Abstract
Purpose
Ultraviolet (UV) acts as low-dose ionizing radiation. Acute UVB exposure causes photokeratitis and induces apoptosis in corneal cells. Astaxanthin (AST) is a carotenoid, present in seafood, that has potential clinical applications due to its high antioxidant activity. In the present study, we examined whether topical administration of AST has preventive and therapeutic effects on UV-photokeratitis in mice.
Methods
C57BL/6 mice were administered with AST diluted in polyethylene glycol (PEG) in instillation form (15 μl) to the right eye. Left eyes were given vehicle alone as controls. Immediately after the instillation, the mice, under anesthesia, were irradiated with UVB at a dose of 400 mJ/cm2. Eyeballs were collected 24 h after irradiation and stained with H&E and TUNEL. In an in vitro study, mouse corneal epithelial (TKE2) cells were cultured with AST before UV exposure to quantify the UV-derived cytotoxicity.
Results
UVB exposure induced cell death and thinning of the corneal epithelium. However, the epithelium was morphologically well preserved after irradiation in AST-treated corneas. Irradiated corneal epithelium was significantly thicker in eyes treated with AST eye drops, compared to those treated with vehicles (p<0.01), in a doses dependent manner. Significantly fewer apoptotic cells were observed in AST-treated eyes than controls after irradiation (p<0.01). AST also reduced oxidative stress in irradiated corneas. The in vitro study showed less cytotoxicity of TKE2 cells in AST-treated cultures after UVB-irradiation (p<0.01). The cytoprotective effect increased with the dose of AST.
Conclusions
Topical AST administration may be a candidate treatment to limit the damages by UV irradiation with wide clinical applications.
Introduction
Ultraviolet (UV) irradiation represents a significant environmental hazard that can cause acute and chronic inflammatory changes in the cornea, lens, and retina of the eye. The sources of UV radiation are not merely from electric welding and tanning lamps but also from sunny days on the sea or in snowy mountains when eyes are left unprotected. In recent decades, the risk of acute photochemically-induced ocular damage has increased due to stratospheric ozone depletion [1]. UVB exposure causes photokeratitis, which is associated with expression of nuclear factor (NF)-κB, prostaglandin E2 (PGE2), and many other inflammatory agents [1]. Acute UVB exposure causes damage deeper than the epithelium, involving all tissues of the cornea [2] and inducing apoptosis in corneal cells [3,4]. Although the energy is much less than that of the gamma rays, ophthalmologists find it is necessary to study ways to prevent the damages caused by UV radiation due to its association with clinical ocular diseases. We have already reported that applying UVB irradiation at 400 mJ/cm2 to mice corneas can be a useful model for studying corneal inflammation [3].
Antioxidant enzymes, such as superoxide dismutase [5] and catalase [6], and pharmacologic antioxidants like N-acetyl cysteine [7,8], inhibit tumor necrosis factor (TNF)-α activation through NF-κB-dependent gene expression [9]. Astaxanthin (AST), 3,30-dihydroxy-b,b-carotene-4,40-dione, a carotenoid without vitamin A activity [10,11], has potential clinical applications due to its antioxidant activity, which is higher than β-carotene and α-tocopherol [10,12,13]. In addition, it has many highly potent pharmacological effects, including anti-tumor, anti-cancer, anti-diabetic, and anti-inflammation activities [12,14-16]. The potent activity of AST has been observed to modulate biologic functions ranging from lipid peroxidation to tissue protection [14,17]. The presence of the hydroxyl (OH) and keto (C=O) on each ionone ring in AST explains its unique feature of antioxidant activity for the protection of both the inner and outer membrane surfaces [10,14,18,19]. AST is found abundantly in the red-orange pigment of marine animals such as salmon (and salmon roe) and the shell of crabs and shrimp. AST and AST-like products are commonly indicated as antioxidants [20] and immune modulators [21]. One of the effects of AST is to scavenge reactive oxygen species [22]. We previously reported that AST showed a dose-dependent anti-inflammatory effect [23,24]. It was also reported that AST inhibited the production of inflammatory mediators by blocking NF-κB activation in vitro [4]. In the present study, we examined whether topical administration of AST has therapeutic effects on UV photokeratitis in mice.
Methods
Animals and reagents
Six- to 8-week-old C57BL/6 male mice were obtained from Clear Japan (Tokyo, Japan). Mice were maintained under specific pathogen-free conditions. All procedures involving animals were performed in accordance with the ARVO resolution on the use of animals in research. AST was purchased from Sigma-Aldrich (Tokyo, Japan).
UV irradiation
The mice were administered with AST diluted in polyethylene glycol (PEG) in instillation form (15 μl) to the right eye once at the following concentrations: 1, 0.1, and 0.01 mg/ml (10 mice per group. Left eyes were instilled with vehicle alone. Immediately after the instillation, anesthetized mice were irradiated with UVB at a dose of 400 mJ/cm2 from a FS-20 (Panasonic, Osaka, Japan) fluorescent lamp. These bulbs have a broad emission spectrum (250–400 nm) with high output primarily in the UVB spectrum (290–320 nm). To evaluate the effects of AST on the corneal surface without UVB damage, control mice received the instillation of 1 mg/ml of AST without irradiation. All experiments were performed in triplicate.
H&E and TUNEL staining
The eyes were dissected from mice 24 h after UVB exposure and fixed with 4% paraformalin. Tissue sections were prepared and stained with hematoxylin-eosin (H&E) for morphological analysis. Other sections were stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to detect apoptotic signaling. Apoptotic cells were detected with a Cell Death Detection Kit (Roche Diagnostics Japan, Tokyo) containing all necessary reagents for staining. Slides imaging, cell counting, and thickness evaluations were performed with BZ-9000 fluorescence microscope (Keyence, Japan) and software bundled with the apparatus. At least 10 sections were used to evaluate the epithelial thickness, measurements of 10 randomly selected areas of epithelium of central cornea was performed and averaged.
Evaluation of the “sunglasses effect” of AST
The “sunglass effect” represents the status when protective properties of a substance are only due to blocking the UV rather than having a cytoprotective effect. To evaluate this effect, and whether AST is capable of rescuing the damaged epithelia after UVB irradiation, we performed additional experiments where mice were first irradiated with UVB at a dose of 400 mJ/cm2 under anesthesia. After 5 min of irradiation, the right eyes were then instilled with AST (1 mg/ml) and the left eyes instilled with vehicle alone.
Detection of reactive oxygen species (ROS)
The eyes were dissected from mice 24 h after UVB exposure and frozen fresh in optimal cutting temperature (OCT) compound with liquid nitrogen. To visualize not only oxidative signaling but also the tissue structure, 4',6-diamidino-2-phenylindole (DAPI) dye (blue) was used to stain the nuclei of the cells. Dihydroethidium (DHE; Sigma-Aldrich, St. Louis, MO), an oxidative fluorescent dye, was used for the immunohistochemical detection of cytosolic superoxide anion (O2-) to evaluate ROS production in corneal epithelium tissue, as reported recently [25].
NF-κB immunohistochemistry staining
The eyes were dissected from mice 24 h after UVB exposure and fixed with 4% paraformaldehyde and then paraffin-embedded. To evaluate the NF-κB positive cells in the corneal epithelium, slides were rehydrated and applied with a 1:50 dilution of NF-κB P65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 12 h, washed with PBS and then applied with 1:100 dilution of secondary goat anti–rabbit antibody dye conjugate (Invitrogen, Carlsbad, CA), which gives red fluorescence. Since NF-κB is widely present in intercellular tissue, to detect its expression in cell nuclei, we stained the section slides with 1:100 dilution of YO-PRO®-1 (Invitrogen), which produces a green signal from the cell nuclei. NF-κB positive (yellow) nuclei were quantified in merged images.
In vitro study
TKE2 cells were used for in vitro study. TKE2 is a murine corneal epithelium-derived progenitor cell line [26,27]. Mouse TKE2 cells were cultured in Defined K-SFM (Keratinocye Serum Free Medium) with L-Glutamine, Phenol Red, and Sodium Pyruvate (Invitrogen), supplemented with 100 U/ml of penicillin and 100 U/ml of streptomycin, (Invitrogen). The cells were seeded onto 24-well plates (5×104 cells/well) at 37 °C in a humidified incubator containing 5% CO2. The cells were treated with 1, 0.1, or 0.01 mg/ml AST. After 6 h of incubation, the wells were irradiated with UVB at a dose of 300 mJ/cm2 at room temperature for 1 min. To prevent UVB radiation absorption, the culture media were removed just before irradiation and replaced with a thin layer of phosphate-buffered saline (PBS). After UVB irradiation, cells were fed with fresh medium. The LDH (lactate dehydrogenase) Cytotoxicity Detection Kit (Roche Applied Science, Penzberg, Germany) was used to determine cytotoxicity 6 h after UVB exposure. Culture supernatants (100 μl) were collected and added to the kit’s work solution. After 30 min incubation, the absorbance was measured with an ELISA reader. All procedures were performed according the user manual of the kit. Analysis was performed in triplicate. To determine the background LDH activity of the medium and spontaneous LDH release, additional plates were prepared with empty medium and unaffected cells. The maximum LDH release was achieved by adding 100 μl of Triton-B to the reference wells. Cytotoxicity percentages were calculated, and Triton-B treated wells with maximum LDH release were assumed to be 100%. All experiments were performed in triplicate with 5 or more wells for each group in each experiment.
Statistical analysis
All data were expressed as the mean±standard deviation (SD) from the respective test or control groups of data. Statistical significances were determined by the paired t-test and non-parametric Mann–Whitney U-test. P values less than 0.05 were considered significant.
Results
Morphological properties
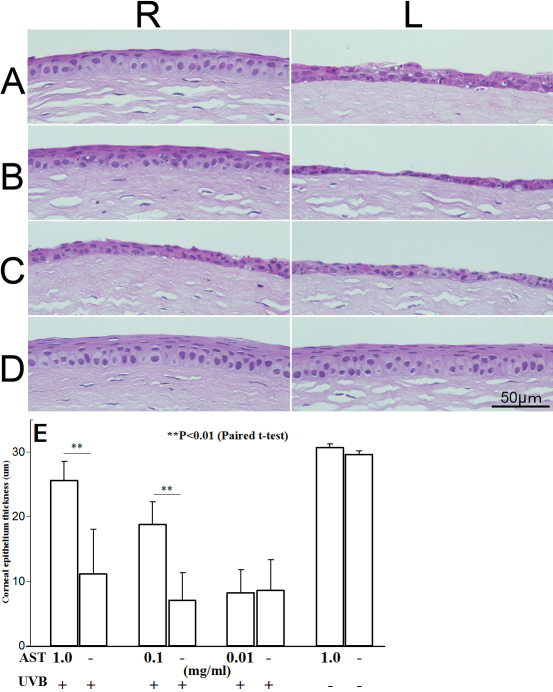
We examined the morphological properties of UVB-irradiated and control corneas using H&E staining. At 24 h after UVB irradiation at a dose of 400 mJ/cm2, thinning and ulceration of the corneal epithelial layer were observed (Figure 1). The corneal epithelial thicknesses were 25.6±2.9, 18.8±3.5, and 8.2±3.6 μm in eyes treated with 1, 0.1, and 0.01 mg/ml of AST, respectively. The mean corneal epithelial thickness in eyes untreated with AST was 8.91±5.3 μm after UVB-exposure. The mean corneal epithelium thickness of non-irradiated eyes was 29.6±0.5 μm. Corneal epithelium was well preserved, and the thickness of the epithelium remained close to normal in the right eyes treated with 1 mg/ml of AST instillation (Figure 1A). Its protective effect decreased gradually in a concentration-dependent manner (Figure 1B,C). The corneas of mice administered with AST without UVB irradiation (Figure 1D) showed no differences from naïve corneas (Figure 1D). The mean values of the epithelium thickness of corneas were calculated and summarized (Figure 1E). Corneal epithelia were significantly thicker in eyes treated with 1 and 0.1 mg/ml AST eye drops compared with vehicle-treated eyes (p<0.01). Irradiated corneas treated with 1 mg/ml AST showed corneal epithelium thickness close to naïve cornea. AST protected corneal epithelium in a dose-dependent manner (Figure 1E).
Figure 1.
Morphological properties of ultraviolet (UV)-irradiated corneas. Eyes were treated with AST eye drops before UV exposure (A: 1 mg/ml, B: 0.1 mg/ml, C: 0.01 mg/ml AST). Control subjects were not irradiated with UVB (D). R: Right eyes were given various concentrations of AST eye drops. L: Left eyes were given vehicle alone as controls. The mean values of corneal epithelial thickness are summarized (E). Epithelia were significantly thicker in right eyes treated with 1 and 0.1 mg/ml AST compared to the left eyes, which served as controls (p<0.01). Protective effects of AST were found to occur in a dose-dependent manner.
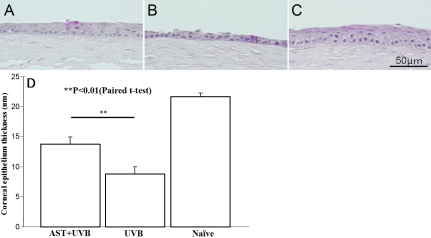
We also examined the morphological properties of UVB-irradiated and control corneas using H&E staining when AST was applied to corneas at a concentration of 1 mg/ml 5 min after UVB irradiation at a dose of 400 mJ/cm2. Twenty-four hours later, the corneal epithelial thickness was 13.7±0.8 μm (Figure 2A). The mean corneal epithelial thickness of vehicle-given corneas was 8.7±1.1 μm after UVB-exposure (Figure 2B). Non-irradiated corneal epithelium was 21.6±0.6 μm thick (Figure 2C). Even when AST was administered after UVB irradiation, the corneal epithelium was significantly thicker than in fellow eyes treated with vehicle (p<0.01, Figure 2D).
Figure 2.
Morphological properties of ultraviolet (UV)-irradiated corneas treated with AST after irradiation. Eyes were treated with AST eye drops within 5 min after UVB exposure (A: 1 mg/ml, B: 0.1 mg/ml). Control subjects were not irradiated with UVB (C). The mean values of corneal epithelial thickness are summarized (D). Epithelia were significantly thicker when treated with 1 mg/ml AST compared to fellow eyes as controls (p<0.01). The protective effects of AST remained, even if eyes were treated after irradiation.
TUNEL staining
We compared TUNEL staining in the corneas of AST-treated and untreated eyes of the mice 24 h after UV exposure. Few TUNEL-positive nuclei were detected in corneas of mice treated with AST at 1 and 0.1 mg/ml compared to untreated mice after UV irradiation (Figure 3A,B). No significant protective effect was observed in eyes treated with AST 0.01 mg/ml (Figure 3C). There were no apoptotic cells detected in corneas without UVB exposure (Figure 3D).
Figure 3.
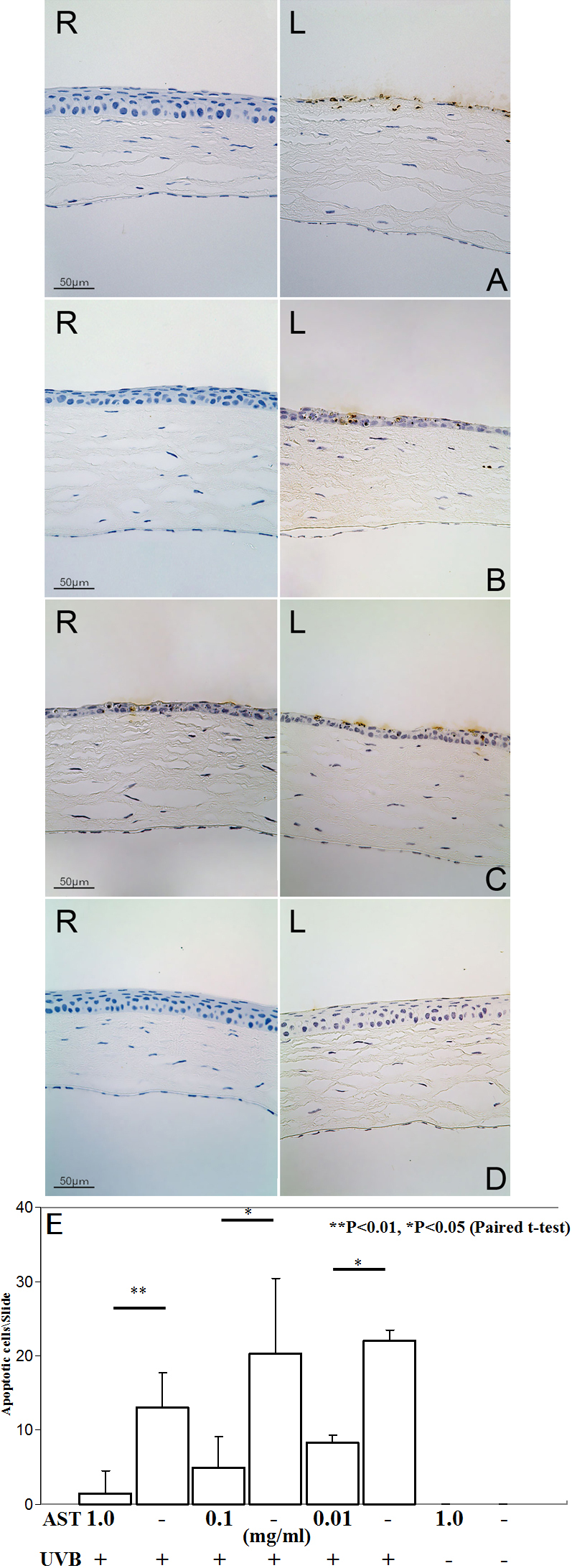
TUNEL labeling at irradiated corneas with and without AST-treatment. Eyes were treated with AST eye drops before UV exposure (A: 1 mg/ml, B: 0.1 mg/ml, C: 0.01 mg/ml AST). Control subjects were not irradiated with UVB (D). R: Right eyes were given various concentrations of AST eye drops. L: Left eyes were given vehicle alone as controls. Numbers of apoptotic corneal cells per slide after UVB exposure is shown (E). Apoptotic cells were significantly fewer in right eye corneas treated with 1 (p<0.01), 0.1 (p<0.05), and 0.01 (p<0.05) mg/ml AST eye drops compared to the left eyes, which served as controls. There were no apoptotic cells detected in corneas without irradiation.
TUNEL-positive cells were counted, and the numbers were 1.5±3.0, 5.0±4.1, and 8.25±0.9 in eyes treated with 1, 0.1, and 0.01 mg/ml of AST, respectively (Figure 3E). The mean number of apoptotic cells was 18.4±4.7 in vehicle-given irradiated corneas. The apoptotic cells were significantly fewer in corneas treated with AST than those with vehicle only (p<0.01).
Detection of reactive oxygen species (ROS)
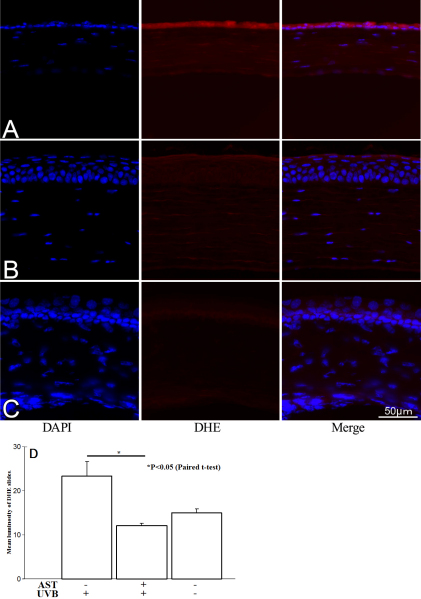
Confocal microscopic images was examined to quantify reactive oxygen species (ROS) production in mouse corneal tissue. ROS production was determined by conversion of DHE to ethidium bromide (EtBr). All images were made in parallel at identical settings (Figure 4). ROS were strongly detected in untreated corneal epithelium after UVB irradiation (Figure 4A). However, the ROS signal was downregulated in AST-treated corneas (Figure 4B) and close to unspecific ROS signaling in naïve corneas (Figure 4C). The mean gray values of the corneal epithelium of DHE stained slides were evaluated by Image J software and summarized in Figure 4D. The mean gray value in vehicle-given irradiated corneas was 23.3±3.3. In eyes treated with 1 mg/ml of AST, after UVB irradiation, the mean gray value was 12.08±0.5. Non-irradiated corneal epithelium showed a 15.04±0.8 mean gray value. AST-treated epithelium showed significantly (p<0.05) lower mean gray values than irradiated corneas did.
Figure 4.
Reactive oxygen species signal expression after UVB exposure. Reactive oxygen species (ROS) were strongly detected (red) in untreated corneal epithelium after UVB irradiation (A). The ROS signal was weak in AST treated corneas (B) and close to unspecific ROS signaling in naïve corneas (C). The mean gray values of the corneal epithelium of DHE stained slides were evaluated by Image J software and summarized (D). Mean gray values were significantly lower in corneas treated with 1 mg/ml AST eye drops than in the vehicle-treated eyes (p<0.05).
NF-κB downregulation by AST
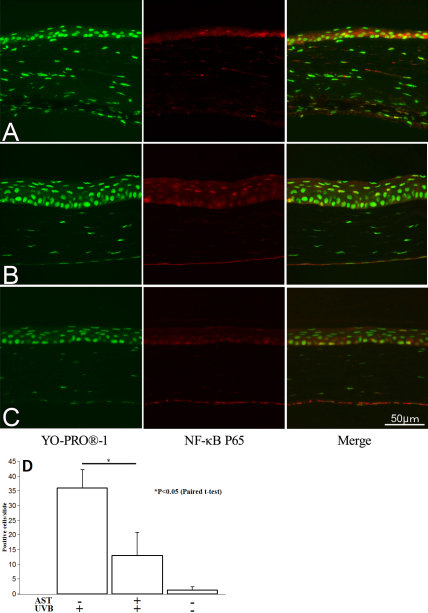
Previously, we reported that AST decreased NF-κB expression in endotoxin-induced uveitis (EIU) and choroidal neovascularization [23,24]. To examine whether AST administration effects NF-κB in corneal epithelium, we immunohistochemically analyzed AST expression in the collected corneal tissues and examined it with confocal microscope. NF-κB positive nuclei (yellow) were found to be 13.0±7.9 in corneal epithelial cells in UV-irradiated mice treated with AST at 1.0 mg/kg (Figure 5A). However, 36.0±6.2 (Figure 5B) of multiple NF-κB positive cells were found in the corneal epithelial cells in UV-irradiated mice untreated with AST. Naïve mice corneal tissues showed weak response to anti-NF-κB antibodies, and only 1.3±1.1 positive nuclei were found (Figure 5C). Expression of NF-κB was significantly downregulated in AST-treated mice after UVB irradiation (p<0.05, Figure 5D).
Figure 5.
NF-κB expression in corneal epithelia. Eyes were given vehicle alone (A) or treated with AST eye drops before UV exposure (B); control subjects were not irradiated with UVB (C). The mean values of NF-κB positive cells (yellow) in corneal epithelial were summarized (D). Expression of NF-κB was significantly downregulated in AST-treated mice after UVB irradiation (p<0.05).
AST suppressed phototoxicity in corneal epithelial cell cultures after UVB irradiation
Next, cytotoxicity was examined in UVB-irradiated TKE2 cells, murine corneal epithelium-derived progenitor cell line, treated or untreated with AST in vitro (Figure 6). The percentages of cytotoxicity after irradiation were 17.6±2.0, 29.5±2.2, 31.7.2±2.8, and 32.7±1.7% in wells containing 1, 0.1, 0.01, and 0 mg/ml of AST, respectively. Cytotoxicity was significantly suppressed by 1 mg/ml (p<0.01) and 0.1 mg/ml (p<0.05) AST administration. The effect was AST concentration-dependent.
Figure 6.
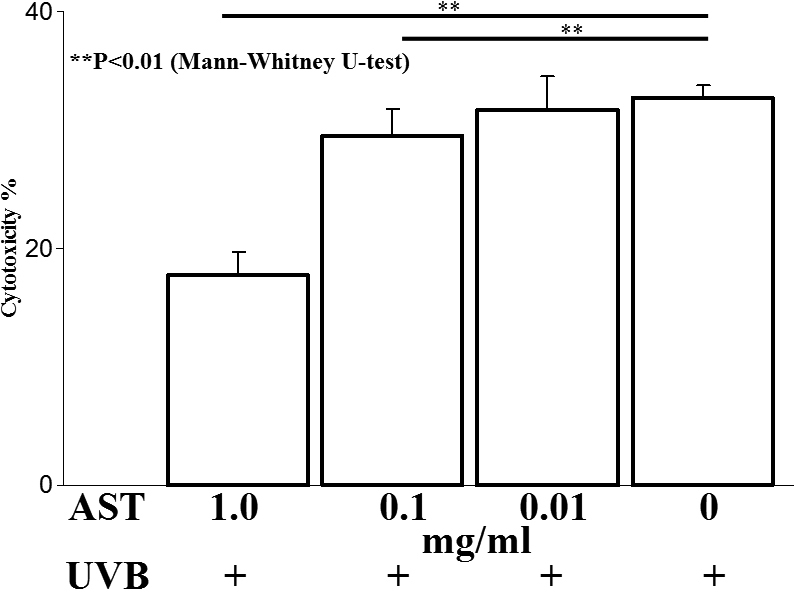
UVB-induced cytotoxicity in TKE2 cells in vitro. AST was added to a culture of UVB-irradiated TKE2 cells. Cytotoxicity after UVB exposure in keratinocyte cultures was significantly decreased by AST in a dose-dependent manner (1 mg/ml: p<0.01, 0.1 mg/ml: p<0.05, and 0.01 mg/ml: not significant).
Discussion
The corneal epithelium serves to protect corneal structures against UV damage by absorbing a substantial amount of UV energy. Though the energy of UV is much less than that of ionizing radiation rays, the injuries on cells and DNA are critical. Excessive UVB irradiation induces various changes of DNA, proteins and cells, through activation of pro-inflammatory mediators including NF-κB. AST can form stable resonance structures by attachment of the carbonyl and hydroxyl groups to its β-ionone ring. It can also remove the chain-carrying lipid peroxyl radicals in the liposomal suspension, protecting cells from oxidative and free radical damages. NF-κB activates proinflammatory cytokines, chemokines, and enzymes that generate mediators of inflammation. It can also activate adhesion molecules that play a key role in the initial recruitment of leukocytes to sites of inflammation. Therefore, activation of NF-κB leads to a coordinated upregulation of many genes whose products mediate the inflammatory loop and perpetuate local inflammatory responses. In our previous studies, AST inhibited the in vivo activation of NF-κB in endotoxin-induced uveitis (EIU) and choroidal neovascularization [23,24]. Therefore, reactive oxygen species-induced oxidative stress may play an important role in NF-κB activation and proinflammatory cytokine production. Our immunohistochemical finding shows that AST has the same effects of reducing NF-κB expression in corneal epithelia. The anti-inflammatory effects of AST, through its suppression of NF-κB activation, may be based on its antioxidant activity, as previous reports showed that several antioxidants efficiently inhibit NF-κB activation induced by lipopolysaccharides (LPS) in cell systems [28-33]. Therefore, these effects appear to be mediated by the powerful radical scavenger properties of the antioxidants, which apparently counteract reactive oxygen intermediates generated by NF-κB activation [30,34,35].
We also showed that the relieving effect of AST on UVB photokeratitis is not derived from light-interception (sunglasses effect) but its direct pharmacological effect (Figure 2). In fact, a previous study showed that AST eye drops did not delay the progression of UVB-induced cataract in rats when AST was not on lens [36]. In addition, UVB irradiation improved AST accumulation in green microalgae [8]. These results strongly suggest that AST can produce a protective effect against UV, and organisms have been using AST to save themselves from harmful UV light.
AST is found abundantly in the red-orange pigment of marine animals such as salmon (and salmon roe) and the shells of crabs and shrimp. Humans have taken in these food products since ancient times, and AST is now available as an oral supplement. Therefore, AST may be free of any harmful side effects. In the present study, we demonstrated that topical AST, instead of systemic administration, is effective in protecting the ocular surface against UV exposure with no adverse effects. Thus, in conclusion, AST might be a promising naturally-derived substance for protecting ocular surfaces from the damages caused by ultraviolet radiation.
Acknowledgments
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS); the Ministry of Education, Culture, Sports, Science, and Technology (MEXT); and the Mishima Memorial Foundation, Japan.
References
- 1.Schein OD. Phototoxicity and the cornea. J Natl Med Assoc. 1992;84:579–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen AP, Chou BR, Hall MG, Jany SE. Ultraviolet-B damages corneal endothelium. Am J Optom Physiol Opt. 1984;61:473–8. doi: 10.1097/00006324-198407000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kitaichi N, Shimizu T, Yoshida K, Honda A, Yoshihisa Y, Kase S, Ohgami K, Norisugi O, Makino T, Nishihira J, Yamagishi S, Ohno S. Macrophage migration inhibitory factor ameliorates UV-induced photokeratitis in mice. Exp Eye Res. 2008;86:929–35. doi: 10.1016/j.exer.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, Yazawa K, Yoshida K, Kase S, Ohno S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–81. doi: 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, Gonzalez-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68:1939–46. doi: 10.1016/j.bcp.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Clark RA, Valente AJ. Nuclear factor kappa B activation by NADPH oxidases. Mech Ageing Dev. 2004;125:799–810. doi: 10.1016/j.mad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Shao DZ, Lee JJ, Huang WT, Liao JF, Lin MT. Inhibition of nuclear factor-kappa B prevents staphylococcal enterotoxin A-induced fever. Mol Cell Biochem. 2004;262:177–85. doi: 10.1023/b:mcbi.0000038233.20276.e0. [DOI] [PubMed] [Google Scholar]
- 8.Woo SH, Park IC, Park MJ, An S, Lee HC, Jin HO, Park SA, Cho H, Lee SJ, Gwak HS, Hong YJ, Hong SI, Rhee CH. Arsenic trioxide sensitizes CD95/Fas-induced apoptosis through ROS-mediated upregulation of CD95/Fas by NF-kappaB activation. Int J Cancer. 2004;112:596–606. doi: 10.1002/ijc.20433. [DOI] [PubMed] [Google Scholar]
- 9.Ohta K, Nakayama K, Kurokawa T, Kikuchi T, Yoshimura N. Inhibitory effects of pyrrolidine dithiocarbamate on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2002;43:744–50. [PubMed] [Google Scholar]
- 10.Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5:333–42. doi: 10.2217/fca.09.19. [DOI] [PubMed] [Google Scholar]
- 11.Jyonouchi H, Sun S, Tomita Y, Gross MD. Astaxanthin, a carotenoid without vitamin A activity, augments antibody responses in cultures including T-helper cell clones and suboptimal doses of antigen. J Nutr. 1995;125:2483–92. doi: 10.1093/jn/125.10.2483. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol. 2000;54:550–5. doi: 10.1007/s002530000416. [DOI] [PubMed] [Google Scholar]
- 13.Spiller GA, Dewell A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J Med Food. 2003;6:51–6. doi: 10.1089/109662003765184741. [DOI] [PubMed] [Google Scholar]
- 14.Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19(3A):1849–53. [PubMed] [Google Scholar]
- 15.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci Biotechnol Biochem. 2007;71:893–9. doi: 10.1271/bbb.60521. [DOI] [PubMed] [Google Scholar]
- 16.Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101(10A):58D–68D. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Choi SK, Park YS, Choi DK, Chang HI. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J Microbiol Biotechnol. 2008;18:1990–6. [PubMed] [Google Scholar]
- 18.Barrow CJ, Shahidi F. Marine nutraceuticals and functional foods. Boca Raton: CRC Press; 2008. [Google Scholar]
- 19.Liu X, Shibata T, Hisaka S, Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 20.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990;22:27–38. [PubMed] [Google Scholar]
- 21.Bennedsen M, Wang X, Willen R, Wadstrom T, Andersen LP. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol Lett. 1999;70:185–9. doi: 10.1016/s0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997;418:91–7. doi: 10.1016/s0014-5793(97)01355-0. [DOI] [PubMed] [Google Scholar]
- 23.Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:2694–701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 24.Izumi-Nagai K, Nagai N, Ohgami K, Satofuka S, Ozawa Y, Tsubota K, Ohno S, Oike Y, Ishida S. Inhibition of choroidal neovascularization with an anti-inflammatory carotenoid astaxanthin. Invest Ophthalmol Vis Sci. 2008;49:1679–85. doi: 10.1167/iovs.07-1426. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–9. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada N, Kawakita T, Mishima K, Saito I, Miyashita H, Yoshida S, Shimmura S, Tsubota K. Clusterin promotes corneal epithelial cell growth through upregulation of hepatocyte growth factor by mesenchymal cells in vitro. Invest Ophthalmol Vis Sci. 2011;52:2905–10. doi: 10.1167/iovs.10-6348. [DOI] [PubMed] [Google Scholar]
- 27.Kawakita T, Shimmura S, Hornia A, Higa K, Tseng SC. Stratified epithelial sheets engineered from a single adult murine corneal/limbal progenitor cell. J Cell Mol Med. 2008;12:1303–16. doi: 10.1111/j.1582-4934.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Németh ZH, Hasko G, Vizi ES. Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-alpha, MIP-1alpha, IL-12, and nitric oxide production and protects from the lethal effect of endotoxin. Shock. 1998;10:49–53. doi: 10.1097/00024382-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330–7. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 33.Lauzurica P, Martinez-Martinez S, Marazuela M, Gomez del Arco P, Martinez C, Sanchez-Madrid F, Redondo JM. Pyrrolidine dithiocarbamate protects mice from lethal shock induced by LPS or TNF-alpha. Eur J Immunol. 1999;29:1890–900. doi: 10.1002/(SICI)1521-4141(199906)29:06<1890::AID-IMMU1890>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Schulze-Osthoff K, Los M, Baeuerle PA. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes. Biochem Pharmacol. 1995;50:735–41. doi: 10.1016/0006-2952(95)02011-z. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz C, Pascual-Salcedo D, Castellanos MC, Alfranca A, Aragones J, Vara A, Redondo JM, de Landazuri MO. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood. 1996;88:3482–90. [PubMed] [Google Scholar]
- 36.Kawada H, Kojima M, Kimura T, Natori S, Sasaki K, Sasaki H. Effect of 5-S-GAD on UV-B-induced cataracts in rats. Jpn J Ophthalmol. 2009;53:531–5. doi: 10.1007/s10384-009-0695-2. [DOI] [PubMed] [Google Scholar]