, social, and conservation value (Davidson et al. 2010). The salmonidae family contains 11 genera and 68 species, including salmon, trout, charr, freshwater whitefishes, ciscos, and graylings (for review, see Davidson et al. 2010) and has experienced a whole-genome duplication approximately 25 to 100 million years ago (e.g., Phillips and Rab 2001). More is known about the biology and genetics of salmonids than in any other groups of fish (Koop and Davidson 2008), which has led to the development of important genomic tools in recent years. For example, linkage maps have been successively developed in the Atlantic salmon Salmo salar to include up to 5650 markers (Danzmann et al. 2008; Moen et al. 2008; Lorenz et al. 2010, Lien et al. 2011). In rainbow trout (Onchorynchus mykiss), multiple generations of linkage maps have been developed using various types of molecular markers, including amplified fragment length polymorphisms (Young et al. 1998; Nichols et al. 2003), single-sequence repeats, or SSR (Rexroad et al. 2008), and SNP (Miller et al. 2011; Lien et al. 2011). Lower resolution linkage maps have also been developed in Arctic charr Salvelinus alpinus (Woram et al. 2004, Moghadam et al. 2007), coho salmon Oncorhynchus kisutch (McClelland and Naish 2008), brown trout Salmo trutta (Gharbi et al. 2006), lake whitefish Coregonus clupeaformis (Rogers et al. 2007), and recently, in brook charr, Salvelinus fontinalis (Timusk et al. 2011).
All these maps exhibit a large number of linkage groups, or LG (>28), because the diploid ancestor of salmonids possessed a karyotype with 48 acrocentric chromosomes (Phillips and Rab 2001) and a span of 390 to 5548 cM with a genome size estimated at 3.3 × 109 bp (Davidson et al. 2010). The investigation of the evolution of the structural genome within the salmonidae family, highlighting chromosomal rearrangements notably between Salvelinus and Onchorynchus species (Timusk et al. 2011). From the available linkage maps, recent QTL investigations were performed for phenotypic traits of interest for production such as hatching time (Robison et al. 2001, Nichols et al. 2007, Miller et al. 2011, Easton et al. 2011), spawning date (O’Malley et al. 2003), length (McClelland and Naish 2010), growth (Nichols et al. 2008; McClelland and Naish 2010; O’Malley et al. 2010; Wringe et al. 2010), early male maturation (Fotherby et al. 2007; Haidle et al. 2008), resistance to pathogens (Moen et al. 2009; for review, see Palti 2009 and Nichols et al. 2010), thermal tolerance (Perry et al. 2001), and swimming behavior (Rogers and Bernatchez 2007).
Brook charr, Salvelinus fontinalis, is one of the most economically important species for freshwater aquaculture in Canada, where it is farmed both for food production and enhancing the sport fishery industry (Page and Burr 1991). In Québec (Canada), approximately 1200 tons are produced annually, which represents approximately 55% of the total aquaculture production. In this context, the aims of the present study were (1) to develop a set of validated SNP markers located in coding genes using RNA-seq, and thus provide the first SNP markers for the genus Salvelinus; (2) to build a linkage map for this species combining these newly developed SNPs and previously published microsatellites; and (3) to identify QTL detection for reproductive physiological traits of interest for aquaculture production. We also compare our genetic map with that recently published by Timusk et al. (2011).
Material and Methods
Biological material and fish crosses
The QTL population was created using two different populations of brook charr. The first one (D) is a domestic population used in aquaculture for more than 100 years in Québec (Canada). The other (L) is an anadromous population originating from the Laval River near Forestville (North of the St. Lawrence River, Québec, Canada). Breeders from the L population were kept in captivity for three generations at the Station aquicole de l’ISMER (Québec, Canada, 48°31′N, 68°28′W) whereas breeders from the D population were obtained from the Pisciculture de la Jacques Cartier (Cap-Santé, Québec, Canada). In 2005, 10 sires of each population (L and D; F0 generation) were crossed with 10 dams (L and D) to generate 10 full-sib outbreed hybrid families (LD) crosses (F1 generation). Then, three F2 families were obtained from the biparental cross of six F1 individuals. The selected F2 family used in the present study for both phenotyping and genotyping was chosen according to its lowest mortality rate (<2%) compared with the other ones and its greater number of individuals.
Fish-rearing conditions
Fish from the F2 hybrid population hatched in January 2008 and were reared in indoor circular tanks in fresh water at the Station aquicole de l’ISMER (Québec, Canada, 48°31′N, 68°28′W), under natural photoperiod and temperature. The density was maintained < 35 kg of fish per m3. Fish were fed daily with commercial pellets with the percent weight per day adjusted according to fish age and water temperature (from 5.3% to 1.5% of body weight). They were individually identified with PIT-tags at the age of 5 months.
DNA library preparation and cDNA sequencing for SNP development
In September 2008, liver tissue was collected from 22 fish, including eight individuals from the freshwater Rupert River population (Québec, 48°44′N, 68°05′W), eight individuals from the Laval River population (Québec, 51°05′N, 73°41′W) (Audet and Bernatchez 2004), and the six parents used to create the three F2 families. Tissue samples were kept at −80° at all times and total RNA was extracted using the Purelink Micro to Midi total RNA purification system (Invitrogen, Inc., ON, Canada). Two micrograms of mRNA were used to perform a full-length cDNA amplification and normalization (Evrogen, Moscow, Russia). The cDNA was synthesized and amplified following the SMART approach (Zhu et al. 2001) and normalized using the duplex-specific nuclease method (Zhulidov et al. 2004). A total of 5 µg of double-stranded cDNA was sequenced (half plate) on a GS-FLX Titanium sequencer at the Genome Québec Innovation Center (McGill University, Montréal, QC, Canada).
Data assembly
Base calling was performed using PyroBayes software according to Quinlan et al. (2008). Primer tag sequences were removed, and sequence data were assembled into contiguous sequences using CLC Genomic Workbench v3.7 (CLC Bio, Denmark). Assembly parameters were set at 97% of similarity between two sequences showing an overlap of 0.6. Because of the duplicated genome of salmonids, such stringent assembly criteria were necessary to reduce probabilities of assembling paralogous genes (Renaut et al. 2010). Nonspecific reads (matching in different contigs) were discarded. Sequences that did not assemble into contigs were removed from further analyses to reduce the impact of sequencing errors. Raw pyroreads are publicly available under the accession number SRX037496 (http://www.ncbi.nlm.nih.gov/sra). Contig sequences, obtained from 454 data assembly, are provided in supporting information, Table S1.
SNP detection, validation, genotyping, and annotation
Assembled contigs were screened for SNPs using CLC Genomic Workbench v3.7 with the following criteria: minimum SNP coverage at 10× and minor allele frequency at 20%; the remaining parameters were left as default. To validate the markers in the mapping population, a large subset of putative SNP (∼1000) was selected from the whole set of putative SNP detected in the contigs according to the following parameters: (1) polymorphic sites had to be surrounded by a fragment of 60 bp to allow the design of polymerase chain reaction (PCR) primers and the genotyping assay; (2) polymorphic sites were not linked to other polymorphic loci in the contig (may reveal the assembly of paralogous sequences).
We then applied four steps to validate a subset (>300) of these 1000 putative markers. (1) A pair of PCR primers surrounding each SNP of interest was designed using Primer 3 software (Rozen and Skaletsky 2000) to generate an amplicon of 250 to 400 bp. PCR amplicons were then ran and visualized on a 1.5% agarose gel. Primer pairs that showed an amplicon size greater than 400 bp were removed to avoid the amplification of intronic regions in subsequent genotyping steps. (2) Amplicons meeting step 1 requirements were sequenced on the six F1 mapping parents in forward and reverse sense using the Big Dye Terminator v3 chemistry (Applied Biosystems, Foster City, CA) to confirm every polymorphic site. Chromatograms were inspected by eye and every monomorphic site was removed from subsequent analysis. (3) iPlex Gold assays for the Sequenom MassARRAY (Sequenom, San Diego, CA) genotyping technology were designed. Sites that did not respect the Sequenom assay the following technical requirements were removed: flanking sequences of 50 bp around the site of interest, no other polymorphic site within these flanking sequences, and a GC content greater than 40% (See File S2).
For each validated SNP, we searched for functional annotation and the nature of the polymorphism (transition vs. transversion, synonymous vs. nonsynonymous mutation). BlastX similarity searches were performed with the NCBI protein database (nr) using the consensus sequences of each contig containing a validated SNP. Only e-values lower than 1 × 10−40 were considered significant. Then, open reading frame (ORF) predictor (Min et al. 2005) was used to predict the most probable ORF for contigs that exhibited a significant Blast hit. Finally, SNPs were characterized as synonymous or nonsynonymous from the deduced ORFs. (For details, see File S3.) (4) Only SNP that met all requirements were multiplexed for genotyping the F1 parents and their F2 progeny using the iPlex Gold assays on the MassARRAY platform (Sequenom, San Diego, CA) according to the manufacturer’s instructions at the Genome Québec Innovation Center (McGill University, Montréal, QC, Canada).
DNA extraction and microsatellite genotyping in the F2 progeny
Genomic DNA was extracted from fin tissues (n = 191) of the selected F2 hybrid progeny using the QIAGEN DNeasy kit according to the manufacturer’s specifications (QIAGEN, Valencia, CA). DNA was quantified using the Quant-iT PicoGreen assay (Invitrogen, Inc., ON, Canada). For microsatellite genotyping, we first selected a set of 101 microsatellite markers that were available from the literature and previously used in the development of a linkage map in the brook charr (see Timusk et al. 2011). These markers cross-amplify in several salmonid species, including Salmo salar, Onchorynchus mykiss, Salvelinus alpinus, and Salmo trutta (Olsen et al. 1998; Sakamoto et al. 2000; Palti et al. 2002; Rexroad et al. 2003; McGowan et al. 2004; Rise et al. 2004; Coulibaly et al. 2005; Thorsen et al. 2005; Gharbi et al. 2006; Phillips et al. 2009). In addition, we included 25 additional microsatellites (Angers et al. 1995; O’Reilly et al. 1996; Scribner et al. 1996; Dehaan and Ardren 2005; Perry et al. 2005; see File S1 for details).
Only the informative markers were kept, that is, heterozygote for at least one of the two F1 parents. These were then multiplexed and amplified by PCR on a ABI 7900HT thermocycler (Applied Biosystems, Foster City, CA) according to the following cycling conditions: initial denaturation of 15 min at 95°, then 35 cycles of 30 sec of denaturation at 94°, 3 min of annealing at 60°, and 1 min of elongation at 72°, followed by a final extension step of 10 min at 72°. The reaction mixture was composed of 2 µL of gDNA (20−50 ng µL−1), 5 µL of Taq PCR Master Mix (QIAGEN, Inc., ON, Canada), and 0.2 µL of each primer (forward and reverse), for a final reaction volume of 8 µL. Forward PCR primers were FAM and HEX labeled with a fluorescent dye and multiplexed according to the size of the PCR products to avoid any overlap in the markers size ranges. PCR products from each sample were diluted 50-fold, and 1 µL of diluted PCR product was mixed with 0.25 µL of ROX GS500 size ladder (Applied Biosystems) and 10 µL of HiDi formamide. Samples were denatured at 95° for 3 min and kept on ice until loading on an ABI 3130 automatic sequencer (Applied Biosystems) according to the manufacturer’s recommendations. GeneMapper software V4.1 (Applied Biosystems) was used to analyze output data and collect microsatellite genotypes.
Sampling and phenotyping
F2 progeny (1+ year-old fish) were first collected in August (n = 91; 223.9 ± 5.5 g) and then in November 2009 (n = 100; 276.7 ± 6.4 g) on 24-hour−fasted fish. After capture, fish were immediately placed in an anesthetic solution (3-aminobenzoic acid ethyl ester, 0.16 g L-1) with constant aeration. They were identified using a pit-tag reader, measured (± 0.1 cm), and weighed (± 0.1 g). In November 2009, blood was sampled for each fish (0.3 mL) by caudal puncture using heparinized syringes. Plasma was obtained by centrifugation (5 min, 5000 rpm, 8500g), and then stored at −80° until analysis. Sperm and eggs were collected from sexually mature animals by stripping. At both sampling periods, adipose fin was cut on all anesthetized fish for genotyping of markers. Fins were individually stored in ethanol (> 95%) until DNA extraction. Anesthetized fish were killed by decapitation according to regulations of Canadian Council of Animal Protection recommendations and protocols approved by the University Animal Care Committee.
Seven phenotypic traits were measured in November 2009 for males and females sampled that were successfully genotyped (n = 86; as discussed in the section SNP detection, validation, genotyping, and functional characterization). Plasma sexual steroids (testosterone in males and females; 17β-estradiol in females; 11-ketotestosterone in males) were measured using commercial kits validated in fish [Testosterone 125I RIA kit (De Montgolfier et al. 2009) and 17β-estradiol 125I RIA kit Ria Kit (Davis et al. 2009), Immuchem Inc, Biomedicals, OH; 11-keto testosterone EIA kit (de Montgolfier et al. 2009), Cayman Chemical Compagny, MI]. The diameter of 10 eggs per female (38 females mature on a total of 40%–95%) was measured using a caliper (± 0.1 mm) under binocular microscope. Sperm from sexually mature males (42 sexually mature males on a total of 46, i.e., 91.3%) was diluted (1:2 × 105) in NaCl 0.9%. Sperm counts and mean spermatozoid head diameter (µm) were measured in triplicates for each male using a Z Series Coulter Counter Cell and Particle Counter (Beckman Coulter Canada Inc., ON, Canada).
Phenotype data analyses
Data normality was checked for each trait using a Shapiro-Wilk test. Phenotypic traits that were not normally distributed were Log10 transformed. The relationships among phenotypes were tested using a correlation matrix Spearman Rho test on both males and females data after removing of outliers (n = 6 and n = 4 for males and females, respectively) using the scatterplot box-plot function of the Statistica software (StatSoft Inc., Tulsa, OK). Statistical significance was considered when P < 0.05. All statistical tests were performed using [R] (v. 2.10.1; The R Foundation for Statistical Computing , 2009, 3-900051-07-0), unless specified otherwise.
Genetic linkage map construction
Before performing linkage mapping, deviation from Hardy-Weinberg Equilibrium (HWE) was tested for every marker using a Pearson’s χ2 test implemented in the SNPassoc [R] package (v.1.6.0) (Gonzalez et al. 2007). Markers that deviated from HWE equilibrium (P < 0.05) were removed from subsequent linkage analyses. Remaining SNP and microsatellite genotype data were formatted in the Crimap input format using a homemade python script (available upon request), and linkage between markers was evaluated using the ‘two points’ option implemented in Crimap. A decreasing LOD (Log10 of the odd ratio) score approach (from 6 to 3, step = 1) was used to reveal the most significant linkages (LOD = 6) to the weakest linkages (LOD = 3) and was used to group linked markers in LGs. This allowed mapping markers showing pseudo-linkage to different LG without any misassociation to another LG. Finally, markers that failed to be assigned with confidence to only one LG were not considered in further analysis. Chromosomal distances were evaluated according to the Kosambi formula (in cM) and estimated using the “build” option. Most probable order of marker inside each LG was estimated using the “flipsn” option. Double recombination events and genotyping errors were revealed using the “chrompic” option. Three linkage maps were built: two sex-specific maps and one consensus map combining information from both sexes. LGs were visualized using Mapchart (http://www.joinmap.nl). The linkage map was compared with the brook charr map recently published by Timusk et al. (2011) that comprises 37 LGs built with 139 microsatellites genotyped in three F2 families. Shared markers between both studies were used to assess homology between linkage groups. Maps were also then compared in terms of length, intermarker distance, and recombination differences between sexes.
QTL detection
QTL analysis was carried out using the [R] package R/qtl [v. 1.18-7, August 2010, http://www.rqtl.org/ (Broman et al. 2003)] on the sex-specific linkage maps. (1) A single QTL analysis was performed using the Haley-Knott regression method (10,000 permutations) to reveal which LGs were carrying QTL. The most probable position of the QTL was defined at the position giving the largest LOD score and this QTL was fixed. (2) A two QTL model based on the Haley-Knott regression was used to refine the QTL detection across the genome with a resolution of 5 cM and eventually to detect two QTL on a single LG linked to a particular trait. (3) The best model fitting our data was used to compute the percent variance explained (PVE expressed in %) by the QTL. The chromosome-wide and the genome-wide thresholds were calculated for each LG using 10,000 permutations. The 1.5 LOD confidence intervals were determined for all analyses following the Bayesian method implemented in the “bayesint” function in R/qtl. The bayesint function calculates an approximate interval (end points around the maximum LOD) for a given chromosome using the genome scan output. Allele effects were determined using the effect plot function in R/qtl with the QTL peak marker or marker nearest to the peak as the reference marker. Additive effects were estimated as half of the differences between two homozygous genotypic values whereas dominance effects were estimated as the deviation of the heterozygous from the average value between two homozygous genotypic values (Lynch and Walsh 1998).
Results
454 data assembly
A total of 388,395 pyroreads with a median length of 237 bp, totaling ∼123 Mb, were obtained from sequencing the cDNA library. A total of 61.2% of these reads were assembled into 6317 contigs of minimal, mean, and maximal sizes of 200, 423, and 2250 bp respectively, with N50 contig size estimated at 673 bp.
SNP detection, validation, genotyping, and functional characterization
A total of 4841 putative SNPs was detected in 1682 contigs (2.87 SNPs/contig) with an average, minimal, and maximal coverage of 18.2, 10, and 411×. Proportions of transitions were A/G – 27.9% and C/T – 28.2% and those of transversions were A/C – 12.9%, G/T –12.3%, A/T –, 12.1% and C/G – 6.6%. Among these 4841 putative SNPs, 1134 were selected with the objective of validating a subset of 300 markers. Amplicon of expected size was obtained for 46% (522/1134) of these, and Sanger sequencing revealed that 42.1% (220/522) of the amplified markers were false positive (no polymorphism). On the remaining 302 markers, 96.3% (291/302) met the technical requirements of the iPlex Gold assays, and 280 of these were successfully multiplexed in nine panels. Each panel contained 28 to 32 SNP markers (Table S2).
Genotype data were obtained for 171 (n = 85 fish sampled in August and n = 86 fish sampled in November) of the 191 F2 progenies (89.5%), and on 96.4% (270/280) of the markers as 10 of these failed to amplify or had >50% of missing data. The overall percentage of missing data for the SNP genotyping was 2.2%. Among the 270 validated SNPs, 45.5% (123/270) exhibited a significant blast hit (e-value < 1×10−40), among which, ORF predictor identified 118 ORFs (95.9%; 118/123). Within these 118 ORFs, 23 ORFs (19.5%; 23/118) showed a frame shift that was introduced during the assembly of raw data into contigs. These 23 ORFs were ignoredm and the SNP characterization was performed on the 95 remaining ORFs. Among these 95 ORFs, 92 SNPs (96.8%) were assigned to coding regions. Finally, 35 loci (38.0% - 35/92) were characterized as nonsynonymous mutations and 57 loci (61.9%; 57/92) as synonymous mutations (details in Table S3).
Microsatellite genotyping
The preliminary genotyping tests performed on a subset of eight individuals (the two mapping F1 parents and six F2 progenies) revealed that 84 markers were polymorphic in at least one of the two F1 parents allowing their mapping. Markers that showed complex banding pattern or that failed to amplify in more than 80% of the progeny were removed. The missing percentage of data (missing genotypes) was estimated at 6.4%.
Linkage map
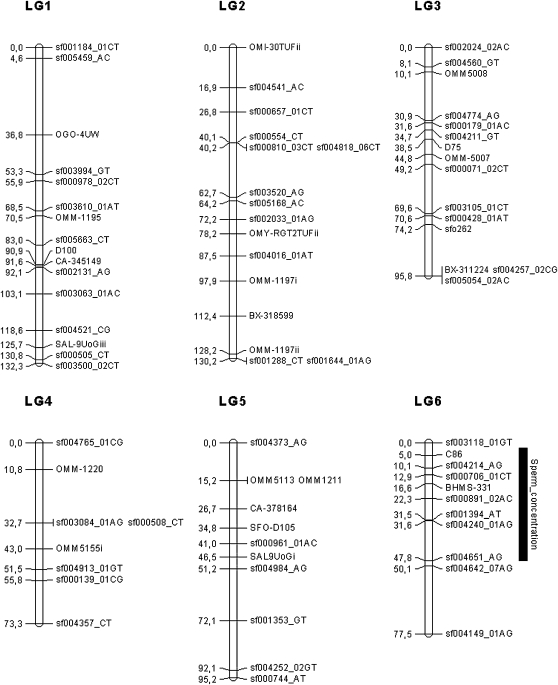
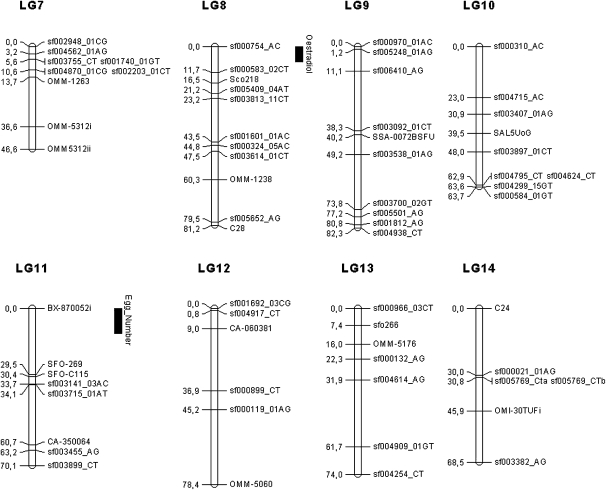
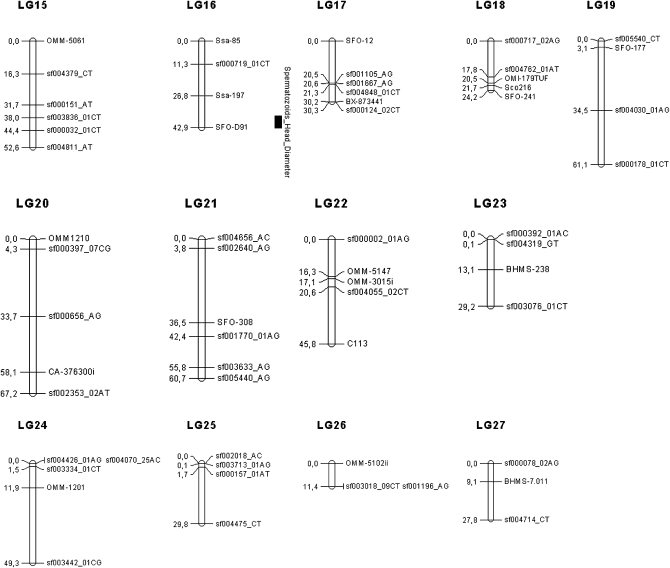
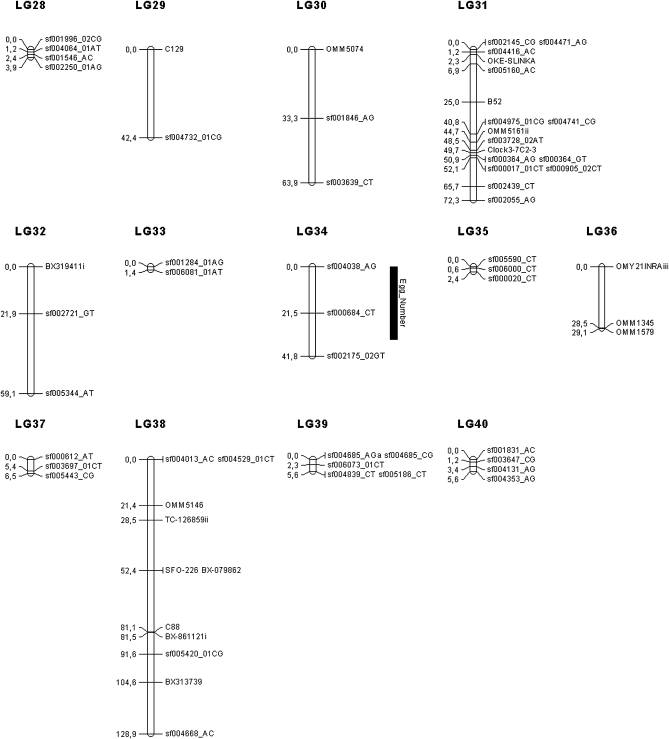
Seventeen markers (three microsatellites and 14 SNP) were removed because they heavily deviated from the HWE. Thus, the dataset used to build the linkage maps included 81 microsatellites and 256 SNPs, for a total of 337 markers. Six markers (1.8%; 6/337) remained unlinked, and a total of 40 LGs was generated. The consensus (sex-averaged) map contained 266 markers (187 SNPs and 79 microsatellites) distributed among the 40 LGs (see Table 1 for details). The LG length ranged from 1.4 to 132 cM, for a total consensus map length of 2047.5 cM. The average marker spacing per LG ranged from 0.7 to 21.3 cM and was estimated at 8.3 cM over the whole genome. The size of each LG was correlated to the number of markers within each LG (R2 = 0.516, P < 0.001), as well as to the average spacing between two markers within the LG (R2 = 0.182, P < 0.01). The recombination rate was approximately 2.19-fold greater in females than in males. The exact position and order of the 266 markers among the 40 LGs are given in Table S5. The sex-specific linkage maps were very similar to the consensus linkage map and included almost the same number of markers (n = 263 in females and n = 261 in males). Markers were generally ordered similarly in the two maps except for eight LGs (LG 2, 6, 10, 14, 17, 24, 31, and 38) when compared to the consensus one. For each sex-specific map, a total of 40 LGs was also obtained.
Table 1 . Description of the 40 LGs with the number and size of markers composing each LG (in cM) obtained for the consensus (sex-average) linkage map in brook charr.
| LG | Size of the LG, in cM | No. Markers in the LG | No. SNP | No. SSR | Average Spacing, in cM |
|---|---|---|---|---|---|
| LG1 | 132.2 | 16 | 11 | 5 | 8.26 |
| LG2 | 130.2 | 16 | 11 | 5 | 8.14 |
| LG3 | 95.7 | 15 | 10 | 5 | 6.38 |
| LG4 | 73.2 | 8 | 6 | 2 | 9.15 |
| LG5 | 95.2 | 11 | 6 | 5 | 8.65 |
| LG6 | 77.5 | 11 | 9 | 2 | 7.04 |
| LG7 | 46.6 | 9 | 6 | 3 | 5.17 |
| LG8 | 81.2 | 11 | 8 | 3 | 7.38 |
| LG9 | 82.3 | 10 | 9 | 1 | 8.23 |
| LG10 | 63.6 | 9 | 8 | 1 | 7.06 |
| LG11 | 70.1 | 8 | 4 | 4 | 8.76 |
| LG12 | 78.4 | 6 | 4 | 2 | 13.06 |
| LG13 | 74 | 7 | 5 | 2 | 10.57 |
| LG14 | 68.5 | 6 | 4 | 2 | 11.41 |
| LG15 | 52.6 | 6 | 5 | 1 | 8.76 |
| LG16 | 42.8 | 4 | 1 | 3 | 10.70 |
| LG17 | 30.3 | 6 | 4 | 2 | 5.05 |
| LG18 | 24.9 | 5 | 2 | 3 | 4.98 |
| LG19 | 61.1 | 4 | 3 | 1 | 15.27 |
| LG20 | 67.2 | 5 | 3 | 2 | 13.44 |
| LG21 | 60.7 | 6 | 5 | 1 | 10.11 |
| LG22 | 45.8 | 5 | 2 | 3 | 9.16 |
| LG23 | 29.2 | 4 | 3 | 1 | 7.30 |
| LG24 | 49.3 | 5 | 4 | 1 | 9.86 |
| LG25 | 29.8 | 4 | 4 | 0 | 7.45 |
| LG26 | 11.5 | 3 | 2 | 1 | 3.83 |
| LG27 | 27.8 | 3 | 2 | 1 | 9.26 |
| LG28 | 3.9 | 4 | 4 | 0 | 0.97 |
| LG29 | 42.3 | 2 | 1 | 1 | 21.15 |
| LG30 | 63.9 | 3 | 2 | 1 | 21.30 |
| LG31 | 72.3 | 17 | 13 | 4 | 4.25 |
| LG32 | 59.1 | 3 | 2 | 1 | 19.70 |
| LG33 | 1.4 | 2 | 2 | 0 | 0.70 |
| LG34 | 41.8 | 3 | 3 | 0 | 13.93 |
| LG35 | 2.4 | 3 | 3 | 0 | 0.80 |
| LG36 | 29.1 | 3 | 2 | 1 | 9.70 |
| LG37 | 6.5 | 3 | 3 | 0 | 2.16 |
| LG38 | 12.9 | 11 | 5 | 6 | 1.17 |
| LG39 | 4.5 | 5 | 5 | 0 | 0.90 |
| LG40 | 5.6 | 4 | 4 | 0 | 1.40 |
| Max | 132.25 | 17 | 13 | 6 | 21.3 |
| Min | 1.40 | 2 | 1 | 0 | 0.70 |
| Average | 51.18 | 6.65 | 4.75 | 1.90 | 8.31 |
| Total | 2047.45 | 266.00 | 190.00 | 76.00 | - |
LG, linkage group; SNP, single nucleotide-polymorphism; SSR, simple sequence repeat.
Phenotyping and QTL identification
The detailed description of the seven phenotypic traits measured is reported in Table 2. In brief, mean egg diameter (± D) was 3.96 ± 0.173 mm, mean spermatozoid head diameter was 2.86 ± 0.05 µm, and mean sperm concentration was 12.20 ± 3.20 109 mL-1. Plasma testosterone was about 1.4-fold greater in males than in females at the time of sampling (20.23 ± 14.46 and 14.49 ± 13.69 ng mL-1 respectively). Mean plasma 11-ketotestosterone and 17β-estradiol were 76.85 ± 167.84 and 18.35 ng mL-1 respectively.
Table 2 . Descriptive statistics of phenotypic traits measured in male (M) and female (F) brook charr from the QTL population (F2 progeny).
| Spermatozoid Head Diameter, µm | Sperm Concentration, ×109 spermatozoids mL-1 | Plasma Testosterone, ng mL-1 | Plasma 11-Keto-testosterone, ng mL-1 | Egg Diameter, mm | Plasma Testosterone, ng mL-1 | Plasma 17β-Estradiol, ng mL-1 | |
|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | F | F | F |
| N | 42 | 42 | 42 | 35 | 38 | 37 | 38 |
| Min | 2.57 | 4.52 | 0.56 | 0.39 | 3.35 | 0.85 | 0.15 |
| Max | 2.92 | 20.12 | 56.42 | 674.14 | 4.30 | 68.89 | 85.93 |
| Mean ± SD. | 2.86 ± 0.05 | 12.20 ± 3.20 | 20.23 ± 14.46 | 76.85 ± 167.84 | 3.96 ± 0.17 | 14.49 ± 13.69 | 6.91 ± 18.36 |
N, number of observations, Min, minimum mean value obtained; Max, maximal mean value obtained, mean ± SD, mean ± SD of the mean.
In the female progeny, three significant QTL were detected at the chromosome wide level: two were detected for the egg diameter in LG 11 and LG 34, and one QTL was detected for the plasma 17β-estradiol in LG 8 (Table 3). For each of these traits, total PVE of the QTL detected was estimated at 26.7% (10.6% and 16.1% for each QTL) and 27.6% for egg diameter and plasma 17β-estradiol, respectively. The most probable positions of these QTL, their respective 95% CI, the closest linked molecular markers (one per QTL) as well as additive and dominance effects are presented in Table 3.
Table 3 . Descriptive statistics, including the LOD score, the position, 95% CI, PVE (%), the associated P-value, and the specific additive, and dominance effects of each QTL linked to every phenotype related to reproductive traits.
| Phenotype | LG | Position(cM) | 95% CI (cM) | LOD | PVE, % | F Value | P Value (F) | Additive Effect | SE | Dominance Effect | SE | Nearest Marker |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg diameter | 11 | 0.0 | 0 - 11 | 5.193 | 10.62 | 4.432 | 0.044 | −0.8930 | 0.0936 | −0.8030 | 0.1345 | BX-870052i |
| 34 | 0.0 | 0 - 34 | 3.824 | 16.16 | 6.179 | 0.043 | 0.3635 | 0.0491 | 0.4824 | 0.3713 | BX319411i | |
| 17β-estradiol | 8 | 0.0 | 0 - 7 | 3.152 | 27.62 | 5.412 | 0.049 | 0.0360 | 0.0056 | 0.9562 | 0.1254 | sf000754_AC |
| Sperm concentration | 6 | 21 | 2 - 48 | 3.831 | 18.58 | 5.543 | 0.052 | 0.0257 | 0.0061 | 0.3895 | 0.0327 | sf000891_02AC |
| Spermatozoid head diameter | 16 | 42.9 | 37 - 42.9 | 5.290 | 14.95 | 9.146 | 0.029 | −1.2260 | 0.0333 | −0.4490 | 0.0190 | SFO-D91 |
Additive and dominance effect give insight in the mode of action of the genes underlying the variance of the traits. Moreover, direction (−/+) of the dominance or additive effects indicates which parent contributes to decrease the trait the phenotypic values in the studied trait. LOD, Log10 of the odd ratio; 95% CI, 95% confidence interval; PVE, percent variance explained; QTL, quantitative trait loci; SE, standard error.
In the male progeny, two QTL were detected, at the chromosome wide level, one for the sperm concentration on LG 6 and one for the average spermatozoid head diameter on LG 16. The PVE associated with these two traits were estimated at 18.58% and 14.95%, respectively. The most probable positions (in cM) of the QTL, their respective 95% CI, and additive and dominance effects are presented in Table 4. All significant QTL regions are depicted in Figure 1. No significant QTL were detected for plasma testosterone neither in male nor in female progeny, neither for the 11-keto-testosterone traits in the male progeny.
Table 4 . Comparison of putative LGs in salvelinus (in cM) between the present study and Timusk et al. (2011); homologies with the rainbow trout LGs also are given.
| LG from Timusk et al. (2011) | LG from Present Study | No. of Shared Markers | Markers | Putative Homology with Rainbow Trout LGa |
|---|---|---|---|---|
| BC-1 | LG24 | 1 | OMM1201 | RT-2p; RT-12q; RT-16q; RT-21q; RT-29p; RT-29c |
| BC-5 | LG18 | 1 | OMI179TUF | RT-8p; RT-19c; RT-19q |
| BC-6 | LG5 | 2 | CA378164, OMM1211 | RT-3c; RT-3q; RT-6p |
| BC-8 | LG15 | 1 | OMM-5061 | RT-2p; RT-3q; RT-7q; RT-10q |
| BC-9 | LG9 | 1 | SSA0072BSFU | RT-14q; RT-19q |
| BC-10 | LG14 | 1 | OMI30TUF/i | RT-10q; RT-18 |
| BC-11 | LG29 | 1 | BHMS7.011 | RT-10p; RT-12c; RT-12q; RT-19c |
| BC-13a and BC-13b | LG7 | 2 | OMM5312/i, OMM5312/ii | RT-8c; RT-14p; RT-14c; RT-20p; RT-20q; RT-21q; RT-22p; RT-24p; RT-24c |
| BC-15 | LG2 | 4 | OMI30TUF/ii, OMM1197/i, OMM1197/ii, OMYRGT2TUF/ii | RT-10p; RT-10c; RT-10q; RT-18 |
| BC-19 | LG11 | 1 | CA350064 | RT-13; RT-22c; RT-23q; RT-27q |
| BC-20 & BC-43 | LG3 | 2 | BX311224, OMM5007 | RT-2q; RT-5q; RT-8c; RT-9p; RT-9q; RT-12p; RT-16p; RT-20p; RT-31c; RT-31q |
| BC-23 | LG17 | 1 | BX873441 | RT-3q; RT-9p; RT-9q; RT-20p |
| BC-25 | UNA | 1 | SalD39SFU | RT-6c; RT-6q; RT-11; RT-12p; RT-16c; RT-26 |
| BC-30 | LG22 | 2 | OMM3015/i, OMM5147 | RT-3p; RT-6p |
| BC-32 | LG13 | 1 | OMM5176 | RT-13; RT-23p; RT-23q |
| BC-34 | LG34 | 1 | BX319411/i | RT-14p; RT-14c; RT-20q |
| BC-36 | LG10 | 1 | SAL5UoG | RT-5p; RT-5c |
Based on the Oxford grid of Timusk et al. (2011) presented in Table S9. LG, linkage group.
Figure 1 .
Linkage map in the brook charr: graphical representation of the consensus linkage map (sex-average) built using 266 markers (187 SNPs and 79 microsatellites), on the basis of 40 LGs, spanning 2047.5 cM. Significant QTL associated with reproduction traits are represented with their 95% confidence interval. LGs were named randomly. Genetic distances are indicated on the left of the LG, whereas marker names are reported on the right.
In both sexes, the 95% CI surrounding the most probable QTL positions were highly variable and ranged from 5.9 cM for the QTL linked to the spermatozoid head diameter (LG 16) to 46 cM for the QTL linked to the sperm concentration (LG 6). Two of the five QTL detected (on LG 8 and 6) were linked to the SNP sf000754_AC and sf000891_2AC. These markers were both transversions (A/C), but no significant annotation (or gene name) was found for them. The three remaining QTL (LG 11 and LG 34 for egg diameter and LG 16 for spermatozoid head diameter) were linked to SSR markers (BX870052i, BX319411i, and SfoD91).
The five QTL identified for the four phenotypic traits displayed different and significant additive and dominance effects. Additive effects ranged from −1.226 ± 0.033 (QTL on LG 16 associated with spermatozoid head diameter) to 0.363 ± 0.049 (QTL on LG 34 associated with egg diameter) and dominance effects ranged from −0.803 ± 0.134 (QTL on LG 11 associated with egg diameter) to 0.956 ± 0.125 (QTL on LG 8 associated with plasma 17β-estradiol concentration).
Discussion
The main objective of this study was to identify QTL associated with reproductive physiological traits that have not or rarely been investigated in previous studies. To this end, we first developed new SNP markers specific to brook charr in coding gene regions and used them in combination with previously published microsatellite markers to build a linkage map. We also phenotyped seven physiological traits for which we searched for QTL association. Thus, for the first time in salmonids, five significant QTL for four reproductive physiological traits were detected, including for egg number, plasma 17β-estradiol in female and sperm concentration, and spermatozoid head diameter in males. Results suggest that these four traits may be under the control of one major gene or a small number of genes, explaining an important variance of these traits. Moreover, results also indicate that genes underlying the phenotypic variance of these traits are under different mode of action (additive vs. dominance) and may be used to predict an increase or a decrease in their phenotypic values in subsequent generations of selective breeding. Admittedly, as for any gene mapping study, increasing the average marker density to detect QTL with a greater accuracy and thus dissecting the genetic architecture of the traits of interest may improve the results of this first-generation linkage map. Finally, this newly developed panel of mapped SNP located in coding gene region will be useful for screening wild populations, especially in the context of investigating the genetic impact of massive stocking of domestic brook charr to support the angling industry throughout eastern North America.
SNP development
Using RNA-seq, a total of 280 SNP markers located in coding regions and annotated for about half of them were identified for the first time in the genus Salvelinus. High-throughput sequencing technologies are increasingly applied for numerous purposes, including developing SNP markers for building linkage maps (e.g., rainbow trout) (Sanchez et al. 2009). To this end, a major constraint when working on nonmodel species is that, although de novo assembly approaches are routinely used (Kumar and Blaxter 2010), the absence of a reference genome makes discerning paralogous genes very difficult. This is particularly challenging in species with complex duplicated genomes also comprising numerous repeated elements such as salmonids (Davidson et al. 2010). To minimize the orthologous/paralogous problem in this study, each putative SNP was resequenced using the Sanger method. This stringent approach (261 SNPs validated >1000 putative sites) was necessary to overcome uncertainties associated with contig assembly. The low validation rate is likely a consequence of the following factors: (1) the greater sequencing error rate of the 454 technology compared to Sanger sequencing; (2) the assembly of paralogous sequences (even if we used restrictive parameters in the assembly); (3) the design of primers over an exon/intron junction for the validation step leading to the amplification of intronic regions, and (4) the technical requirements for the Sequenom assay. Low SNP validation rate similar to what we obtained here has been previously reported in other salmonids, including chum salmon, O. keta (validation success rate of 13.3%; 37/202, Seeb et al. 2011), and rainbow trout (validation success rate of 28.9%; 139/480 for Abadia-Cardoso et al. 2011 and 48%; 183/384 of validation success for Sanchez et al. 2009). Clearly, such a low validation rate is a major constraint to the efficient development and application of SNP markers using the method we used here, and alternative approaches may be better suited in future steps toward densifying the brook charr genetic map.
Indeed, during the time course of this study, new methods allowing the genotyping of thousands of SNP directly from next-generation sequencing sequences have been developed and recently applied (Elshire et al. 2011, also reviewed in Davey et al. 2011). However, the application of such “genotype by sequencing” methods for genetic mapping has been limited to very few species thus far (e.g., Baxter et al. 2011; Chutimanitsakun et al. 2011). As the first application to genetic mapping in fishes, the so-called “RAD-sequencing” approach has been used to build a linkage map comprising 8400 SNP markers in the longnose gar (Lepisosteus osseus) (Amores et al. 2011). Also, Miller et al. (2012) have recently produced the first genetic map for salmonids (rainbow trout) using this same method. One potential drawback with these new methods as currently applied is that, unlike the approach we used that specifically targeted coding regions, most detected SNPs are located in noncoding regions and thus remain anonymous, although new protocols are being adapted for cDNA RAD-sequencing (Davey et al. 2011). Also, the challenge posed by the recently duplicated genome of polyploidy salmonids remains the same, although the number of markers from which high-quality ones can be validated is substantially increased. Thus, there is little doubt that future development in brook charr genetic mapping and QTL detection will benefit from these new methods, as it will likely be the case for any other species.
QTL detection for reproductive physiological traits
Up to now, QTL analyses linked to reproductive traits in salmonids mainly focused on age at sexual maturation (e.g. Moghadam et al. 2007). Here, five significant QTL were detected for other traits related to reproduction, including two QTL associated with egg number, as well as with plasma 17β-estradiol and spermatozoid head diameter. The 17β-estradiol is synthesized from the aromatization of testosterone in the ovarian granulosa cells (Kagawa et al. 1983). It is subsequently released into the bloodstream and is responsible for growth and maturation of oocytes (Peter and Yu 1997). In particular, it triggers hepatic synthesis of vitellogenin, the yolk precursor protein (Patino and Sullivan 2002). For each of the five QTL, the closet underlying molecular marker has been identified. Three of them are microsatellites (BX870052i, BX319411i, and SfoD91) and two are SNP markers (sf000754 and sf000891) associated with plasma 17β-estradiol and sperm concentration. Unfortunately, these two SNPs were not associated with any significant annotation. Clearly, the availability of a fully sequenced and annotated genome in other salmonids such as Atlantic salmon (Davidson et al. 2010) and rainbow trout (Palti et al. 2011) will be of paramount importance to refine the annotation of the SNP markers developed here and to investigate the potential functional role of SNP underlying QTL in salmonid species.
The PVE for identified QTL ranged from moderate to large effects (10.6%–27.6%). The low number of QTL identified per trait, combined to the high PVE may suggest that the expression of these traits is under the control of one major or a limited number of genes. However, this should be interpreted cautiously because the limited number of QTL may also reflect a lack of power because of the limited number of progeny and markers that could be analyzed here. Namely, the limited number of progeny may influence QTL detection experiments by underestimating the QTL number and overestimating their respective effects (Beavis 1998; Xu 2003). Also, most likely because of the limited of markers, the most probable location of the five QTL was identified with relatively large 95% confidence intervals.
Comparison with other linkage maps
Our brook charr linkage map comprises 40 LGs, which is very close to the haploid number of chromosome in the species (2n = 84). With 171 genotyped individuals, and based on the linkage map previously published for this species (Timusk et al. 2011), we expected a maximal mapping resolution of 0.58 cM (100/171) and an average marker spacing ranging from 8 to 10 cM (total length of the map estimated from 2400 cM to 3000 cM /300 markers) This expectation was reached with a marker spacing average per LG that ranged from 0.7 to 21.3 cM and a marker spacing average estimated at 8.3 cM over the whole genome. The marker spacing also reached the minimal expectation estimated at a spacing average of 10 cM over the whole genome that is needed for an accurate QTL detection (Darvasi et al. 1993).
As mentioned previously, the present map is composed of 40 LGs, including a total of 266 markers (both SNP and SSR), whereas Timusk et al. (2011) reported a map composed of 37 evident LGs determined by the mapping of 139 SSR loci. In both studies also, significant differences in recombination rates between sexes were identified, resulting in a female/male ratio of 2.19:1 in this study vs. 3.47:1 in Timusk et al. (2011). Such strong differential recombination rates between sexes are typical of all salmonid species investigated thus far. Sakamoto et al. (2000) were the first to report this phenomenon in salmonids with a ratio of 3.25:1 ratio observed in rainbow trout. Subsequently, a 1.38:1 female/male ratio was reported in Atlantic salmon, (Lien et al. 2011), 6.4:1 in brown trout, (Gharbi et al. 2006), and 2.6:1: in Arctic charr (Woram et al. 2004).
Such large differences in female/male recombination rates could be explained by the differential sex-specific alignment of chromosomes during meiosis (Lee and Wright 1981). This could also result in segregation distortion (non-Mendelian segregation of markers), which was observed in this study (17 markers comprising 3 SSRs and 14 SNPs) as well as by Timusk et al. (2011) (33 loci in 9 LGs). Although the number of markers shared between this study and that of Timusk et al. (2011) was relatively modest, comparison between the two maps was possible. Thus, a total of 18 LGs sharing 24 SSR markers was apparently homologous between both studies (Table 4). Also, the comparison between brook charr, Arctic charr, and rainbow trout genetic maps done by Timusk et al. (2011) allowed us to assign homology within this triplet of species for the 18 common LGs. However, direct comparison of linkage maps has to be interpreted cautiously, given the low number of shared SSR markers between studies. Further comparison with other available maps that were developed with SNP markers (e.g., Atlantic salmon and zebrafish Danio rerio) proved impossible given the very low number of shared markers (<5%) between brook charr and Atlantic salmon as well as the high level of divergence between zebrafish and brook charr. Clearly, further investigation of the synteny within the salmonid family using gene-based markers will be of prime interest while the complete genome sequence of the Atlantic salmon and rainbow trout genome soon become available (Davidson et al. 2010; Lien et al. 2011).
Supplementary Material
Acknowledgments
We thank R. Gagné, S. Granier, H. Segouani, and G. Côté for their invaluable help during the phenotyping experiments and sample collection; E. Normandeau for his help in bioinformatics; L. Papillon and Nathalie N. Brodeur for their help with microsatellite genotyping; and A. Montpetit and A. Belisle from Genome Québec Innovation Center (McGill University, Montreal, Canada) for their help in cDNA library sequencing and SNP genotyping. This work was founded by a Partnership grant (Strategic program) of the Natural Sciences and Engineering Research Council of Canada (NSERC) to L.B., N.D., and C.A. This is a contribution to the research program of Réseau Aquaculture Québec (RAQ). The authors have no conflict of interest to declare and note that the sponsors of the issue had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Communicating editor: A. H. Paterson
Literature Cited
- Abadia-Cardoso A., Clemento A. J., Garza J. C., 2011. Discovery and characterization of single-nucleotide polymorphisms in steelhead/rainbow trout, Oncorhynchus mykiss. Mol. Ecol. Resources 11: 31–49 [DOI] [PubMed] [Google Scholar]
- Amores A., Catchen J., Ferrara A., Fontenot Q., Postlethwait J. H., 2011. Genome evolution and meiotic maps by massively parallel DNA sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics 188: 799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers B., Bernatchez L., Angers A., Desgroseillers L., 1995. Specific microsatellite loci for brook charr (Salvelinus fontinalis Mitchill) reveal strong population subdivision on a microgeographic scale. J. Fish Biol. 47: 177–185 [Google Scholar]
- Audet C., Bernatchez L., 2004. The Laval strain, why and how. L’Aquicole 10: 9–11 (in French) [Google Scholar]
- Baxter S. W., Davey J. W., Johnston J. S., Shelton A. M., Heckel D. G., et al. , 2011. Linkage mapping and comparative genomics using Next-Generation RAD Sequencing of a non-model organism. PLoS ONE 6: e19315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis W., 1998. QTL analyses: power, precision and accuracy, pp. 145–162 in Molecular Dissection of Complex Trait, edited by A. H. Patterson. CRC Press, New York [Google Scholar]
- Broman K. W., Wu H., Sen Ő., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890 [DOI] [PubMed] [Google Scholar]
- Chutimanitsakun Y., Nipper R. W., Cuesta-Marcos A., Cistue L., Corey A., et al. , 2011. Construction and application for QTL analysis of a restriction site associated DNA (RAD) linkage map in barley. BMC Genomics 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly I., Gharbi K., Danzmann R. G., Yao J., Rexroad C. E., III, 2005. Characterization and comparison of microsatellites derived from repeat-enriched libraries and expressed sequence tags. Anim. Genet. 36: 309–315 [DOI] [PubMed] [Google Scholar]
- Danzmann R., Davidson E., Ferguson M., Gharbi K., Koop B., et al. , 2008. Distribution of ancestral proto-Actinopterygian chromosome arms within the genomes of 4R-derivative salmonid fishes (Rainbow trout and Atlantic salmon). BMC Genomics 9: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvasi A., Weinreb A., Minke V., Weller J. I., Soller M., 1993. Detecting marker-QTL linkage and estimating QTL gene effect and map location using a saturated genetic map. Genetics 134: 943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. W., Hohenlohe P. A., Etter P. D., Boone J. Q., Catchen J. M., et al. , 2011. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 12: 499–510 [DOI] [PubMed] [Google Scholar]
- Davidson W. S., Koop B. F., Jones S. J., Iturra P., Vidal R., et al. , 2010. Sequencing the genome of the Atlantic salmon (Salmo salar). Genome Biol. 11: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. K., Visitacion N., Riley L. G., Hiramatsu N., Sullivan C. V., et al. , 2009. Effects of o,p’-DDE, heptachlor, and 17-beta-estradiol on vitellogenin gene expression and the growth hormone/insulin-like growth factor-I axis in the tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. Part Toxicol. Pharmacol. 149(4): 507–514 [DOI] [PubMed] [Google Scholar]
- de Montgolfier B., Faye A., Audet C., Cyr D. G., 2009. Seasonal variations in testicular connexin levels and their regulation in the brook trout, Salvelinus fontinalis. Gen. Comp. Endocrinol. 162: 276–285 [DOI] [PubMed] [Google Scholar]
- Dehaan P. W., Ardren W. R., 2005. Characterization of 20 highly variable tetranucleotide microsatellite loci for bull trout (Salvelinus confluentus) and cross-amplification in other Salvelinus species. Mol. Ecol. Notes 5: 582–585 [Google Scholar]
- Easton A. A., Moghadam H. K., Danzmann R. G., Ferguson M. M., 2011. The genetic architecture of embryonic developmental rate and genetic covariation with age at maturation in rainbow trout Oncorhynchus mykiss. J. Fish Biol. 78: 602–623 [DOI] [PubMed] [Google Scholar]
- Elshire R. J., Glaubitz J. C., Sun Q., Poland J. A., Kawamoto K., et al. , 2011. A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS ONE 6: e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotherby H. A., Mogahadam H. K., Danzmann R. G., Ferguson M. M., 2007. Detection of quantitative trait loci for body weight, condition factor and age at sexual maturation in North American Atlantic salmon (Salmo salar) and comparative analysis with rainbow trout (Oncorhynchus mykiss) and Arctic charr (Salvelinus alpinus). Aquaculture 247: 256–257 [DOI] [PubMed] [Google Scholar]
- Gharbi K., Gautier A., Danzmann R. G., Gharbi S., Sakamoto T., et al. , 2006. A Linkage map for brown trout (Salmo trutta): chromosome homeologies and comparative genome organization with other salmonid fish. Genetics 172: 2405–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. R., Armengol L., Sole X., Guino E., Mercader J. M., et al. , 2007. SNPassoc: an R package to perform whole genome association studies. Bioinformatics 23: 644–645 [DOI] [PubMed] [Google Scholar]
- Haidle L., Janssen J., Gharbi K., Moghadam H., Ferguson M., et al. , 2008. Determination of quantitative trait loci (QTL) for early maturation in rainbow trout (Oncorhynchus mykiss). Mar. Biotechnol. 10: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. E., 2008. Sequencing breakthroughs for genomic ecology and evolutionary biology. Mol. Ecol. Notes 8: 3–17 [DOI] [PubMed] [Google Scholar]
- Jaari S., Li M.-H., Merila J., 2009. A first-generation microsatellite-based genetic linkage map of the Siberian jay (Perisoreus infaustus): insights into avian genome evolution. BMC Genomics 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Young G., Nagahama Y., 1983. Relationship between seasonal plasma estradiol-17β and testosterone levels and in vitro production by ovarian follicles of amago salmon (Onchorhynchus rhodurus). Biol. Reprod. 29: 301–309 [DOI] [PubMed] [Google Scholar]
- Koop B. F., Davidson W. S., 2008. Genomics and the Genome duplication in Salmonids, pp. 77–86 in Fisheries for Global Welfare and Environment, edited by K. Tsukamoto, T. Kawamura, T. Takeuchi, T. D. Beard Jr., and M. J. Kaiser. 5th World Fisheries Congress 2008 [Google Scholar]
- Kumar S., Blaxter M. L., 2010. Comparing de novo assemblers for 454 transcriptome data. BMC Genomics 11: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. M., Wright J. E., 1981. Mitotic and meiotic analyses of brook trout, Salvelinus fontinalis. J. Hered 72: 321–327 [DOI] [PubMed] [Google Scholar]
- Lien S., Gidskehaug L., Moen T., Hayes B. J., Berg P. R., et al. , 2011. A dense SNP-based linkage map for Atlantic salmon (Salmo salar) reveals extended chromosome homeologies and striking differences in sex-specific recombination patterns. BMC Genomics 12: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S., Brenna-Hansen S., Moen T., Roseth A., Davidson W. S., et al. , 2010. BAC-based upgrading and physical integration of a genetic SNP map in Atlantic salmon. Anim. Genet. 41: 48–54 [DOI] [PubMed] [Google Scholar]
- Lynch M., Walsh B., 1998. Genetics and Analysis of Quantitative Traits. Sinuaer Associates, Sunderland, MA [Google Scholar]
- McClelland E. K., Naish K. A., 2008. A genetic linkage map for coho salmon (Oncorhynchus kisutch). Anim. Genet. 39: 169–179 [DOI] [PubMed] [Google Scholar]
- McClelland E. K., Naish K. A., 2010. Quantitative trait locus analysis of hatch timing, weight, length and growth rate in coho salmon, Oncorhynchus kisutch. Heredity 105: 562–573 [DOI] [PubMed] [Google Scholar]
- McGowan C. R., Davidson E. A., Woram R. A., Danzmann R. G., Ferguson M. M., et al. , 2004. Ten polymorphic microsatellite markers from Arctic charr (Salvelinus alpinus): linkage analysis and amplification in other salmonids. Anim. Genet. 35: 479–481 [DOI] [PubMed] [Google Scholar]
- Miller K. M., Li S., Kaukinen K. H., Ginther N., Hammill E., et al. , 2011. Genomic signatures predict migration and spawning failure in wild canadian salmon. Science 331: 214–217 [DOI] [PubMed] [Google Scholar]
- Miller M. R., Brunelli J. P., Wheeler P. A., Liu S., Rexroad C. E., 3rd, et al. , 2012. A conserved haplotype controls parallel adaptation in geographically distant salmonid populations. Mol. Ecol. 105: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X. J., Butler G., Storms R., Tsang A., 2005. OrfPredictor: predicting protein-coding regions in EST-derived sequences. Nucleic Acids Res. 33: W677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen T., Hayes B., Baranski M., Berg P. R., Kjoglum S., et al. , 2008. A linkage map of the Atlantic salmon (Salmo salar) based on EST-derived SNP markers. BMC Genomics 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen T., Baranski M., Sonesson A., Kjoglum S., 2009. Confirmation and fine-mapping of a major QTL for resistance to infectious pancreatic necrosis in Atlantic salmon (Salmo salar): population-level associations between markers and trait. BMC Genomics 10: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam H., Poissant J., Fotherby H., Haidle L., Ferguson M., et al. , 2007. Quantitative trait loci for body weight, condition factor and age at sexual maturation in Arctic charr (Salvelinus alpinus): comparative analysis with rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Mol. Genet. Genomics 277: 647–661 [DOI] [PubMed] [Google Scholar]
- Nichols K., Young W., Danzmann R., Robison B., Rexroad C., et al. , 2003. A consolidated linkage map for rainbow trout (Oncorhynchus mykiss). Anim. Genet. 34: 102–115 [DOI] [PubMed] [Google Scholar]
- Nichols K. M., Broman K. W., Sundin K., Young J. M., Wheeler P. A., et al. , 2007. Quantitative Trait Loci x Maternal Cytoplasmic Environment Interaction for Development Rate in Oncorhynchus mykiss. Genetics 175: 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols K., Felip-Edo A., Wheeler P., Thorgaard G., 2008. The genetic basis of smoltification-related traits in Oncorhynchus mykiss. Genetics 179: 1559–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols K. M., Neale B. D., Gratten J., Alastair J. W., McRae A. F., et al. , 2010. Association genetics, population genomics, and conservation: revealing the genes underlying adaptation in natural populations of plants and animals, pp. 392 in Molecular Approaches in Ntural Resource Conservation and Management, edited by J. A. DeWoody, J. W. Bickham, C. H. Michler, K. M. Nichols, O. E. Rhodes, Jr. et al.. Cambridge University Press, Cambridge [Google Scholar]
- O’Malley K., Sakamoto T., Danzmann R., Ferguson M., 2003. Quantitative trait loci for spawning date and body weight in rainbow trout: testing for conserved effects across ancestrally duplicated chromosomes. J. Hered. 94: 273–284 [DOI] [PubMed] [Google Scholar]
- O’Malley K. G., McClelland E. K., Naish K. A., 2010. Clock genes localize to quantitative trait loci for stage-specific growth in juvenile coho salmon, Oncorhynchus kisutch. J. Hered. 101: 628–632 [DOI] [PubMed] [Google Scholar]
- O’Reilly P., Hamilton L., McConnell S., Wright J., 1996. Rapid analysis of genetic variation in Atlantic salmon (Salmo salar) by PCR multiplexing of dinucleotide and tetranucleotide microsatellites. Can. J. Fish. Aquat. Sci. 53: 2292–2298 [Google Scholar]
- Olsen J., Bentzen P., Seeb J., 1998. Characterization of seven microsatellite loci derived from pink salmon. Mol. Ecol. 7: 1087–1089 [PubMed] [Google Scholar]
- Page L. M., Burr B. M., 1991. A Field Guide to Freshwater Fishes of North America North of Mexico. Houghton Mifflin Company, Boston [Google Scholar]
- Palti Y., Fincham M. R., Rexroad C. E., 2002. Characterization of 38 polymorphic microsatellite markers for rainbow trout (Oncorhynchus mykiss). Mol. Ecol. Notes 2: 449–452 [Google Scholar]
- Palti Y., 2009. Aquaculture genomics, p. 103–145 Molecular Research in Aquaculture, edited by Overturf K., Wiley-Blackwell, New York [Google Scholar]
- Palti Y., Genet C., Luo M.-C., Charlet A., Gao G., et al. , 2011. A first generation integrated map of the rainbow trout genome. BMC Genomics 12: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino R., Sullivan C. V., 2002. Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol. Biochem. 26: 57–70 [Google Scholar]
- Perry G. M. L., Danzmann R. G., Ferguson M. M., Gibson J. P., 2001. Quantitative trait loci for upper thermal tolerance inoutbred strains of rainbow trout (Oncorhynchus mykiss). Heredity 83: 333–341 [DOI] [PubMed] [Google Scholar]
- Perry G. M. L., King T. L., St-Cyr J., Valcourt M., Bernatchez L., 2005. Isolation and cross-familial amplification of 41 microsatellites for the brook charr (Salvelinus fontinalis). Mol. Ecol. Notes 5: 346–351 [Google Scholar]
- Peter R. E., Yu K. L., 1997. Neuroendocrine regulation of ovulation in fishes: basic and applied aspects. Rev. Fish Biol. Fish. 7: 173–197 [Google Scholar]
- Phillips R., Keatley K., Morasch M., Ventura A., Lubieniecki K., et al. , 2009. Assignment of Atlantic salmon (Salmo salar) linkage groups to specific chromosomes: Conservation of large syntenic blocks corresponding to whole chromosome arms in rainbow trout (Oncorhynchus mykiss). BMC Genet. 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. B., Rab P., 2001. Chromosome evolution in the Salmonidae (Pisces): an update. Biol. Rev. Camb. Philos. Soc. 76: 1–25 [DOI] [PubMed] [Google Scholar]
- Quinlan A. R., Stewart D. A., Stromberg M. P., Marth G. T., 2008. Pyrobayes: an improved base caller for SNP discovery in pyrosequences. Nat. Methods 5: 179–181 [DOI] [PubMed] [Google Scholar]
- Renaut S., Nolte A., Bernatchez L., 2010. Mining transcriptome sequences towards identifying adaptive single nucleotide polymorphisms in lake whitefish species pairs (Coregonus spp. Salmonidae). Mol. Ecol. 19: 115–131 [DOI] [PubMed] [Google Scholar]
- Rexroad C. E., 3rd, Lee Y., Keele J. W., Karamycheva S., Brown G., et al. , 2003. Sequence analysis of a rainbow trout cDNA library and creation of a gene index. Cytogenet. Genome Res. 102: 347–354 [DOI] [PubMed] [Google Scholar]
- Rexroad C., Palti Y., Gahr S., Vallejo R., 2008. A second generation genetic map for rainbow trout (Oncorhynchus mykiss). BMC Genet. 9: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rise M. L., von Schalburg K. R., Brown G. D., Mawer M. A., Devlin R. H., et al. , 2004. Development and application of a salmonid EST database and cDNA microarray: data mining and interspecific hybridization characteristics. Genome Res. 14: 478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison B. D., Wheeler P. A., Sundin K., Sikka P., Thorgaard G. H., 2001. Composite interval mapping reveals a major locus influencing embryonic development rate in rainbow trout (Oncorhynchus mykiss). J. Hered. 92: 16–22 [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Bernatchez L., 2007. The genetic architecture of ecological speciation and the association with signatures of selection in natural lake whitefish (Coregonus sp. Salmonidae) species pairs. Mol. Biol. Evol. 24: 1423–1438 [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Isabel N., Bernatchez L., 2007. Linkage maps of the dwarf and normal lake whitefish (Coregonus clupeaformis) species complex and their hybrids reveal the genetic architecture of population divergence. Genetics 175: 375–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H., 2000. Primer3 on the WWW for general users and for biologist programmers. Bioinformatics Methods and Protocols. Methods Mol. Biol. 132: 365–386 [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Danzmann R., Gharbi K., Howard P., Ozaki A., et al. , 2000. A microsatellite linkage map of rainbow trout (Oncorhynchus mykiss) characterized by large sex-specific differences in recombination rate. Genetics 155: 1331–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C., Smith T., Wiedmann R., Vallejo R., Salem M., et al. , 2009. Single nucleotide polymorphism discovery in rainbow trout by deep sequencing of a reduced representation library. BMC Genomics 10: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner K. T., Gust J. R., Fields R. L., 1996. Isolation and characterization of novel salmon microsatellite loci: cross-species amplification and population genetic applications. Can. J. Fish. Aquat. Sci. 53: 833–841 [Google Scholar]
- Seeb J. E., Pascal C. E., Grau E. D., Seeb L. W., Templin W. D., et al. , 2011. Transcriptome sequencing and high-resolution melt analysis advance single nucleotide polymorphism discovery in duplicated salmonids. Mol. Ecol. Resour. 11: 335–348 [DOI] [PubMed] [Google Scholar]
- Thorsen J., Zhu B., Frengen E., Osoegawa K., de Jong P., et al. , 2005. A highly redundant BAC library of Atlantic salmon (Salmo salar): an important tool for salmon projects. BMC Genomics 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timusk E. R., Ferguson M., Mogahadam H. K., Norman J. D., Wilson C., et al. , 2011. Genome evolution in the fish family salmonidae: generation of a brook charr genetic map and comparisons among charrs (Arctic charr and brook charr) with rainbow trout. BMC Genet. 68: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woram R., McGowan C., Stout J., Gharbi K., Ferguson M., et al. , 2004. A genetic linkage map for Arctic charr (Salvelinus alpinus): evidence for higher recombination rates and segregation distortion in hybrid vs. pure strain mapping parents. Genome 47: 304–315 [DOI] [PubMed] [Google Scholar]
- Wringe B. F., Devlin R. H., Ferguson M. M., Moghadam H. K., Sakhrani D., et al. , 2010. Growth-related quantitative trait loci in domestic and wild rainbow trout (Oncorhynchus mykiss). BMC Genet. 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., 2003. Theoretical basis of the Beavis effect. Genetics 165: 2259–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. P., Wheeler P. A., Coryell V. H., Keim P., Thorgaard G. H., 1998. A detailed linkage map of rainbow trout produced using doubled haploids. Genetics 148: 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Y., Machleder E. M., Chenchik A., Li R., Siebert P. D., 2001. Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction. Biotechniques 30: 892–897 [DOI] [PubMed] [Google Scholar]
- Zhulidov P. A., Bogdanova E. A., Shcheglov A. S., Vagner L. L., Khaspekov G. L., et al. , 2004. Simple cDNA normalization using kamchatka crab duplex-specific nuclease. Nucleic Acids Res. 32: e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.