Abstract
Background
In the U.S. older adults have low rates of follow-up colonoscopy after a positive screening FOBT result. The long-term outcomes of these real world practices and their associated benefits and burdens are unknown.
Methods
Longitudinal cohort study of the 212 patients 70 years or older with a positive screening FOBT at 4 Veteran Affairs facilities in 2001, followed through 2008. We determined the frequency of downstream outcomes during the 7 years of follow-up, including procedures, colonoscopic findings, outcomes of treatment, complications, and mortality based on chart review and national VA and Medicare data. Net burden or benefit from screening and follow-up was determined according to each patient’s life expectancy. Life-expectancy was classified into three categories: best (age 70-79 and Charlson=0), average, and worst (age 70-84 and Charlson ≥ 4 or age ≥ 85 and Charlson ≥ 1).
Results
56% (118/212) of patients received follow-up colonoscopy, which found 34 significant adenomas and 6 cancers. 10% (12/118) experienced complications from colonoscopy or cancer treatment. 46% (43/94) of those without follow-up colonoscopy died of other causes within 5 years of FOBT while 4 died of colorectal cancer. 87% (26/30) of patients with worst life expectancy experienced net burden from screening, 70% (92/131) with average life expectancy, and 65% (35/51) with best life expectancy (P for trend = 0.048).
Conclusions
Over a 7-year period, older adults with best life-expectancy are less likely to experience net burden from current screening and follow-up practices than those with worst life expectancy. The net burden could be decreased by better targeting FOBT screening and follow-up to healthy older adults.
INTRODUCTION
In many real world settings, less than 60% of patients receive colonoscopy within 1 year of a positive FOBT result.1-4 A recent study found many older patients without follow-up are in poor health or decline follow-up suggesting they should not have been screened in the first place. 5 In addition, rates of follow-up have shown minimal improvement over the last decade.3,4,6,7 Despite persistently low rates of follow-up colonoscopy in older adults with positive screening FOBT results, the long-term outcomes of these real world screening and follow-up practices have not been described.3,4,8
The benefit of finding asymptomatic cancer or precancerous polyps that would have caused symptoms years later must be weighed against immediate burdens of follow-up procedures and treatments stemming from a positive screening result.9 Randomized trials of FOBT suggest that a person should have a life expectancy of at least 5 years to derive survival benefit from screening, otherwise they are only subject to the potential burdens.9,10 We are not aware of any studies of real world practices that follow older patients for more than 3 years after a positive screening FOBT result to determine whether patients lived long enough to potentially benefit from detecting large adenomas or early stage colorectal cancer, or whether they only experienced burdens from follow-up procedures and treatments (e.g., false-positive results, repeated testing, complications).3,4 Such information about the benefits and burdens experienced by older adults with positive FOBT results would help to guide individualized screening and follow-up decisions in clinical practice.
We describe 7-year outcomes following a positive screening FOBT result in older adults in real world clinical practice. We took a novel approach of combining VA and Medicare claims and chart review to follow the downstream outcomes after a positive FOBT result among patients 70 years or older screened at 4 geographically diverse VA’s. Downstream outcomes included follow-up testing, polypectomies, cancer diagnoses and treatment, procedural complications and overall mortality. Net benefit or burden resulting from these screening and follow-up practices was determined according to each patient’s predicted life-expectancy.11 The goal is to inform how clinical practice could improve to maximize the net benefit of FOBT screening and follow-up in older adults.
METHODS
Data Sources and Patients
We conducted a longitudinal cohort study of 212 adults aged ≥ 70 years who had a positive screening FOBT result during 2001 at 4 VA facilities (Minneapolis, Durham, Portland, and West Los Angeles) and followed them for 7 years to determine downstream outcomes. We chose to focus on positive screening FOBT results as FOBT is the most common colorectal cancer screening test within the VA.12 To identify our cohort, we used outpatient claims from the VA National Patient Care Database to identify all patients ≥ 70 years who had a screening FOBT between 1/1/01 and 12/31/01 at the 4 facilities and met our inclusion and exclusion criteria (n=2410).5 All patients had continuous enrollment in Medicare Parts A and B and fee-for-service coverage during 1/1/00-12/31/02. Patients were excluded if they had a history of colorectal cancer or polyps, inflammatory bowel disease, colectomy, or colostomy, or were not due for screening.5 We used claims from 6 months before their FOBT and chart review to exclude patients with signs or symptoms that would justify performance of FOBT for non-screening purposes (e.g., iron-deficiency anemia, gastrointestinal bleeding, abdominal pain, change in bowel habits, and unexplained weight loss).5 212/2410 (9%) patients had positive screening FOBT results which were extracted from the Veterans Health Information Systems and Technology Architecture (VISTA) laboratory package. If any FOBT cards were positive for occult blood, the FOBT was considered “positive.”
We used National VA Data Systems as well as inpatient and outpatient Medicare claims through 12/31/02, to capture follow-up testing within one year of a positive FOBT result inside and outside the VA system.5 Age was determined on the date of the screening FOBT. Comorbidity was measured using the Deyo adaptation of the Charlson Comorbidity Index, derived from administrative data.13,14 Charlson-Deyo scores were calculated from VA and Medicare inpatient and outpatient claims during the 12 months before the date of the FOBT. Race and gender were obtained from both VA and Medicare data.
Next, three investigators (CK, MC, and LW) reviewed VA medical records to determine the long-term outcomes of screening and follow-up through 12/31/08. The VA computerized medical records system contains notes from all inpatient and outpatient visits. These notes contain information about follow-up that occurs outside the VA per patient report to their VA clinician.15
Outcomes of Screening
First, we categorized patients as having received follow-up colonoscopy related to their positive FOBT result or not. Patients who did not receive colonoscopy over the 7 years or had a colonoscopy for GI bleeding or other symptoms unrelated to their positive FOBT result were categorized as the “no follow-up colonoscopy” group. Patients with follow-up colonoscopy were categorized according to whether they were diagnosed with cancer, significant adenoma, or non-significant/normal findings. Significant adenoma was defined as one or more large adenomas (≥1 cm), 3 or more small adenomas (<1 cm), or any adenoma with villous pathology based on standard GI guidelines.16 Non-significant or normal findings included findings of diverticuli, less than 3 small adenomas (<1cm), angiodysplasia, hyperplastic polyps, and normal mucosa.
Next we determined downstream outcomes over 7 years, in those with and without follow-up colonoscopy. Outcomes included cancer diagnoses, complications from treatment, frequency of follow-up testing, and 5-year mortality. Cancer diagnoses included cancer stage and treatment. Complications included those chart-documented as related to colonoscopy or cancer treatment (e.g., pain/discomfort, gastrointestinal bleeding, infection, and death). Frequency of follow-up testing included the number of repeat FOBT tests, sigmoidoscopies, barium enemas, and/or colonoscopies patients received during the 7 years. Five-year mortality was determined from the VA Vital Status File. 17
Next 2 authors (CK, LW) reviewed each patient’s outcomes to determine whether they experienced net benefit or net burden from screening and follow-up. Patients were considered to have experienced net benefit if they had a significant adenoma/colorectal cancer on follow-up and lived at least 5 years, even if they had complications or repeat procedures.18,19 Net benefit or burden was indeterminate for 1) patients with 1-2 small adenomas who lived at least 5 years because some clinicians believe this group potentially benefitted from screening20-22 or 2) patients who had a colonoscopy outside of the VA without an available pathology report and lived at least 5 years. All other patients, including those with normal findings on follow-up colonoscopy (i,e., false-positive FOBT results), those without follow-up colonoscopy (i.e., had a positive FOBT result that was not worked-up), and those who died within 5 years of their FOBT (i.e., subjected to tests for an asymptomatic disease that would never have affected them), were defined as experiencing net burden. While net burden may be small in some cases (e.g. those without follow-up who did not develop cancer), some older adults do experience embarrassment and discomfort from performing FOBT23,24 and a positive test result may be anxiety-provoking.25
Statistical Analyses
To describe downstream outcomes we observed patients from the date of their positive FOBT result until death or the end of the study period (12/31/08). To determine the association between life expectancy and net benefit from screening, we stratified patients into 3 subgroups a priori: Best Life Expectancy–the youngest and healthiest patients (Age 70-79 and Charlson score = 0) who were expected to live > 10 years; Average Life Expectancy–the younger patients with increasing comorbidity and the oldest healthiest patients (age 70-79 and Charlson = 1-3 or age ≥80 and Charlson score = 0) who were expected to live 5-10 years; and Worst Life Expectancy–the sickest and oldest patients (age 70-84 and Charlson ≥ 4 or age ≥ 85 and Charlson ≥ 1) who were expected to live < 5 years.13 Differences between patients receiving net burden and net benefit were determined according to worsening life expectancy using the Cochran-Armitage chi-square test for trend. We used SAS, version 9.1 (SAS Institute, Cary, North Carolina) for all analyses.
The Committee on Human Research at the University of California, San Francisco; the Committee for Research and Development at the San Francisco VA Medical Center; and the Institutional Review Board at the Minneapolis VA Medical Center approved the study.
RESULTS
Participant Characteristics
The mean age of the 212 patients was 76.4 years (range 70 to 89 years). Consistent with the elderly veteran population, 99.5% (211/212) were men and 84.9% (180/212) white (Table 1). 31.1% (66/212) patients died within 5 years of their FOBT result. 5-year mortality was 5.9% (3/51) for patients with best life expectancy, 38.2% (50/131) for patients with average life expectancy, and 46.7% (14/30) for patients with worst life expectancy which equates to a life expectancy of 5.4 years.
Table 1.
Characteristics of Patients Aged 70 years and Older with a Positive FOBT Results (N=212)
| Characteristic | N (%) |
|---|---|
| Age, years | |
| 70-74 | 75 (35.4) |
| 75-79 | 93 (43.9) |
| ≥ 80 | 44 (20.8) |
|
| |
| Male Gender | 211 (99.5) |
|
| |
| Race/Ethnicity | |
| White | 180 (84.9) |
| Black | 30 (14.2) |
| Other | 2 (0.9) |
|
| |
| Married | 145 (68.4) |
|
| |
| Charlson categories* | |
| 0 | 66 (31.1) |
| 1-3 | 119 (56.1) |
| 4+ | 27 (12.7) |
|
| |
| VA Site | |
| A | 57 (26.9) |
| B | 119 (56.1) |
| C | 21 (9.9) |
| D | 15 (7.1) |
|
| |
| Lived in ZCTA in which ≥ 25% of Adults Had a College Education** | 56 (26.4) |
|
| |
| Median Income of ZCTA (dollars) | 22,378 (9,810- 75,050) |
|
| |
| Anti-coagulated with Coumadin | 26 (12.3) |
|
| |
| History of Prior CRC Screening | 117 (55.2%) |
|
| |
| Life Expectancy Groups | |
| Best Life-Expectancy (70-79 and Charlson =0) | 51 (24.1) |
| Average Life-Expectancy (70-84 and Charlson=1-3 or ≥85 years and Charlson=0) |
131 (61.8) |
| Worst Life-Expectancy (70+ and Charlson ≥ 4 or 85+ and Charlson≥1) | 30 (14.2) |
<1% of patients in our cohort had dementia at the time of their screening FOBT test.
ZTCA=ZIP Code Tabulation Areas
Outcomes of Screening
Patients with Follow-up Colonoscopy
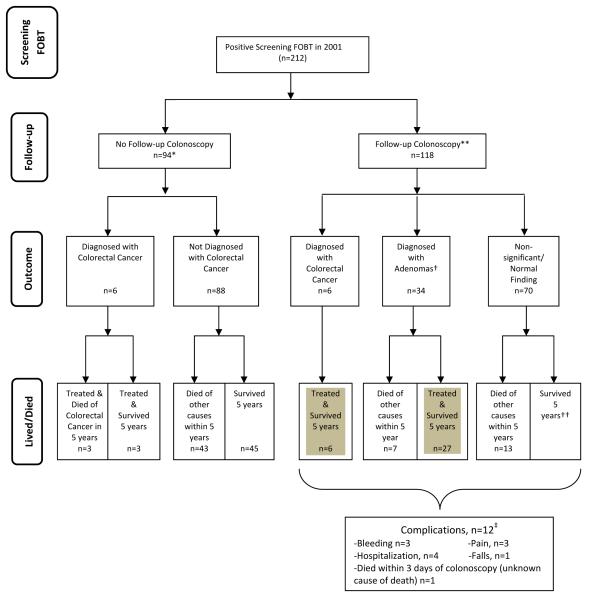
Among the 118 patients who had follow-up colonoscopy over the 7 year period, 6(5.1%) patients had cancer, 34 (29%) had significant adenoma, and 70(59%) had neither cancer nor significant adenoma (Figure 1). One patient with a normal colonoscopy developed an interval colorectal cancer 5 years later which was successfully treated but he died a year later of congestive heart failure. Eight patients had colonoscopies outside the VA without an available pathology report so it is unclear if they had a significant adenoma or not. No subsequent notes indicated that any of these patients were ever diagnosed with colorectal cancer. 17% (20/118) of these patients died within 5 years of causes other than colorectal cancer. We also assessed complications of follow-up. 10% (12/118) of patients who underwent colonoscopy or cancer treatment experienced complications as described in Figure 1. Three of the 6 patients with cancer had complications from surgical treatment of their cancer (Table 2)–although all eventually recovered and survived more than 5 years after their positive FOBT. Lastly, 29.4% (10/34) patients with significant adenomas had three or more follow-up tests over the 7 years, compared to 20.0% (14/70) of patients with non-significant/normal colonoscopy results.
Figure 1. Flowchart of the Long-term Outcomes Following a Positive Screening FOBT Result (n=212).
* Among the 94 patients who did not have a follow-up colonoscopy related to working-up their positive FOBT result, 10 patients ultimately underwent colonoscopy to work-up symptoms that developed over the 7-year period (e.g., hematochezia, unexplained weight loss, anemia) and 2 patients underwent screening colonoscopy many years later without any mention of their positive FOBT result from 2001. The 3 patients in the no follow-up colonoscopy group who died of colorectal cancer all had refused follow-up colonoscopy.5
**8 patients did not have a pathology report available from their colonoscopy performed outside of VA to determine size or type of polyps detected. Therefore we were unable to definitively classify them as “significant adenoma” versus “non-significant/normal findings.” None of these 8 patients have any evidence in their medical records that they ever were diagnosed with colorectal cancer. Overall, 17% (35/212) patients received follow-up colonoscopy outside the VA healthcare system.
†Significant adenoma was defined as having an adenoma ≥1 cm, 3 or more adenomas or any adenoma with villous features.
††One patient was diagnosed with an incidental colorectal cancer and survived more than 5 years.
‡12 patients had complications from colonoscopy: three of the 34 patients with significant adenomas had notable bleeding following polypectomy–one required hospital admission; another had a vasovagal episode following epinephrine for bleeding; and one had the procedure aborted and required a repeat colonoscopy. Two patients had complications from other testing after their colonoscopy–one had a fall after a barium enema, requiring transfer to the emergency department but was otherwise unharmed and the other had a sigmoidectomy to treat a large adenoma complicated by a hypoxic event and a 3-week hospitalization. Three of the 45 patients with normal colonoscopies had discomfort with the colonoscopy. Lastly, colonoscopy may have contributed to the death of 1 patient who died within 3 days of his colonoscopy although the exact cause of death is unknown per the medical records. Gray shading indicates patients who potentially benefited from screening.
Table 2.
Outcomes of Patients Diagnosed with Colorectal Cancer According to Whether They Received Follow-up of their Positive FOBT Result (N=13)
| Prognostic Group |
Clinical Course |
|---|---|
| Patients who had a follow-up colonoscopy | |
| Best Life Expectancy |
74 year-old active man with no Charlson comorbidities who had a follow-up colonoscopy which found T2N1M0 colorectal cancer in 2002. He underwent curative hemicolectomy without complications. He had 2 normal colonoscopies since surgery and remains alive and healthy. |
| Best Life Expectancy |
73 year-old healthy man with no Charlson comorbidities who had a normal follow-up colonoscopy. In 2006 he suddenly developed hematochezia and colonoscopy found T1N0M0 colorectal cancer for which he underwent curative hemicolectomy. He remained healthy until 2007 when he was diagnosed with congestive heart failure and died later that year from heart failure. |
| Best Life Expectancy |
Healthy 71 year-old man with no Charlson comorbidities who had a follow-up colonoscopy which found rectal cancer. He underwent curative right hemicolectomy complicated by recurrent colostomy leakage. He remained relatively healthy until 2007 when he was diagnosed with lung cancer and died shortly thereafter. |
| Average Life Expectancy |
72 year-old man with emphysema who had a follow-up colonoscopy which found T1N0M0 colorectal cancer for which he underwent curative hemicolectomy without complications. He has had 3 normal follow-up colonoscopies during the study period. He developed renal failure in 2008 but remains alive. |
| Average Life Expectancy |
81 year-old man with chronic obstructive pulmonary disease who had a follow-up colonoscopy which found a 3cm colorectal cancer without metastases. He underwent curative hemicolectomy, developed delirium requiring prolonged hospitalization, but eventually recovered. He had progressive functional decline since surgery but remains alive. |
| Average Life Expectancy |
81 year-old with diabetes and mild heart failure who had a follow-up colonoscopy which found near obstructing colorectal cancer. He was referred for surgery but became unresponsive and required emergent colectomy. He recovered from surgery but developed progressive renal failure and remains alive in a nursing home. |
| Worst Life Expectancy |
74 year-old man with severe rheumatoid arthritis on chronic steroids, emphysema, and prostate cancer who had a follow-up colonoscopy which found a T1N0M0 colorectal cancerous polyp which was removed. Given a possibly inadequate resection, he had a sub- total colectomy complicated by repeat hospitalizations for recurrent wound infections and bowel obstruction. His chronic obstructive pulmonary disease worsened but he remains alive. |
| Patients who did not have a follow-up colonoscopy | |
| Best Life Expectancy |
77 year-old active man with prostate cancer on androgen deprivation therapy (this comorbidity was missed by claims data) who had a positive FOBT 2001. He was sent to GI clinic but adamantly refused colonoscopy since “he has prostate cancer, he doesn’t want to know if he has colon cancer.” He agreed to repeat FOBT 4 years later which was positive and at that time was persuaded to have a colonoscopy which found T3N0M0 colon cancer. He underwent curative hemicolectomy 2006 and remains alive. |
| Best Life Expectancy |
79 year-old healthy man with no Charlson comorbidities who had a positive FOBT 2001 but repeatedly declined colonoscopy over the next 6 years as his hematocrit slowly fell. In 2007 he developed hematochezia and colonoscopy found two obstructing colorectal cancers. Colonoscopy was complicated by aspiration requiring intubation delaying surgery for several weeks. He underwent a hemicolectomy in 2007 which was complicated by renal failure and sepsis and he died soon after in the ICU as a result of his late stage colorectal cancer. |
| Average Life Expectancy |
75 year-old active man with diabetes who had a positive FOBT 2001 and his VA physician recommended colonoscopy. Instead he had a sigmoidoscopy in 2001 which as done privately and was normal per patient report. He felt well until he developed hematochezia in 2006 and colonoscopy found Stage 3 colorectal cancer treated with resection, chemotherapy, and radiation, and he remains alive without recurrence. |
| Average Life Expectancy |
76 year-old man with a history of a myocardial infarction and coronary stents who had a positive FOBT 2001 and was referred for colonoscopy. Instead he had a barium enema and sigmoidoscopy due to his heart disease, which both found an obstructing colorectal cancer. Further work-up found widely metastatic disease. He underwent palliative hemicolectomy 2001 complicated by a myocardial infarction, abdominal abscesses, and delirium requiring prolonged hospitalization. He enrolled in hospice 2002 and died shortly afterwards of metastatic colorectal cancer. |
| Average Life Expectancy |
79 year-old obese man with diabetes on coumadin for atrial fibrillation who had a positive FOBT 2001 and scheduled for colonoscopy but he suddenly developed abdominal pain and narrow caliber stools so had a barium enema 2001 which found T3N1M0 colorectal cancer. He was treated with hemicolectomy and declined adjuvant therapy. He was diagnosed with metastatic disease 2001 and died shortly afterwards of metastatic colorectal cancer. |
| Worst Life Expectancy |
81 year-old man with stage III congestive heart failure, emphysema, diabetes with end-organ damage who had a positive FOBT 2001 but refused colonoscopy since he had one in 1987 and never wanted another. He developed progressive anemia and had a colonoscopy 2002 which found Stage 2 colorectal cancer. He underwent hemicolectomy which was complicated by pneumonia and he died shortly afterwards in the hospital. |
Patients with No Follow-up Colonoscopy
Among the 44.3% (94/212) who had no follow-up colonoscopy over the 7-year study period (Figure 1), 6.4% (6/94) were eventually diagnosed with colorectal cancer, of whom 4 died within a few months of treatment (Table 2). 45.7% (43/94) of patients who did not get a follow-up colonoscopy died within 5 years of causes other than colorectal cancer. 57.4% (54/94) of patients underwent some form of follow-up other than colonoscopy, such as repeat FOBT or sigmoidoscopy and 59.3% (32/54) of those patients had more than one follow-up test over the study period but never a colonoscopy.
Benefits and Burdens
15.6% (33/212) of patients were diagnosed with cancer or significant adenomas on follow-up colonoscopy and lived at least 5 years, and were defined as receiving net benefit (see Table 3). Since it is controversial whether the 22 patients with non-significant adenomas (<1cm) who lived at least 5 years benefitted from screening we categorized them as receiving indeterminate benefit as well as the 8 patients for whom we did not have pathology reports from their colonoscopy. The remaining 149 patients were defined as receiving net burden, including: 10 patients who had adenomas removed but died within 5 years; 45 patients with no adenomas of any kind found on colonoscopy (i.e. unequivocal false positives); and 94 patients who did not have a follow-up colonoscopy. The magnitude of net burden varied, ranging from false positive results in patients without cancer to cancers not found because patients declined follow-up (6.4%).
Table 3.
Downstream Outcomes Classified According to Benefits and Burdens from Screening and Follow-up Practices for Patients Aged 70 years and Older with a Positive FOBT Result (N=212)
| Downstream Outcomes (N) | Examples | Net Benefit or Net Burden |
|---|---|---|
|
| ||
| Follow-up Colonoscopy (N=118) | ||
| Cancer (N=6) | ||
|
| ||
| FOBT led to diagnosis of CRC and patient lived ≥5 yrs (N=6) |
74 year-old man, status post coronary artery bypass graft, otherwise healthy, with a curative resection for rectal cancer. Two repeat colonoscopies were negative for recurrence. He remains healthy |
Net Benefit |
|
| ||
| Significant Adenoma (N=34) | ||
|
| ||
| 1) FOBT led to diagnosis of a significant adenoma and patient lived ≥5 years (N=27) |
79 year-old man who was relatively healthy with a large adenoma (1.5 cm) on colonoscopy. He had 4 repeat colonoscopies over 7 years which found adenomas. He remains healthy. |
Net Benefit |
| 2) FOBT led to diagnosis of a significant adenoma but patient died within 5 years (n=7) |
74 year-old man with severe heart disease with an implanted cardiac defibrillator, dependent in several instrumental activities of daily living and a history of multiple falls who had 2 adenomas, one large (2 cm), found on colonoscopy. He suffered a broken hip in 2003 and died in early 2004. |
Net Burden |
|
| ||
| Insignificant/Normal Findings (N=78) |
||
|
| ||
| 1) FOBT found 1-2 small non-villous adenomas or indeterminate pathology and person lived ≥5 years (n=30) |
76 year-old man who was independent in instrumental activities of daily livings with 2 small adenomas (3mm, 8mm) found on colonoscopy. He had several additional procedures: Repeat FOBTs in 2002 and 2004 and repeat colonoscopy in 2005- all negative. He remains healthy. |
Indeterminate |
| 2) FOBT found no adenoma and person lived ≥5 years (N=35) |
76 year-old man who had peripheral neuropathy with 2 hyperplastic polyps found on colonoscopy. He had repeat testing with a normal colonoscopy in 2006 and no further screening was recommended. He remains alive but quite ill. |
Net Burden |
| 3) FOBT found no significant adenomas and person died within 5 years (N=13) |
75 year-old man with end-stage chronic obstructive pulmonary disease on home oxygen with multiple ER visits for dyspnea with 1 hyperplastic polyp found on colonoscopy. He was diagnosed with inoperable3-vessel coronary disease and severe aortic stenosis in 2004 but had a repeat colonoscopy which was negative. His angina worsened and he died in 2005. |
Net Burden |
|
| ||
| No Follow-up Colonoscopy (N=94) | ||
| 1) Had No Other Work-Up after +FOBT (N= 40) |
73 year-old man status post stroke with aphasia and left hemiparesis, coronary disease status post coronary artery bypass graft, and chronic obstructive pulmonary disease, who had screening at a preventive visit despite physician note indicating patient had < 5 year life expectancy and did not recommend screening. His physician recommended against further work-up and he progressively declined and died in 2002 in hospice. |
Net Burden |
| 2) Had other work-up after +FOBT (N=54) |
80 year-old man with gout and arthritis who had a positive FOBT in 2001, followed by repeat negative FOBT in 2004. He never had follow-up colonoscopy. He moved to a VA nursing home in 2007 and remains alive. |
Net Burden |
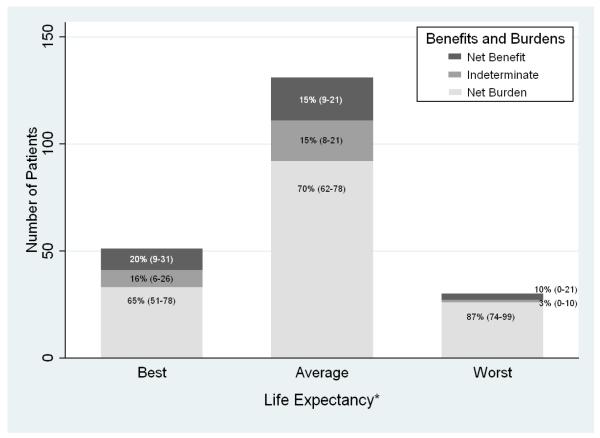
Also, the net burden of these screening and follow-up practices varied across the 3 prognostic groups (Figure 2). 87% of those with worst life expectancy experienced net burden compared to 70% of those with average life expectancy and 65% of those with best life expectancy (P for trend =0.048 ). Conversely, 20% (10/51) of patients with best life expectancy experienced net benefit versus 15.3% (20/131) with average life expectancy and 10.0% (3/30) with worst life expectancy (p for trend=0.25).
Figure 2. Benefits and Burdens of Screening and Follow-up Practices According to Life Expectancy among 212 Patients Aged 70 Years and Older with a Positive Screening FOBT Result*.
*Net benefit was assigned to those patients diagnosed with a significant adenoma or colorectal cancer on follow-up colonoscopy who lived at least 5 years after screening, even if they experienced complications from screening or treatment. Net benefit or burden was indeterminate if 1) patients had 1-2 small adenomas and lived at least 5 years or 2) patients had a colonoscopy outside of the VA without an available pathology report and lived at least 5 years . Net burden was assigned to patients who failed to receive colonoscopy after their positive FOBT result, who had a false-positive FOBT result (i.e.,non-significant findings on follow-up colonoscopy) , or who had a significant adenoma detected but died within 5 years . Among patients with best life expectancy who experienced net burden the most common reason was failure to get a follow-up colonoscopy (55%; 18/33) whereas among patients with average or worst life expectancy the most common reason for net burden was dying within 5 years of their FOBT (54%;64/118). 5-year mortality was 5.9% (3/51) for patients with best life expectancy, 38.2% (50/131) for patients with average life expectancy, and 46.7% (14/30) for patients with worst life expectancy which equates to a life expectancy of 5.4 years.
DISCUSSION
Even over 7 years after a positive FOBT result, only a little over half of older adults received follow-up colonoscopy. Among those who received follow-up colonoscopy, more than a quarter had significant adenomas or cancer detected and treated and lived > 5 years, potentially benefitting from screening, while approximately 59% (70/118) had no significant findings on follow-up and 10% experienced complications from colonoscopy or cancer treatment. Among patients who did not receive follow-up colonoscopy, nearly half died of other causes within 5 years while 4% ultimately died of colorectal cancer. Patients with worst life expectancy (life-expectancy of 5.4 years) were more likely to experience net burden compared to patients with average or best life expectancy. As current guidelines from the USPSTF and other groups encourage individualized decision-making in patients over 75,16,26 our study provides data about the consequences following the choice to pursue or forego follow-up of a positive FOBT result.
The low rate of follow-up colonoscopy found in this study (56%) is similar to that of other studies in older adults even though the follow-up period in other studies ranged from only a few months to 3 years whereas we extended the follow-up period to 7 years.1-4,7,8 Patients may not get follow-up colonoscopy because of the risks of colonoscopy and treatment and other competing causes of mortality, as perceived either by the physician27 or the patient.5 We found that nearly half of those without follow-up colonoscopy died of causes other than colorectal cancer within 5 years, suggesting that the decision to forego follow-up colonoscopy was appropriate for those patients. It also suggests such decisions are occurring after FOBT results are known rather than following recommendations to avoid screening FOBT if there is no intention to follow-up a positive result with colonoscopy. As the use of screening colonoscopy increases, colonoscopy decisions will be made up front and will need to be better targeted than FOBT or the number of colonoscopies performed in people who die within 5 years will increase.
Among our patients with follow-up colonoscopy, about a third had colorectal cancer or significant adenomas, on par with other studies.10,28,29 Strul et al. found the overall adenoma rate in older adults to be 26%.30 Our study indicates that older patients without follow-up had a 4% risk of ultimately dying of colorectal cancer in the next 5 years. On the other hand, undergoing follow-up procedures is not without risk. While prior studies have suggested minimal complications from colonoscopy in older adults, these studies only followed patients over a short period.4,31-35 We found complications of follow-up encompassed more than immediate events. In our cohort, four of the 40 older patients with cancer or significant adenomas were hospitalized for several weeks as a result of complications from treatment. In addition, 23% (24/104) of those with follow up colonoscopy, excluding those with cancer, had 3 or more additional tests over 7 years. However, while older adults are often at greatest risk for complications from colonoscopy and colorectal cancer treatment due to their increasing comorbidity36, they may be most likely to benefit from screening, especially if they have a substantial life expectancy.
While our study is not a randomized trial, the lengthy follow-up allows us to identify patients who most likely received net benefit from real world screening and follow-up practices, i.e., those with significant disease treated as a result of screening and lived more than 5 years. The choice of a 5 year survival time to achieve benefit is based on the natural history of polyps18 and randomized trials that shows survival benefit begins around 5 years after the start of FOBT screening.10 In addition, several of the cancers and adenomas found in our study were large (>3cm) such that it is reasonable to expect that they would have caused symptoms within 5 years. As with all screening tests, FOBT does not benefit most patients because most do not have cancer or significant adenomas. Yet, more than 15.6% (33/212) of patients aged 70 years and older had colorectal cancer or significant adenomas successfully treated and lived more than 5 years suggesting that a significant minority received net benefit from current practices.
It makes intuitive sense that patients with the best life expectancy are more likely to benefit from screening than those with the worst. This has been shown in cost-effectiveness analyses,37,38 which are based on numerous methodological assumptions, whereas our study uses real world data to describe outcomes of screening according to life expectancy. We found older patients with best predicted life expectancy were less likely to experience net burden from screening than those with the worst. Our study supports guidelines that recommend using life expectancy to guide colorectal cancer screening decisions in older adults and argues against one-size-fits-all interventions that simply aim to increase overall screening and follow-up rates.16,26 We used the well-validated Charlson-Deyo comorbidity index because it strongly predicts long-term mortality.14 In our cohort it effectively stratified patients into groups with widely differing 5-year mortality rates, ranging from 6% for patients with best life expectancy to 47% for those with worst (life expectancy of 5.4 years) for whom most would not recommend screening.36 However, more comprehensive prognostic indices (e.g. incorporating functional status) over more than 5 years are needed to better guide physicians as they target screening and follow-up to those older adults with substantial life expectancies.39,40
This study has several limitations. First, our cohort is primarily comprised of men who use the VA, so the generalizability of our findings to non-white men, women and persons not in the VA is uncertain. However, the VA is the largest healthcare system in the U.S. so outcomes of screening and follow-up in this system are likely to have generalizable lessons for US healthcare. Second, our sample size was small because only 212 (9%) patients had positive screening FOBT results at the 4 participating facilities in 2001. Third, although we used Medicare claims data for the first year of follow-up, we relied on chart review for later outcomes which may miss some complications or testing outside the VA, and we were unable to find pathology results for 8 patients with a colonoscopy outside the VA, although all 8 remained alive without colorectal cancer. Fourth, we defined patients as having benefitted from screening if they had colorectal cancer or significant adenomas detected on follow-up colonoscopy and treated AND survived more than five years. Others may argue for a longer or shorter survival length to define benefit in older adults.10,41
In conclusion, systematic reviews of colorectal cancer screening have called for more studies to assess the net benefit of real world colorectal cancer screening practices to improve appropriate use and minimize burdens of screening.39 Our study employed a novel method of following patients with a positive screening FOBT result for 7 years to determine the net benefit and burden of real world screening and follow-up practices in older adults. We demonstrated that older adults with substantial life expectancies are less likely to experience net burden than those with limited life expectancies. Therefore, through individualized decision-making the percentage of patients experiencing net burden could be decreased by better targeting FOBT screening and follow-up to healthy older adults.
ACKNOWLEDGMENT
Dr. Walter is supported by a VA Health Services Research and Development grant IIR-04-427 and by grant 1R01CA134425 from the National Cancer Institute. Dr. Kistler was supported by the VA Quality Scholars Program and the T32 AG000212-16 grant from the National Institute on Aging during the first portion of the study. She is currently supported by the Agency for Healthcare Research and Quality Mentored Career Development Program in Comparative Effectiveness Development grant K12 HS19468-01, and by the Cecil G. Sheps Center for Health Services Research and the Lineberger Cancer Center at the University of North Carolina at Chapel Hill.
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication. Dr. Kistler and Dr. Walter had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Citations
- 1.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Screening for colorectal neoplasia: physicians’ adherence to complete diagnostic evaluation. Am J Public Health. 1993 Nov;83(11):1620–1622. doi: 10.2105/ajph.83.11.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin B, Hess K, Johnson C. Screening for colorectal cancer. A comparison of 3 fecal occult blood tests. Arch Intern Med. 1997 May 12;157(9):970–976. [PubMed] [Google Scholar]
- 3.Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010 Sep-Oct;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lurie JD, Welch HG. Diagnostic testing following fecal occult blood screening in the elderly. J Natl Cancer Inst. 1999 Oct 6;91(19):1641–1646. doi: 10.1093/jnci/91.19.1641. [DOI] [PubMed] [Google Scholar]
- 5.Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of Follow-up After Fecal Occult Blood Testing in Older Adults: Inappropriate Screening or Failure to Follow Up? Arch Intern Med. 2010 Oct 11; doi: 10.1001/archinternmed.2010.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JB, Stellato TA, Guy BB, Gordon NH, Berger NA. A critical analysis of the largest reported mass fecal occult blood screening program in the United States. Am J Surg. 1991 Jan;161(1):101–105. doi: 10.1016/0002-9610(91)90368-n. discussion 105-106. [DOI] [PubMed] [Google Scholar]
- 7.Garman KS, Jeffreys A, Coffman C, Fisher DA. Colorectal cancer screening, comorbidity, and follow-up in elderly patients. Am J Med Sci. 2006 Oct;332(4):159–163. doi: 10.1097/00000441-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Shields HM, Weiner MS, Henry DR, et al. Factors that influence the decision to do an adequate evaluation of a patient with a positive stool for occult blood. Am J Gastroenterol. 2001 Jan;96(1):196–203. doi: 10.1111/j.1572-0241.2001.03475.x. [DOI] [PubMed] [Google Scholar]
- 9.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001 Jun 6;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 10.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996 Nov 30;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 11.Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009 Apr 7;150(7):465–473. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006 Jun;15(6):1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994 Nov;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Singh H, Kadiyala H, Bhagwath G, et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol. 2009 Apr;104(4):942–952. doi: 10.1038/ajg.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009 Jan-Feb;59(1):27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 17.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winawer SJ, Zauber AG, O’Brien MJ, et al. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer. 1992 Sep 1;70(5 Suppl):1236–1245. doi: 10.1002/1097-0142(19920901)70:3+<1236::aid-cncr2820701508>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer. 2004 Aug 10;111(1):147–151. doi: 10.1002/ijc.20241. [DOI] [PubMed] [Google Scholar]
- 20.Brooks DD, Winawer SJ, Rex DK, et al. Colonoscopy surveillance after polypectomy and colorectal cancer resection. Am Fam Physician. 2008 Apr 1;77(7):995–1002. [PubMed] [Google Scholar]
- 21.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008 Mar 18;148(6):419–426. doi: 10.7326/0003-4819-148-6-200803180-00004. [DOI] [PubMed] [Google Scholar]
- 22.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006 May;130(6):1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Hoogewerf PE, Hislop TG, Morrison BJ, Burns SD, Sizto R. Health belief and compliance with screening for fecal occult blood. Social Science & Medicine. 1990;30(6):721–726. doi: 10.1016/0277-9536(88)90257-2. [DOI] [PubMed] [Google Scholar]
- 24.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal Cancer Screening in Older Men and Women: Qualitative Research Findings and Implications for Intervention. Journal of Community Health. 2000;25(3):263–278. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 25.Parker MA, Robinson MHE, Scholefield JH, Hardcastle JD. Psychiatric morbidity and screening for colorectal cancer. J Med Screen. 2002 March 1;9(1):7–10. doi: 10.1136/jms.9.1.7. 2002. [DOI] [PubMed] [Google Scholar]
- 26.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 27.Jimbo M, Myers RE, Meyer B, et al. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009 Jan-Feb;7(1):11–16. doi: 10.1370/afm.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008 Jun;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 29.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993 May 13;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 30.Strul H, Kariv R, Leshno M, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006 Feb;101(2):255–262. doi: 10.1111/j.1572-0241.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 31.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009 Jun 16;150(12):849–857. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 32.Zerey M, Paton BL, Khan PD, et al. Colonoscopy in the very elderly: a review of 157 cases. Surg Endosc. 2007 Oct;21(10):1806–1809. doi: 10.1007/s00464-007-9269-x. [DOI] [PubMed] [Google Scholar]
- 33.Lukens FJ, Loeb DS, Machicao VI, Achem SR, Picco MF. Colonoscopy in octogenarians: a prospective outpatient study. Am J Gastroenterol. 2002 Jul;97(7):1722–1725. doi: 10.1111/j.1572-0241.2002.05832.x. [DOI] [PubMed] [Google Scholar]
- 34.Arora A, Singh P. Colonoscopy in patients 80 years of age and older is safe, with high success rate and diagnostic yield. Gastrointest Endosc. 2004 Sep;60(3):408–413. doi: 10.1016/s0016-5107(04)01715-8. [DOI] [PubMed] [Google Scholar]
- 35.Karajeh MA, Sanders DS, Hurlstone DP. Colonoscopy in elderly people is a safe procedure with a high diagnostic yield: a prospective comparative study of 2000 patients. Endoscopy. 2006 Mar;38(3):226–230. doi: 10.1055/s-2005-921209. [DOI] [PubMed] [Google Scholar]
- 36.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006 Nov 7;145(9):646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 37.Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006 May 24;295(20):2357–2365. doi: 10.1001/jama.295.20.2357. [DOI] [PubMed] [Google Scholar]
- 38.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005 Oct;129(4):1163–1170. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 39.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010 May 18;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 40.Lewis CL, Griffith J, Pignone MP, Golin C. Physicians’ decisions about continuing or stopping colon cancer screening in the elderly: a qualitative study. J Gen Intern Med. 2009 Jul;24(7):816–821. doi: 10.1007/s11606-009-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010 Sep 9;363(11):1076–1079. doi: 10.1056/NEJMsb1002538. [DOI] [PubMed] [Google Scholar]