Abstract
Objective
Leukemia initiating cells (LICs) have been the subject of considerable investigation because of their ability to self-renew and maintain leukemia. Thus, selective targeting and killing of LIC would provide highly efficient and novel therapeutic strategies. Here we explored whether we could use a canine leukemia cell line (G374) derived from a dog that received HOXB4 transduced repopulating cells to study leukemia in the murine xenograft model and the dog.
Materials and Methods
G374 cells were infused in dogs intravenously (IV) and in NOD/SCID and NOD/SCID/IL2Rγnull mice either IV or directly into the bone cavity (IF). Animals were bled to track engraftment and proliferation of G374 cells, and were sacrificed when they appeared ill.
Results
We found that canine LICs are capable of sustained in vitro self-renewal while maintaining their ability to induce AML that resembles human disease in both dogs and mice. Furthermore, we developed a novel strategy for the quantification of LIC frequency in large animals and showed that this frequency was highly comparable to that determined by limited dilution in mouse xenotransplants. We also demonstrated, using single cell analysis, that LICs are heterogenous in their self-renewal capacity and regenerate a leukemic cell population consistent with a hierarchical leukemia model.
Conclusions
The availability of this novel framework should accelerate the characterization of LICs and the translation of animal studies into clinical trials.
Introduction
Leukemia initiating cells (LICs) have been the subject of considerable investigation since their discovery by Bonnet and Dick in 1997 [1] and the important implication that such cells must the target for curative therapies. At the same time the existence of LICs and the associated hierarchical model of leukemia have attracted some controversy concerning the origin, frequency and real therapeutic significance. LICs have been documented to exist by in vivo transplantation assays which provide evidence of both their self-renewal capabilities and ability to reconstitute a phenotypic and functional leukemic cell hierarchy. Murine models are the most frequently used systems for LIC studies due to their low cost, easy handling, and the ability to maintain xenografts of human cancers. However, possible limitations of the current models have been suggested that would limit direct extrapolation from mice to humans and thus, data obtained in these systems need to be critically analyzed.
Human/mice xenograft models have unique constraints, such as those derived from the foreign background presented to human cells and may not, therefore, reflect the true intrinsic properties of cancer cells in humans, selecting for cells that can propagate in a niche-independent manner [2]. The effect of the microenvironment on leukemic cells is underscored by the work of Feuring-Buske et al, who showed significantly enhanced patients’ AML cell engraftment and increased frequency of LICs in NOD/SCID (NS) mice transgenic for the human growth factor genes SCF, interleukin-3 and granulocyte macrophage-colony-stimulating factor, when compared with standard NS mice [3]. In addition, migration of LICs to a supportive microenvironment is essential for successful engraftment, and thus ineffective LIC transiting to the niche could contribute to underestimates of LIC frequency [4]. Although there are no studies on the influence of route of administration on LIC frequency, assays of normal hematopoietic cells based on direct intrafemoral injection (IF) have been shown to be more efficient in detecting a novel class of short term repopulating cells that are either rare or poorly detected with traditional intravenous injection (IV)–based assays [5, 6]. Another potential limitation of the xenograft model is residual activity of the host immune system, which again could lead to an underestimation of LIC frequency by allowing only cells with immunoevasive capacity to grow in this setting. Indeed, immune recognition is an important determinant of host resistance to xenotransplantation as demonstrated by increased engraftment and frequency of LIC from AML patients’ samples in permissive xenograft models such as the NOD/SCID/β2 microglobulin-null (NOD/SCID/β2mnull) mice, which have a reduced innate immunity because of a lack of natural killer cell activity [3], and NOD/SCID/IL2Rγnull (NSG) mice, which lack mature lymphocytes and natural killer cell [7-9]. Of note, NSG mice are long-lived compared with other immunodeficient strains, allowing for long-term follow-up on these animals [10]. Whereas syngeneic mouse models remove potential cross-species barriers to engraftment, and may more faithfully recapitulate LIC interactions with the microenvironment, the biology of mouse and human cells is not identical, and several observations highlight that species-specific differences do exist in the mechanisms of malignant transformation [11-13].
Contrary to classical small animal models, a large animal system such as the outbred dog provides several advantages. Human and dogs share an ancestrally retained pathogenetic basis for cancer [14] and thus canine cancers more accurately mimic human diseases than do their rodent counterparts [15]. Spontaneous leukemias are rare but occur in dogs, and thus provide a large animal system in which to investigate drug activities. Dogs with spontaneous leukemia have been used to show the ability of DMAPT, an orally bioavailable parthenolide analog, to eradicate acute AML stem and progenitor cells [16]. However, spontaneous canine leukemias are usually only detected at advanced stages, and thus the window of opportunity to evaluate treatment regimens is generally very brief, underscoring the need for longer lived models.
We have recently reported the induction of leukemia in dogs transplanted with autologous HOXB4-transduced CD34+ cells [17, 18]. In primary transplants, the dogs developed overt leukemia after 2 years [17]; however, accidental infusion of 2 dogs with HOXB4-leukemic cells from one of the primary transplants (G374) quickly developed lethal myeloid leukemia, demonstrating transmission and rapid expansion of the leukemic clone in vivo [18]. In this study we used a large animal model and a mouse xenotransplantation model to functionally characterize LICs contained in a cell line derived from the bone marrow (BM) of the G374 dog (G374 cell line). We determined the ability of the cells to recapitulate the original disease in dogs and mice, and exploited a strategy for their quantification and assessment of LIC self-renewal in this large animal leukemia model. Our findings support a model in which LIC frequency is a function of both a hierarchical leukemic population and stochastic processes impacting on LIC self-renewal. This line now provides a new and powerful resource for further functional and molecular investigations of leukemic stem cells.
Materials and Methods
Establishment of canine leukemia cell line, retroviral marking and single cell cloning
A canine leukemia cell line (G374) was established by ex vivo culturing of bone marrow cells harvested from dog G374 at the time of death due to leukemia. G374 cells were cultured in IMDM supplemented with 12.5% FBS, 12.5% horse serum, 1% penicillin/streptomycin with cytokines; canine SCF, human FL and TPO, each at 100 ng/ml.
To investigate the frequency of LICs in the dog, we transduced G374 cells (GFP+) with a YFP expressing lentiviral vector at a low multiplicity of infection (MOI) so that single proviral integrations per transduced cell were most likely. Thus by counting the number of proviral integrations ultimately present in a sample of the leukemic animal, one achieves an estimation of the number of LICs (clones) contributing to the leukemia. This number expressed as a proportion of the total number of transduced cells in the transplant innoculum provides a measure of the frequency of LIC in the leukemic line (in this case, 10 marked clones detected in the leukemic recipient/104 marked cells transplanted gives an estimated LIC frequency of 1/1000). Three days after transduction, 1×106 G374 cells (1% YFP+) together with 1×107 autologous CD34+ cells were transplanted into a dog. Seven weeks later, when the animal developed overt leukemia, GFP+YFP+ cells were sorted for LAM-PCR analysis. To investigate the frequency of LIC in the bulk G374 cells, we conducted single cell cloning experiment. Single cells were sorted into each well of U-bottomed 96 well plates. The presence of a single cell was confirmed visually. Two weeks later, cells in the wells where large colonies were observed were transferred to a 24-well plate for further culture. Expanded cells from some clones were also used for in vivo transplantation studies.
Animals
Dogs were housed at the Animal Health Resources unit of the Fred Hutchinson Cancer Research Center. All dog experiments and manipulations were approved by the IACUCs of the Fred Hutchinson Cancer Research Center. NOD/ShiLtSz-Prkdcscid (NOD/SCID, termed NS) and NOD.Cg-Prkdcscid Il2rgtm1Wj1/SzJ (NOD/SCID/IL2Rγnull, termed NSG) mice were bred and housed at the animal resource center at the British Columbia Cancer Research Centre of the British Columbia Cancer Agency. All mouse experimental procedures were approved by the University of British Columbia Animal Care Committee.
Transplantation of G374 cells and monitoring of the recipients
Lethally irradiated (920cGy) dogs (n=3) were infused with G374 cells cultured ex vivo for over 1 year. Dogs were bled frequently to track engraftment and proliferation of G374 cells (GFP+) by FACS. Animals were sacrificed when they appeared ill, and at the time of death BM was processed for morphological analysis.
In xenotransplant studies, 8-10 wk old NS and NSG mice were sublethally irradiated (325 and 315 cGy respectively) 24h prior to transplantation. G374 cells were injected either into the tail vein (IV) or directly into the bone cavity (IF). For IF injections, a 27g needle was used to drill a hole in the right femur, followed by injection of cells in 25ul using a Hamilton syringe with a 28.5g needle. In limiting dilution assays where mice received less than 1×106 G374 cells, 1×106 irradiated human carrier cells were co-transplanted. Mice were bled at monthly intervals to track engraftment and proliferation of G374 cells (GFP+) by FACS, and were sacrificed when they appeared ill. At the time of death, blood, BM and spleen (after weighing) were processed and analyzed by FACS for the presence of GFP+ leukemia cells after staining with canine CD45 and CD34 antibodies for an accurate assessment of leukemic burden (CD45+GFP+) and the phenotype (CD45+CD34+). For morphological analysis, peripheral blood smears and cytopsins of bone marrow and spleen cells were prepared. Slides were stained with Wright Giemsa and reviewed by 2 independent investigators. Digital pictures were taken with a Nikon Coolpix 4500 camera (Nikon).
Integration site analysis
To determine the LIC frequency of G374 cells in the dog, BM GFP+YFP+ cells from the dog that received YFP-expressing lentiviral vector marked G374 cells (GFP+) were sorted by FACS and genomic DNA was extracted for integration site analysis. Integration site analysis was performed using LAM-PCR as described previously [19]. PCR product was separated with 4-12% PAGE gel and individual bands were counted.
Results
The canine leukemia cell line G374 has sustained in vitro self-renewal potential and causes leukemia in dogs that resemble human AML
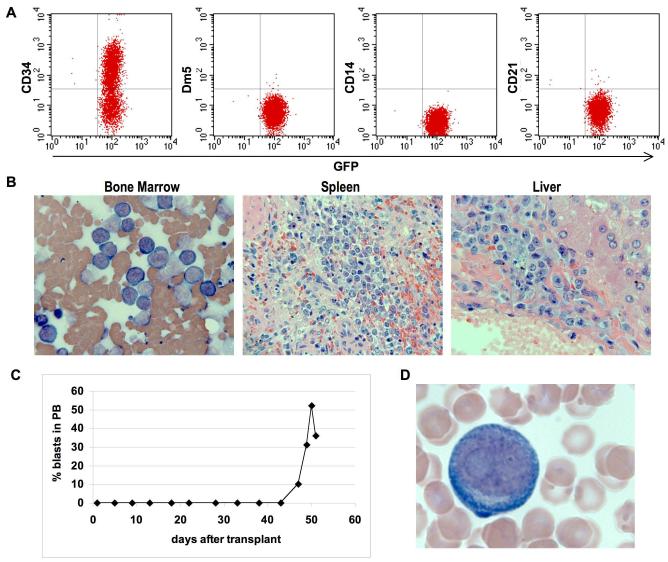
We have recently reported the isolation of a HOXB4-derived canine AML clone (G374 cell line) derived from the BM of a dog that developed leukemia 2 years after transplantation with HOXB4-overexpressing cells [17]. Accidental infusion of primary G374 cells into 2 dogs induced rapid lethal leukemia (the dogs were euthanized 80 and 126 days post transplant), demonstrating the ability of these cells to extensively expand in vivo [18]. To see whether the G374 clone can be continuously grown in vitro while retaining leukemogenic potential in vivo, cells cultured for a period of over one year were injected into two dogs. The cells demonstrated sustained self-renewal potential in vitro in the presence of SCF, FL and TPO, with a doubling time of ~24 hours. FACS analysis shows that these cells are undifferentiated progenitors, with more than 50% of the cells expressing CD34, but not other lineage-specific markers such as Dm5, CD14 and CD21 (Fig. 1A). Transplantation of 108 or 107 G374 cells into dogs G902 and G966 respectively, induced lethal leukemia with a phenotype that resembles human AML, 12 (Fig. 1B) and 51 (Fig. 1C & D) days post transplant, respectively. These data demonstrate that the G374 cell line can be extensively grown in vitro while retaining the ability to recapitulate the original disease in vivo.
Figure 1.
Long-term proliferation of the canine leukemia cell line (G374) in vitro and induction of leukemia in dogs. (a) Immunophenotype of G374 line. (b) HE stained bone marrow, spleen and liver sections in dog transplanted with 108 G374 cells. (c and d) flow cytometric analysis of blood for GFP+ cells and Wright-Giemsa stained blood smear in dog transplanted with 107 G374 cells.
The G374 line demonstrates leukemogenic potential and extensive self-renewal capacity in mice
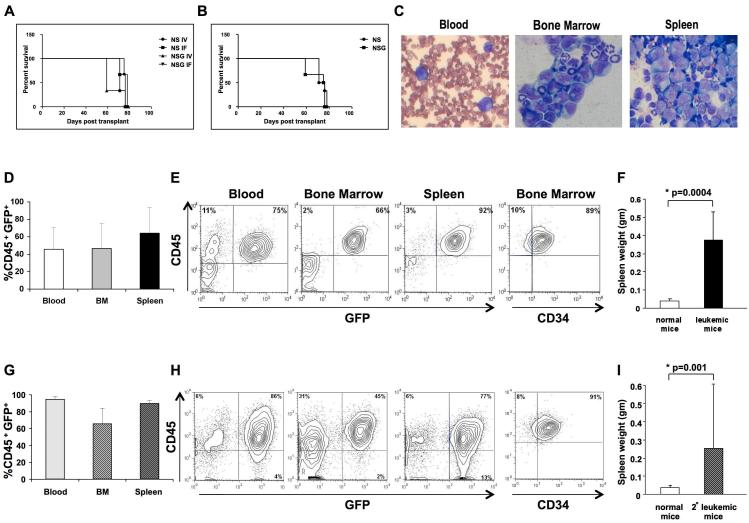
To determine whether the G374 line can be studied in mouse xenotransplants, we performed intravenous (IV) and intrafemoral (IF) transplantation of 5 and 2.5 million cells into NOD/SCID (NS) and NOD/SCID/IL2Rγnull (NSG) mice. Independent of the strain and route of transplantation, all 12 mice injected developed leukemia. The median latency to diagnosis of disease for NS and NSG mice injected IV was 71 and 59 days, respectively; NS and NSG mice injected IF had a median latency of 78 and 76 days, respectively. Kaplan-Meier comparison of survival curves showed that there was no significant differences between groups (p = 0.5, Fig. 2A and B).
Figure 2.
The G374 line induces leukemia in immunodeficient mice. NS and NSG mice were transplanted with G374 cells IV or IF, 3 mice per group. Mice were sacrificed when they showed signs of illness. Kaplan-Meier survival analysis is shown for each cohort of mice (a) and for each strain (b). (c) Wright-Giemsa-stained peripheral blood smear and cytospins of bone marrow and spleen from a representative mouse showing many myeloid blasts. Flow cytometric analysis of blood, BM and spleen of all primary (d) and secondary (g) mice. Immunophenotype of cells from a representative primary (e) and secondary (h) mouse are stained with canine CD45 and CD34 antibodies. Spleen weight (±SEM) in primary (f) and secondary (i) mice in comparison with normal mice. The statistical analysis was performed using two-tailed t-test.
In all animals, G374 cells underwent extensive expansion and organ infiltration resulting in lethal disease typical of acute leukemia (Fig 2C). Analysis of blood, BM and spleens of these mice at the time of sacrifice revealed accumulation of a large number of GFP-expressing CD45+ leukemic cells (45.8 ± 25.1%, 46.6 ± 28.6% and 64.2±29.4% respectively (Fig. 2D and 2E)). Up to 89% of the cells in the BM expressed the primitive CD34 marker (Fig. 2E). All mice had splenomegaly, with spleen weight of 0.373gm ± 0.16gm in leukemic versus 0.038gm ±0.01gm in normal mice (p=0.0004) (Fig. 2F). The ability of the leukemic clone to self-renew in mice was confirmed by secondary transplantation of cells from the primary recipient that received 2,500,000 G374 cells IF. In the first experiment, 4 mice received secondary transplants; 1 with 2,000,000 and 3 with 500,000 GFP+ BM cells, all mice developed leukemia. Only one of these mice was available for analysis, and therefore a more detailed phenotypic analysis of secondary leukemia was performed using BM cells of a primary recipient transplanted IF with 100,000 clonally expanded G374 cells (clone 3). Five of seven mice that received secondary transplants (2 with 10,000, 2 with 1,000 and 1 with 100 GFP+ BM cells) developed leukemia with a median latency to diagnosis of 5.5 months. Disease was similar to the primary recipients, with high level of GFP-expressing CD45+ leukemic cells in blood (95±2.8%), BM (66±18.2%) and spleen (91.7±1.5%) (Fig. 2G and 2H). These mice also had splenomegaly (0.256gm±0.99gm, p=0.001) (Fig. 2I).
Together, these results demonstrate the ability of the G374 cells to engraft and self-renew in mice, establishing a framework for further analysis of these cells in a practical xenotransplantation model.
The G374 line contains LIC detectable at comparable frequencies in both dogs and mice
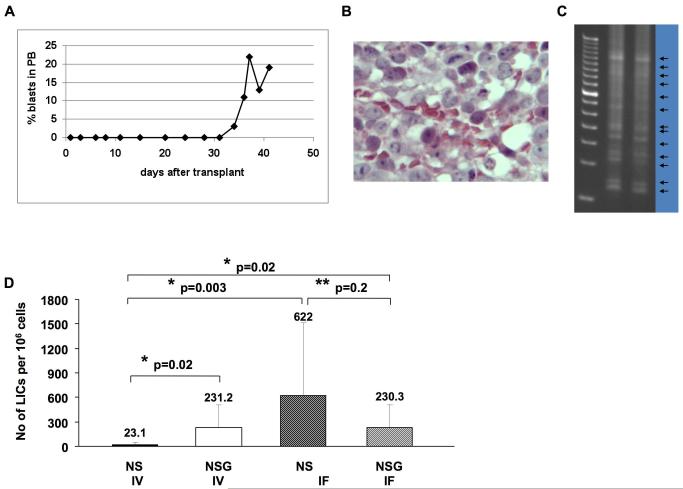
The human/mouse xenotransplantation model is criticized for underestimating the number of LICs. As the use of a large number of large animals for limiting dilution assays is not practical or ethical, we developed a novel strategy based on the tracking of integration of YFP marked cells. A lethally irradiated dog was transplanted with 1 million G374 cells, 1% of which (104) were marked with the YFP virus following infection at a very low MOI and thus likely to yield single proviral integration in successfully transduced cells. To support animal survival after transplantation, 1×107 unprocessed autologous CD34+ cells were co-transfused. This dog died of myeloid leukemia 6 weeks after transplantation with 19% blast cells in blood (Fig. 3A) and a large amount of blast cells in the BM (Fig. 3B). LAM-PCR analysis showed more than 10 unique integrations, estimating the presence of at least 10 YFP-marked leukemic clones (assuming a single integration event per clone, and relatively high sensitivity of the LAM-PCR assay for detecting most of the marked clones) within 104 YFP-marked cells engrafted this dog (Fig. 3C), and thus corresponding to a detectable LIC frequency of 1 in 1,000.
Figure 3.
The G374 line contains high number of LICs in dog (a, b and c) and mice (d). Flow cytometric analysis of blood for GFP+ cells (a) and HE stained BM section (b) of a dog transplanted with YFP-marked (1%) 106 G374 cells. (c) LAM-PCR analysis to track YFP marked LICs in the BM. (d) The number of calculated LICs per 106 G374 cells in NS and NSG transplanted IV or IF. The number of LICs in NSG mice transplanted IV or IF (p=0.02) and NS mice transplanted IF (p=0.003) are greatly increased when compared to the cohort of NS mice transplanted IV. LIC frequency was calculated by Poisson statistics and the method of maximum likelihood using L-Calc software (StemCell Technologies).
To further investigate the frequency of LICs, we performed limiting dilution assays in the more practical xenotransplantation model by transplantation into NS and NSG mice IV and IF. When comparing the LIC readout of the IV route between the 2 strains, the number of LICs was significantly greater (p=0.02) in the NSG (1 in 4,341) than NS mice (1 in 43,267) (Table 1 and Fig. 3D). These results indicate that, similar to human/mouse xenografts, the frequency of dog LICs is underestimated in the standard NS model [20, 21]. Moreover, as shown in human/mouse xenografts [5], injection of the cells directly into the BM of NS mice increased their ability to engraft and self-renew, increasing the detected frequency of LICs to 1 in 1,609 (Table 1 and Fig. 3D). No difference was seen in the LIC readout between IV (1 in 4,326) and IF (1 in 4,341) administration in the NSG mice (Table 1 and Fig. 3D). Strikingly, by using IF injection and/or NSG recipients, the estimated LIC frequency was similar to that measured in the canine transplant setting of 1 in 1,000.
Table 1.
Differential leukemia initiating cell frequency in the G374 line by different assays.
Limiting dilution assay was performed in NS and NSG mice transplanted with variable numbers of G374 cells IV or IF. The proportion of positive mice in each group was determined by FACS analysis of GFP+ leukemia cells in blood, BM and spleen, and splenomegaly in mice that showed signs of illness or died. LIC frequencies and 95% CI (Confidence Interval) were calculated by Poisson statistics and the method of maximum likelihood using L-Calc software (StemCell Technologies).
| Mouse Strain | Cell Dose | No of mice with leukemia/ no of mice tested |
Frequency (95% CI) |
|---|---|---|---|
|
NS
IV |
100,000 | 2/2 | 1 in 43,267 (1 in 9,332-1 in 200,617) |
| 10,000 | 0/2 | ||
| 1,000 | 0/2 | ||
| 100 | 0/1 | ||
|
| |||
|
NSG
IV |
1,000,000 | 2/2 | 1 in 4, 326 (1 in 933-1 in 20,061) |
| 100,000 | 2/2 | ||
| 10,000 | 2/2 | ||
| 1,000 | 0/2 | ||
| 100 | 0/2 | ||
|
| |||
|
NS
IF |
2,500,000 | 1/1 | 1 in 1,609 (1 in 281-1 in 9,211) |
| 500,000 | 2/2 | ||
| 100,000 | 3/3 | ||
| 10,000 | 3/3 | ||
| 1,000 | 1/2 | ||
| 100 | 0/2 | ||
| 10 | 0/2 | ||
|
| |||
|
NSG
IF |
500,000 | 1/1 | 1 in 4, 341 (1 in 939-1 in 20,080) |
| 100,000 | 2/2 | ||
| 10,000 | 2/2 | ||
| 1,000 | 0/2 | ||
| 100 | 0/2 | ||
| 10 | 0/2 | ||
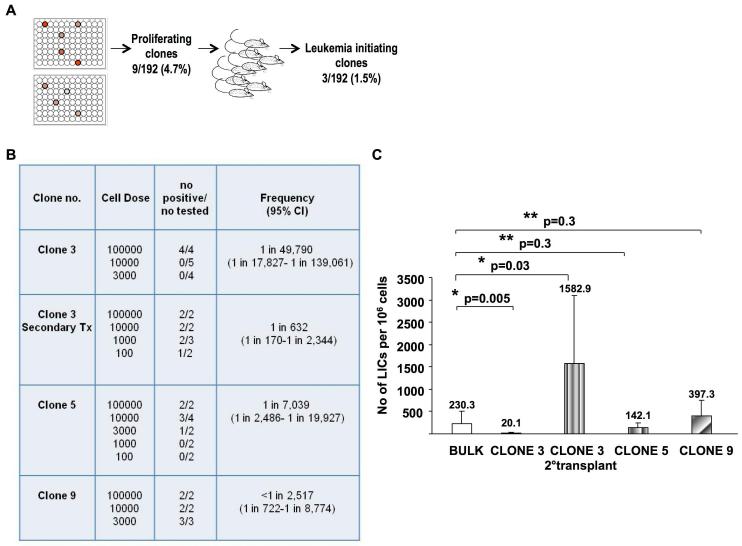
In vitro subcloning of the G374 line reveals increased number of LIC and heterogeneity in their self-renewal potential
To determine whether the number of LICs revealed by limiting dilution assay is a reflection of heterogeneity in LICs’ self-renewal potential and/or inefficiency of LIC detection due to their low probability of self-renewal, we utilized an additional experimental approach. G374 cells were subcloned by seeding of single cells in 2 96-well plates. Nine clones (~5%) of these single cells expanded in culture over 2 weeks (Fig. 4A). The leukemogenic potential of all 9 subclones was tested in NSG mice injected IF. Interestingly, 30% of the expanded clones (1.5% of all plated) were leukemogenic, and this was independent of their proliferation kinetics (data not shown), suggesting LICs in the G374 line are somewhat more frequent than estimated by transplant assays in the dog or NSG transplant models. Limiting dilution studies performed with the leukemogenic clones revealed that the number of LICs within these subclones was similar to the bulk population in 2 clones, ranging from 1 in 2,517 to 1 in 7,039 in clones 9 and 5 respectively, (p=0.3), but significantly lower in clone 3, 1 in 49,790 (p=0.005), thus indicating heterogeneity in LIC’s self-renewal potential (Fig. 4B and 4C). Secondary transplant of clone 3 revealed a LIC frequency of 1 in 632 again consistent with reestablishment of a leukemic cell hierarchy in vivo although the increased frequency detected compared to immediately after in vitro expansion suggest that LICs can show heightened self-renewal properties in vivo (Fig. 4B and 4C).
Figure 4.
The G374 line contains LICs with heterogeneous self renewal potential. (a) Schematic diagram of subcloning of 192 single cells, 9 of which proliferated and 3 of these induced leukemia in NSG mice transplanted IF. (b) Limiting dilution analysis performed with 3 leukemogenic clones in NSG mice transplanted IF. LIC frequencies and 95% CI (Confidence Interval) were calculated by Poisson statistics and the method of maximum likelihood using L-Calc software (StemCell Technologies). (c) The number of calculated LICs within the subclones was similar to the bulk population in clone 5 and 9 (p=0.3), significantly lower in the primary recipients of clone 3 (p=0.005), and significantly higher in the secondary recipients of this clone (p=0.03).
Discussion
In this study we used a novel large animal model in conjunction with mice xenotransplants to functionally characterize LICs contained in the HOXB4-induced G374 canine leukemic cell line. We showed that G374 LICs are capable of sustained in vitro self-renewal while maintaining their ability to induce AML that resembles human disease in both dogs and mice. Furthermore, we exploited a strategy for the quantification of LICs’ frequency in large animals and showed that the frequency of LICs is comparable to that measured by limited dilution in mouse xenotransplants. We also demonstrated, using single cell analysis, that G374 LICs are heterogenous in their self-renewal capacity and regenerate a leukemic cell population in vivo and in vitro consistent with a hierarchical leukemia model. The availability of this novel framework should accelerate the characterization of LICs and the translation of animal studies into clinical trials.
Standard anti-cancer drugs are aimed at killing the bulk AML population; however, targeting the LICs that sustain the disease may prove to be a more efficient therapy. A limitation in the development of such strategy arises from the lack of suitable experimental models, which have been difficult to accomplish due to poor engraftment of human AML LICs into mice [22] constraints inherent in the quantity and variability associated with studying human samples, and physiological differences between humans and mice that make the xenograft an unreliable prediction model for evaluation of therapies. On the other side, large animal models such as the outbred dog share many biochemical and physiological characteristics with humans; however, they present major limitations due to high vivarium costs and constraints in the number of animals making it difficult to perform large scale studies. Thus, a combination of both models could provide a more effective framework. In this regard, Guzman et al. have tested the effect of the orally bioavailable parthenolide analog DAMPT on LICs from three dogs diagnosed with CD34-positive acute leukemia and in NS mice transplanted with primary dog leukemic cells [16]. More recently, Stoica et al. have used a Boxer dog and nude mice xenografts to identify and characterize a cancer stem cell population from glioblastoma multiforme [23]. In both cases, however, they used spontaneous sick dogs and primary cancer cells, which limit the length of the studies and the quantity of study material available, respectively. In this context, the ability of the G374 line to sustain extensive self-renewal in vitro while retaining LIC activity in both dogs and mice provides unlimited number of cells and allows the generation of de novo AML that closely resemble the human disease in both large and small animal model.
Knowing how many and how effectively LICs are targeted by a treatment will be crucial for evaluating potential targeted therapies. The xenotransplantation model is the most used for determining LIC frequency; however, estimated numbers may vary with the immune status of the recipients, as well as the route of administration. Regarding the former, in studies on human AML, a 40-fold reduced number of CD34+CD38− AML cells were required to initiate leukemia in NS mice compared with SCID mice [1]. Moreover, while CD34+CD38− AML cells could form secondary neoplasms in NS mice, they failed to do so in SCID hosts, indicating that the recipient immune status highly influences the LIC phenotype and function in this malignancy [1]. A high frequency of engraftment of peripheral blood [24] and cord blood [25-27] stem cells has been described in the highly immunocompromised NSG mice. When the same strain of mice was used to study melanoma cancer cells, Quintana et al. found that 15–25% of the tumor cells exhibited cancer stem cell activity [28]. Our findings of increased LIC frequency as measured in NSG mice compared with NS are in accordance with these data. As for route of administration it is known that as their normal counterpart cancer initiating cells reside in the niche and that their inability to reach the niche can lead to underestimation of cell frequency [29]. In this regard, we showed increased LIC frequency in the NS model when cells were injected directly into the BM. To avoid the potential setbacks introduced by xenotransplantation, some researchers prefer to evaluate frequency using syngeneic systems. For example, Kelly et al. performed limiting dilution assays of lymphoma and AML cells in syngeneic mice, and found that as many as 10% of the transferred cells could initiate malignant growth in the transplanted mouse [21]. However, other studies showed that when genetically comparable leukemia models are studied, syngeneic and xenogeneic approaches can yield similar calculations of frequency [30, 31]. Due to the need of large number of animals, evaluation of LIC frequency by limiting dilution is not feasible in dogs. Based on the tracking of integration of YFP marked cells, we determined a LIC frequency of approximately 1 in 1,000, which was similar to that measured in NS mice using IF injection or in the most permissive xenotransplant model, (NSG) by IF or IV injection thus providing validation for use of the xenostranplant model to efficiently detect LIC.
The LIC pool in AML has been shown to be composed of distinct LICs that are hierarchically organized because of heterogeneity in longevity of the produced clone and self-renewal capacity [32]. This notion is very important, as cancer pathways may function differently in each LIC subclass than in the bulk leukemic blasts, thus different responses to a given therapy may result. Using single clone analysis we showed that only a small subset of cells in the G374 line are capable of sustained growth, consistent with a hierarchical leukemic cell population. Interestingly, only a subset of those cells capable of sustained growth also had detectable LIC, again consistent with the leukemic stem cell model. Interestingly, there was no correlation between the LIC content and the proliferation kinetics of individual clones, unlike that documented by Brummendorf et al, for clonally expanded human fetal liver cells where an inverse correlation was observed between the growth kinetics and the yield of phenotypically defined primitive cells [33]. This might suggest interesting differences in the mechanisms underlying self-renewal decisions in normal versus leukemic stem cells meriting future investigation. Of further interest, measurement of the LIC frequency within expanded clones revealed substantial heterogeneity in agreement with studies by which demonstrated a hierarchy of human AML LICs, and thus adding further strength to the biological relevance of the G374 leukemia model. Intriguingly, clonal expansion analysis indicated that overall up to 1.5% of cells in the G374 line can fulfill the criteria for LIC or some 10x higher than the frequency determined by limiting dilution from bulk populations. Thus, as shown already by Somervaille and Cleary [34], by comparison with single cell isolation and transplantation experiments, limit dilution analysis appeared to underestimate the number of cells with LIC potential and thus to overestimate the steepness of the leukemia hierarchy. In conclusion, we describe here a novel framework that provides unlimited number of cells with leukemogenic potential for studies in large and small animal models. Its availability should prove useful for the characterization of LICs and for the acceleration of proof-of-concept studies that will translate to clinical trials.
Acknowledgments
The authors would like to thank to Ling-Yi Chen, Donald Lai and Christy Brookes for technical assistance. This work was supported by grants from the National Institutes of Health (Bethesda, MD, USA) HL36444, HL74162 to H-P K); the Terry Fox Foundation (to RKH) and the Canadian Stem Cell Network (Cancer Stem Cell project to RKH).
Footnotes
Conflict of interest: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- [2].Kennedy JA, Barabe F. Investigating human leukemogenesis: from cell lines to in vivo models of human leukemia. Leukemia. 2008;22:2029–2040. doi: 10.1038/leu.2008.206. [DOI] [PubMed] [Google Scholar]
- [3].Feuring-Buske M, Gerhard B, Cashman J, Humphries RK, Eaves CJ, Hogge DE. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia. 2003;17:760–763. doi: 10.1038/sj.leu.2402882. [DOI] [PubMed] [Google Scholar]
- [4].Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- [5].Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9:959–963. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- [6].McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259–1261. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- [7].Agliano A, Martin-Padura I, Mancuso P, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- [8].Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- [9].Sanchez PV, Perry RL, Sarry JE, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009;23:2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- [11].Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Forster A, Pannell R, Drynan LF, et al. Engineering de novo reciprocal chromosomal translocations associated with Mll to replicate primary events of human cancer. Cancer Cell. 2003;3:449–458. doi: 10.1016/s1535-6108(03)00106-5. [DOI] [PubMed] [Google Scholar]
- [13].Schwieger M, Lohler J, Fischer M, Herwig U, Tenen DG, Stocking C. A dominant-negative mutant of C/EBPalpha, associated with acute myeloid leukemias, inhibits differentiation of myeloid and erythroid progenitors of man but not mouse. Blood. 2004;103:2744–2752. doi: 10.1182/blood-2003-07-2280. [DOI] [PubMed] [Google Scholar]
- [14].Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans--man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- [15].Brooks MSD. The Genetics of the Dog. CABI Publishing; Wallington: 2001. Genetics aspects of diseases in dogs. [Google Scholar]
- [16].Guzman ML, Rossi RM, Neelakantan S, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang XB, Beard BC, Trobridge GD, et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J Clin Invest. 2008;118:1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thakar MS, Zhang XB, Beard BC, et al. Transmission and expansion of HOXB4-induced leukemia in two immunosuppressed dogs: implications for a new canine leukemia model. Exp Hematol. 2009;37:1157–1166. doi: 10.1016/j.exphem.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beard BC, Dickerson D, Beebe K, et al. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- [20].Dick JE, Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int J Hematol. 2005;82:389–396. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- [21].Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- [22].Bonnet D. Curr Protoc Stem Cell Biol. John Wiley & Sons, Inc.; 2008. Vivo Evaluation of Leukemic Stem Cells through the Xenotransplantation Model. 2009. [DOI] [PubMed] [Google Scholar]
- [23].Stoica G, Lungu G, Martini-Stoica H, Waghela S, Levine J, Smith R., 3rd Identification of cancer stem cells in dog glioblastoma. Vet Pathol. 2009;46:391–406. doi: 10.1354/vp.08-VP-0218-S-FL. [DOI] [PubMed] [Google Scholar]
- [24].Shultz LD, Lyons BL, Burzenski LM, et al. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-scid IL2R{gamma}null Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- [25].Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma cnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- [26].Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McDermott SP, Eppert K, Lechman E, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010 doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- [28].Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- [30].Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- [31].Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. doi: 10.1126/science.1149590. author reply 1722. [DOI] [PubMed] [Google Scholar]
- [32].Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- [33].Brummendorf TH, Dragowska W, Zijlmans J, Thornbury G, Lansdorp PM. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J Exp Med. 1998;188:1117–1124. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]