Abstract
Background
Thrombosis is the major risk factor for death in patients with paroxysmal nocturnal hemoglobinuria. Previous case reports indicate that venous thrombosis in patients with paroxysmal nocturnal hemoglobinuria is amenable to thrombolysis.
Design and Methods
We reviewed the outcome of thrombolytic therapy for patients with paroxysmal nocturnal hemoglobinuria who had thromboses refractory to anticoagulation at our institutions.
Results
In this study of 41 patients who had at least one thrombotic event, we confirmed a very high incidence of recurrence despite anticoagulation. Nine patients with thrombosis were regarded as eligible for administration of intravenous tissue plasminogen activator, which was effective in reversing thrombi in all of 15 occasions in which it was given. Serious hemorrhagic complications developed in three cases. At last follow-up visit, of the nine patients treated, three had died, and six were in very good to excellent condition in terms of clinical outcome and radiological findings. The only patient in whom thrombolysis may have contributed to a fatal outcome also had complications of “heparin induced thrombocytopenia with thrombosis”, which we diagnosed in three additional patients. In our review of the literature, nine out of 15 patients treated with thrombolysis have had a good outcome.
Conclusions
Although it is associated with a significant but manageable risk of bleeding, systemic thrombolysis is a highly effective treatment for reversing venous thromboses in patients with paroxysmal nocturnal hemoglobinuria.
Keywords: thrombolysis, paroxysmal nocturnal hemoglobinuria, treatment, outcome
Introduction
Thrombosis, together with intravascular haemolysis and cytopenias, makes up the classical triad of paroxysmal nocturnal hemoglobinuria (PNH). From the clinical point of view, thrombosis in PNH may be anything from immediately life-threatening to an incidental radiological finding; but it is certainly the most significant cause of mortality.1–4 Among patients with PNH, the risk of thrombosis is much higher in Western countries than in the Far East,5 and within the United States it is higher among African-American and Latin American patients.1 It is likely that inherited factors (e.g. the factor V Leiden allele6) also play an additive or synergistic role, and the same is true of acquired states, particularly pregnancy and oral contraception.7
Given that eventually up to 40% of patients with PNH will develop thrombosis, PNH is among the most severe acquired thrombophilic states.1,3,4,8 However, three developments have the potential to alleviate the weight of thrombosis on disease outcome: (i) primary prevention with warfarin,2 (ii) anti-complement therapy,9 and (iii) thrombolytic therapy with plasminogen activators.7,10–19 The first two measures have been discussed elsewhere;2,20 here we focus on the third development, by presenting nine case histories and by reviewing the literature on 15 previously published cases.
Rapid infusion of thrombolytics is a well established treatment for acute myocardial infarction. These agents also have a role in patients who have had arterial cerebral vascular accidents,21,22 in patients who are in hemodynamic shock from pulmonary emboli, and in patients with venous thrombosis at other sites.23–28,29,30 As for PNH, there have been a number of papers in the literature,10–15,17–19,31,32 most of them on isolated cases. Here we report our experience with thrombolytic therapy in a highly selected group of nine patients with PNH, all of whom had severe thrombotic complications, which were regarded as potentially fatal if not treated, or which were progressing despite anticoagulation: in this respect, tissue plasminogen activator (tPA) can be regarded as a second-line treatment for patients with PNH complicated by venous thrombosis.
Design and Methods
Patients were referred to Memorial Sloan-Kettering Cancer Center, or to Bellevue Hospital/NYU Langone Medical Center, or to Careggi University Hospital in Florence, from 1995 to present. Institutional Review Board approval for the use of protected health information was obtained prior to the composition of the manuscript, in accordance with Health Insurance and Portability and Accountability Act (HIPAA) guidelines. The diagnosis of PNH was always confirmed by flow cytometry. All of the patients in this series had hemolytic PNH (see the recent classification by Parker et al.33), with large populations of glycosylphosphatidylinositol (GPI)-negative red blood cells and granulocytes (Table 1A). When thrombosis was suspected clinically, it was always documented through appropriate imaging studies.
Table 1A.
Patients who received thrombolytic therapy: baseline data.
Patients were regarded as eligible for thrombolytic therapy with tPA when they met the following criteria: (i) a diagnosis of PNH confirmed by flow cytometry; (ii) occlusive thrombosis of more than one intra-abdominal and/or intracranial vein; (iii) estimated time since onset of thrombosis less than 2 months; and (iv) lack of response (or progression of thrombotic occlusion) upon anticoagulant treatment. As a rule, patients with severe thrombocytopenia and/or active bleeding were excluded.
The patients selected for treatment were admitted to the intensive care unit for monitoring, and anticoagulation was temporarily discontinued. Prior to treatment, large bore intravenous catheters were inserted to minimize the need for venipuncture for blood samples, and intramuscular and intra-arterial punctures were avoided. tPA was administered as an intravenous infusion at a dose of 1 mg/kg over the course of 24 h. Because tPA may not be stable for the duration of the infusion once it is reconstituted, it is advisable that the pharmacy prepare four consecutive infusions of 0.25 mg/kg to be administered over 6 h each. Platelets were transfused if the platelet count was below 50×109/L. As indicated in the case reports, we administered fresh-frozen plasma if the International Normalized Ratio (INR) was high, to reverse the effects of warfarin, or if a low plasminogen level was thought to be limiting for tPA-stimulated fibrinolytic activity (as this may be the case in severe liver disease, we recommend checking plasminogen levels in all patients with severe Budd-Chiari syndrome receiving tPA). After the first 24-h infusion, parenteral anticoagulation agents were generally resumed, and repeat imaging studies were performed to assess response. The patient was carefully monitored for signs of hemorrhage based on clinical examination, serial hematocrit measurements and, when appropriate, imaging. Additional courses of tPA were then administered as deemed necessary if there was persistent thrombosis, provided there was no serious hemorrhage.
Results
Forty-one patients had documented thrombosis on at least one occasion. The mean age of the first thrombotic episode, based on history or observation, was about 32 years. Recurrence of thrombosis is well known in PNH; in this study we confirmed that patients who had had one thrombosis were indeed at a very high risk of a second thrombosis, even if they were on long-term anticoagulants (Figure 1). In some cases we observed thromboses even during the follow-up of patients regularly attending our clinic. In other cases, the patient was referred already having had thromboses; if the patient’s history suggested that thromboses were long-standing (e.g., for a period of years), they were regarded as not amenable to thrombolysis. In total, nine patients (Table 1B) were determined to have had a recent symptomatic occlusive thrombosis (e.g. duration of a few weeks to a few months) unresponsive to anticoagulants: i.e., they met the four criteria stated above and they received thrombolytic therapy. Of 32 other patients with hemolytic PNH, 29 did not meet these criteria, as detailed in Online Supplementary Table S1. The remaining three of these 32 patients did meet some of the criteria but they were not given thrombolytic therapy because of special complications: patient 5 (Online Supplementary Table S1) had bleeding due to splenic rupture following splenic vein thrombosis; patient 17 (Online Supplementary Table S1) had a hepatic adenoma with possible hemorrhage; patient 14 (Online Supplementary Table S1) had had a recent termination of pregnancy. Of these patients, the first died of hemorrhagic shock; and the other two were referred for a liver transplant.
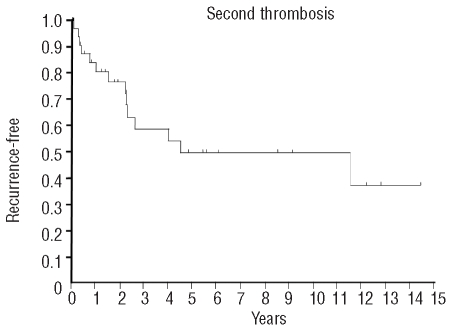
Figure 1.
Kaplan-Meier curve representing recurrent thrombotic events in patients already treated for one thrombotic event. Thirty-five patients were followed for a median of 2.2 years. Median time to recurrence was approximately 4.5 years, and 14 patients eventually had recurrences. To our knowledge, all of these patients were supposed to have been on anticoagulation after their initial thrombosis, with the exception of one patient who was briefly instructed to stop warfarin for a procedure shortly before a recurrent thrombosis.
Table 1B.
Patients who received thrombolytic therapy: clinical course.
Case summaries of patients receiving tissue plasminogen activator
Patient 1 presented with severe Budd-Chiari syndrome and tense ascites, initially treated with heparin, complicated by heparin-induced thrombocytopenia with thrombosis (HITT). Based on the history, the onset of thrombosis may have been up to 6 weeks earlier. Imaging demonstrated complete occlusion of all three hepatic veins and the inferior vena cava (IVC). Heparin was replaced with lepirudin and fresh-frozen plasma was given to correct a low plasminogen level. After five courses of tPA, the IVC and one of the three thrombosed hepatic veins recanalized. Additional courses were not given because of a very large flank hemorrhage. Despite initial incomplete resolution based on radiographic studies, there was clinical resolution of hepatic dysfunction and ascites, and the patient was discharged on warfarin. Six months later, there was documented patency of all thrombosed veins and further clinical improvement, and the patient was still well at her last follow-up.
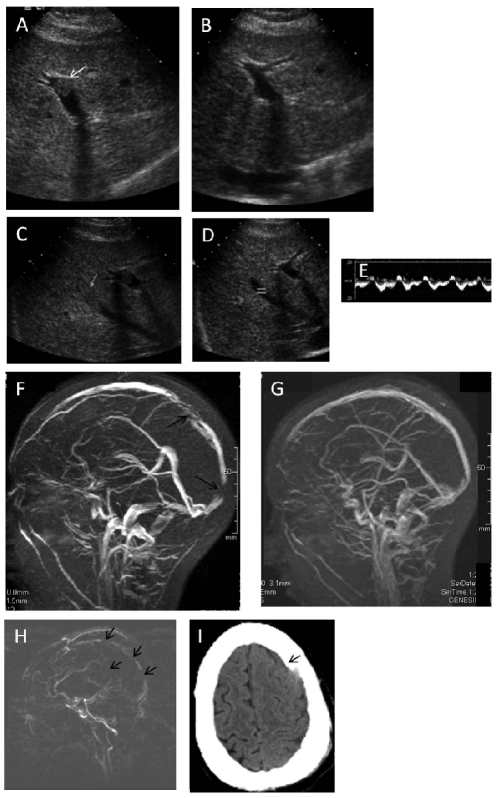
Patient 2 received three cycles of intravenous tPA for intra-cerebral and intra-abdominal venous thromboses in the first trimester of pregnancy (Figure 2). She had a good response, and being well aware of the high risk of recurrence, the patient elected for a surgical termination of pregnancy. Three months later, despite a therapeutic INR, she did in fact develop a recurrent sagittal sinus thrombosis, which was again reversed with two cycles of tPA. The patient remained clinically stable for 5 years on enoxaparin, later replaced with fondaparinux. Subsequently, on routine imaging she was found to have non-occlusive thrombosis of the splenic and portal veins, which resolved following treatment with eculizumab. This case is remarkable for the concurrence of three risk factors: PNH, pregnancy, and factor V Leiden allele.
Figure 2.
Selected radiological images. (A) Portal vein thrombosis, patient 2. (B) Portal vein, patient 2, after tPA (C) Middle hepatic vein thrombosis, patient 2. (D) Middle hepatic vein, patient 2, after tPA, with restored flow (E), demonstrated by Doppler. (F) Magnetic resonance (MR) venogram, patient 2. (G) MR venogram, patient 2, after tPA. (H) MR venogram, patient 4, showing extensive venous occlusions. (I) Head computed tomography, patient 4, after tPA, showing a subdural hematoma. Arrows indicate sites of thrombosis in panels A,C, F, and H, and indicate the hematoma in panel I.
Patient 3 had been dependent upon red blood cell transfusions for severe hemolytic PNH for 7 years before developing a partial occlusion of the portal vein, which, despite treatment with dalteparin, progressed to involve the hepatic veins within several weeks, felt to be due to an HIT antibody. He was given a direct thrombin inhibitor, and the thromboses nearly completely resolved with tPA. He was discharged on warfarin, and has remained on warfarin for the past 7 years. Six years ago he started eculizumab, with a dramatic improvement in his hemoglobin level, transfusion requirements, and quality of life.
Patient 4 presented with thromboses in the IVC, the right and left hepatic veins, the portal vein, the superior mesenteric vein, and the right renal vein. She was initially treated with intravenous heparin and then three 24-h infusions of tPA. During this time, a falling platelet count was attributed to HIT antibodies, and she was found to have cerebral vein thrombosis (Figure 2). She was treated with lepirudin and fresh-frozen plasma to raise the plasminogen level, and achieved almost complete resolution of her thromboses. She then developed bilateral subdural hematomas associated with both subfalcine herniation to the right and downward herniation, including cerebellar tonsillar herniation as well as obstructive hydrocephalus of the right lateral ventricle. The lepirudin and tPA infusions were stopped, and she underwent successful evacuation of the left subdural hematoma. Approximately 48 h later, anticoagulation with lepirudin was resumed after computed tomography of the head revealed resolution of the hydrocephalus without any new hematomas, and the IVC, the portal vein, hepatic, and the right renal veins remained patent, as determined by ultrasound/Doppler imaging. While the patient was receiving lepirudin, warfarin was started, but 2 days later the patient developed altered mental status, and magnetic resonance venography of the brain revealed recurrent dural sinus thrombosis. In addition, there were left basal ganglia/internal capsule and left occipital non-hemorrhagic infarcts. Attempts at directed intra-sinus thrombolysis were unsuccessful, and the patient died a few days later.
Patient 5 presented with acute renal failure secondary to renal vein and IVC thrombosis. This was treated successfully with two courses of systemic tPA 2 weeks after presentation. The patient was then subsequently maintained on warfarin. Two years later, she presented with headache and a sub-therapeutic INR and was found to have a left transverse sinus thrombosis. She received intravenous heparin with resolution of her symptoms but persistent occlusion and development of venous collaterals. Four years later, during a brief hiatus in anticoagulation for a medical procedure, she developed extension of the venous sinus thrombosis with new occlusion of the superior, straight and right transverse sinuses. She was again was treated with intravenous heparin without thrombolysis, with some improvement despite residual thromboses. Seven years later she is clinically stable and is receiving eculizumab and anticoagulation.
Patient 6 had asymptomatic intra-abdominal thromboses identified on presentation. She was started on warfarin with unsatisfactory compliance. Two years later, she developed an acute abdomen; 20 cm of edematous bowel were resected. Thromboses were identified in small vessels on histology, and she was treated with enoxaparin. Two weeks later, she was found to have thromboses in two hepatic veins, which were thought to be new. She received tPA, which resulted in recanalization. She was started again on enoxaparin, but 1 month later she was found to have a symptomatic non-traumatic frontal subdural hematoma. Anticoagulation was discontinued, and approximately 2½ weeks later, she developed recurrent bowel edema, once again requiring resection. Mesenteric and sub-serosal hemorrhage and edema were seen histologically, with no intravascular thrombi. Two weeks after the second operation, and 2 months after the administration of tPA, the patient died. Given the time interval between tPA and the subdural hematoma and the concurrent anticoagulation, it is not clear to what extent tPA contributed to this outcome, and it was not considered likely that this hemorrhagic complication contributed directly to the patient’s death.
Patient 7 developed hepatic vein thrombosis while on oral contraception. She received heparin and tPA with a good radiological response. However, her platelet count soon began to fall, she had a positive test for HIT antibodies, and developed splenic infarctions; this patient is the subject of a separate case report.16 The patient received direct thrombin inhibitors and ultimately required a splenectomy for rupture. She developed a sympathetic pleural effusion with some evidence of hemorrhage into the effusion. This ultimately resolved and the patient remained stable for 2 years with self-injection of fondaparinux, despite incomplete compliance and moderate thrombocytopenia. However, she then developed IVC and hepatic vein thrombosis and received a second course of tPA, resulting in radiological improvement. She was then started on eculizumab, which was discontinued because of inadequate compliance. She presented 6 months later with pain, weight gain, edema, and ascites. Imaging revealed a 5 cm thrombosis in the IVC extending from the level of the renal veins to the level of the hepatic veins. She received one course of tPA, but developed severe epistaxis. She was started again on eculizumab and follow-up imaging showed improvement in the IVC thrombosis. However, she had signs of persistent partial Budd-Chiari syndrome, with external IVC compression attributed to hepatic congestion. This responded to the placement of an IVC stent, she mobilized the edema fluid sufficiently for discharge, and she stabilized on fondaparinux and eculizumab and continued to improve clinically, despite extensive residual vascular occlusions. She has been compliant with anticoagulation and anticomplement therapy for the past year. She now has a performance status of 100% and has been able to work.
Patient 8 had a history of hepatic vein thrombosis treated for 1 year with warfarin, and was referred for tPA treatment after developing thrombosis of the IVC. With tPA he achieved partial recanalization of the hepatic veins, and an intrahepatic porto-caval stent was inserted. Within the following year, he had three relapses of thrombosis within the stent, requiring three further courses of tPA, each time with partial recanalization. The last course was administered, with intensive platelet support, in spite of severe thrombocytopenia, and this time the infusion was complicated by an intra-parenchymal cerebral hemorrhage and left-sided hemiplegia. After neurosurgical evacuation the patient gradually recovered neurological function, with some permanent visual field loss. Anticoagulation was discontinued, eculizumab was started, and the patient is now clinically well, with good hepatic function.
Patient 9 underwent splenectomy for painful splenic infarctions, complicated by a portal vein thrombosis and thrombocytosis, and the JAK2V617F mutation was identified. He was given hydroxyurea, warfarin, and eculizumab, which resulted in partial normalization of lactate dehydrogenase levels and urine color. Approximately 10 months later, despite a therapeutic INR, he developed severe Budd-Chiari syndrome, with complete hepatic vein obstruction. The warfarin was reversed, he was started on lepirudin, and was given three courses of tPA over 1 week. Although an immediate partial recanalization was achieved, with relief of symptoms, further courses were not given because of the development of a painful psoas muscle hemorrhage. He was found to have an unusually rapid clearance of eculizumab, which was then given weekly, as well as daily fondaparinux. Eventually, flow was restored in all three hepatic veins and, at last imaging over 1 year later, he remained free of thrombosis. He eventually made an excellent functional recovery, with transient setbacks due to listeria sepsis and soft tissue and lower gastrointestinal tract bleeding associated with the addition of aspirin. However, due to the myeloproliferative disorder, he developed severe neutrophilia, which was treated with decitabine. He remained heavily dependent on red blood cell transfusions, developed massive iron overload despite chelation, and ultimately died from hepatic failure, despite having patent vessels.
Summary of outcome of patients who received thrombolytic therapy
Treatment with intravenous tPA proved effective in resolving one or more thrombi in venous vessels in all cases. In only one case (patient 4) was infusion of tPA closely associated in time with a fatal outcome, and in this case recurrent thrombosis and HITT also played a role. In patient 6, tPA may have indirectly contributed to death, because, after successful thrombolysis, the patient developed a subdural hematoma, for which reason anticoagulants were discontinued, whereupon she developed progressive pathology of the bowel, which was suspected to be due to thrombosis. In patient 9, death must be attributed to his progressive myeloproliferative syndrome. Two patients suffered large soft tissue hematomas; one patient developed a hemorrhagic pleural effusion on one occasion and epistaxis during a subsequent course of tPA: none of these episodes was life-threatening. Four of the patients suffered from HITT, which was documented by a positive enzyme-linked immunosorbent assay for antiplatelet factor 4 antibodies in the setting of declining platelet counts. In all of these cases HITT probably contributed to progressive or refractory thromboses that required thrombolytic therapy. A total of three cases of central nervous system hemorrhage were documented.
Summary of patients not receiving thrombolytic therapy
Among 32 patients not receiving tPA, the most common reason it was not given (13 patients) was because the thrombosis was known to be old without any recent symptomatic exacerbation (Online Supplementary Table S1). The second most common reason that tPA was not given is that the thromboses occurred in sites that we did not consider to represent indications for thrombolysis--e.g., deep vein thromboses of the extremities or pulmonary emboli that were responding to anticoagulation (7 patients). Two patients had thromboses that were non-occlusive, four patients had obvious contraindications (exclusion criteria), and four patients were treated surgically or by other invasive procedures. Eight of the 32 patients described in Online Supplementary Table S1 have died.
Review of previous case reports
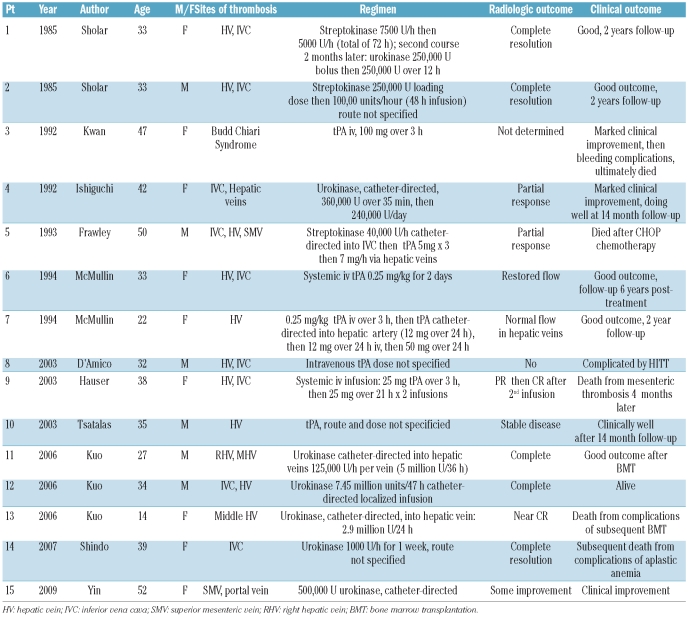
We performed a PubMed search using the terms “fibrinolysis”, “thrombolysis”, or “tissue plasminogen activator”, cross referenced with the terms “PNH” or “paroxysmal nocturnal hemoglobinuria”. We found 11 reports between 1985 and 2009 describing a total of 15 patients (Table 2). tPA, urokinase and streptokinase were used in six, six and three patients, respectively. In seven patients, therapy was delivered as a localized infusion via a catheter placed in or near the thrombosed vessel; one patient received localized therapy followed by systemic therapy because of an inadequate initial response, and in the remaining seven patients, thrombolytics were either administered systemically or the route was not specified. Eight patients achieved a complete or near complete radiologic response, three patients had a partial response, two patients did not respond, and in one patient response was not determined. Overall, nine patients had a good clinical outcome; hemorrhagic complications were observed in only one patient.
Table 2.
Summary of the literature on thrombolytic therapy in patients with PNH.
Discussion
Thrombolytic therapy is highly effective in paroxysmal nocturnal hemoglobinuria
The most significant finding from this study is that in a series of nine patients, at least partial resolution of thrombosis was documented by appropriate imaging on all 15 occasions in which tPA was used. For the purpose of proving the efficacy of a specific form of treatment for a serious complication of a very rare disease one is facing a priori a major difficulty, in the sense that it is practically impossible to conduct a randomized clinical trial (in fact, there is only one, the eculizumab trial34 in the entire literature on the management of PNH). In order to circumvent this difficulty, we used the following approach: (i) we adopted strict eligibility criteria (see above); (ii) we assessed the therapeutic response by extensive imaging; and (iii) in each case we used previous existing documentation, so that each patient became to some extent his or her own control. Thus, although our patients were not part of a formal trial, in most cases there had been a considerable time interval without improvement before tPA was instituted. Specifically, tPA was offered as second-line treatment to those patients in whom anticoagulation was failing. Data from the literature (Table 2) support our finding that systemic administration of tPA can resolve thrombosis in PNH patients.
Thrombolytic therapy is associated with a high risk of bleeding
A second significant point from this work is that we were able to obtain an estimate of the risk of serious bleeding, which was three instances in 15 discrete admissions in which tPA was administered, or about 20%. This would be a higher rate of this complication than typically reported for the treatment of myocardial infarction.21 However, of the three cases of serious bleeding – all of which were intracranial – in one case the underlying cerebral vein thrombosis was probably contributing, along with HITT, and in another case, the intracranial hemorrhage was not diagnosed until weeks after the tPA had been given and cannot, therefore, be definitively attributed to this treatment. Curiously, there is almost no mention of bleeding complications in the previous case reports of thrombolysis in PNH. We found relatively minor bleeding, such as into soft tissues, around catheter sites, and epistaxis to be common and controllable, as expected from the experience with cardiology patients. We cannot say whether catheter-directed therapy would have avoided any of the major or minor bleeding episodes.
Heparin-induced thrombocytopenia with thrombosis
The third important finding of this series is that HITT seems to be common in PNH patients receiving unfractionated heparin and also occurred in one case receiving low molecular weight heparin. Indeed, HITT has been reported occasionally in patients with PNH.10,16,35 We observed this complication in four out of our series of nine cases, far in excess of what one might have expected from chance alone. We have not had instances of HITT in patients who did not receive thrombolytic therapy: the most likely explanation is that such patients rarely received heparin. We suggest avoidance of heparin products in patients with PNH until the reasons for a high incidence of HITT are understood. Fondaparinux very rarely causes HITT and should be preferred; alternatively, the new oral direct thrombin and factor Xa inhibitors may prove useful.
Systemic versus catheter-administered tissue plasminogen activator
In the recent literature, tPA has featured prominently in the management of venous thrombosis mainly for the purpose of preventing the so-called post-thrombosis syndrome.23,25,26,29,30 Indeed, catheter-directed therapy is gaining in acceptance for the treatment of deep vein thrombosis of the extremities.29,30 However, there has been no trial comparing catheter-directed therapy versus systemic tPA. The use of catheter-directed therapy has also been emphasized with respect to Budd-Chiari syndrome, and it has been claimed that systemic thrombolytic therapy is not effective for this condition, based on two out of three patients who did not respond.28 However, there is no anatomic reason why hepatic vein thrombosis should not be amenable to systemic thrombolysis, since this therapy has succeeded with portal vein thrombosis,27 which may be difficult to access by a catheter approach.
Whether and when thrombolytic therapy is indicated for deep vein thrombosis in a limb has not yet been established, but it may well be that when it is indicated, catheter-directed therapy will be the modality of choice. Although we too have not obtained or indeed sought formal proof that systemic thrombolysis is superior to catheter-directed therapy, there are several reasons why we believe that in PNH this is so: (i) we have documented here the efficacy of systemic tPA in PNH, including eight cases of hepatic vein occlusion. On two occasions we demonstrated that cerebral vein thrombosis can also respond to systemic tPA (see patient 2, Table 1); (ii) deep vein thrombosis of the limbs is the exception rather than the rule in PNH; (iii) it is not uncommon for patients with PNH to have thromboses in several sites (see patients 1, 2, 4, 5, 9 in Table 1): in such cases the advantage of systemic therapy is obvious. Furthermore, even when thrombosis is only demonstrated in one site, there might be additional thromboses in smaller vessels; (iv) an experienced interventional radiologist is not always available; (v) once a large central vein has been cannulated, it may be more risky to administer systemic tPA later if this fails or if thromboses appear in multiple sites; conversely, administering intravenous tPA does not preclude subsequent catheter-directed therapy. In practice, most of our patients required several 24-h infusions, and at least one additional administration of systemic tPA should be administered before the approach is abandoned; (vi) the original rationale for preferring tPA to other thrombolytic agents was that it is targeted to sites where fibrin36 and activated clotting factors37 are present: i.e., it was designed so as not to require local delivery.
Selection of patients for thrombolysis
Rapid reversal of ischemia is mandatory for coronary artery thrombosis or arterial stroke, and the temporal selection criteria for treatment is measured in terms of hours of duration of symptoms.21,22 For venous thrombosis, however, the critical issue is reversal of venous congestion, and recanalization may be achieved as long as the thrombosis has not become irreversibly fibrotic. The temporal selection criteria are, therefore, typically measured in terms of weeks of duration.29,30 Based on this, we thought that slow administration over 24 h would be safer than the rapid infusions given for myocardial infarction or stroke, although we cannot be certain of this point. Here we confirm, using this protocol, that responses to tPA can be seen even when the thromboses have been present for several weeks to a few months. Even in patients who have had chronic thromboses, acute exacerbations may be due to new superimposed thromboses, and they may still improve clinically with tPA, patient 7 (Table 1) being an example of this.
Nevertheless, thromboses of long duration remained the most important reason for excluding patients from this treatment (Online Supplementary Table S1). This highlights an important point – that we have seen patients who did not receive tPA but who did well as a result of endogenous thrombolysis and spontaneous collateralization, which we believe plays a beneficial role also in patients who have had a partial response to tPA (e.g. patients 1, 7, and 9; Table 1). We believe that reserving tPA as a second-line treatment for those patients with failing anticoagulation (nowadays combined with anticomplement therapy), will avoid “over treating” those whose thrombosis would otherwise have recanalized or collateralized without thrombolytic therapy. It should be emphasized, however, that exclusion of appropriate candidates for thrombolysis is a potential hazard of this selection process as well, given that tPA was almost certainly life-saving for patients 1 and 9, who had been moribund, and probably also for patient 7, who had had massive edema from recurrent thromboses, and possibly also for patient 5, who had been developing renal insufficiency due to renal vein and IVC thrombosis.
Treatment recommendations
Here we list some recommendations: (i) the work-up of a patient with PNH, even if there is no clinical history or evidence of thrombosis, should include an abdominal Doppler ultrasound or magnetic resonance venography as well as a magnetic resonance venogram of the intracranial dural venous sinuses. Whether the studies are positive or negative, they will be a useful baseline, should an acute episode develop; (ii) when a patient with PNH develops complaints suggestive of thrombosis, there should be a low threshold for diagnostic imaging, with the aim of identifying new thrombotic events before they become fibrotic and refractory to the effects of anticoagulation or tPA; (iii) once a decision to give tPA has been made, early administration is obviously desirable, but there is no known time cut-off, and we have found that thromboses may be reversible for at least 6 weeks; (iv) thrombocytopenia is not an absolute contraindication to tPA, provided that platelet transfusions can be given; (v) the risk of hemorrhage due to thrombolysis must be weighed against the very high radiographic response rate we have observed. (vi) given the surprisingly high rate of occurrence of HITT in PNH patients, heparin-derived products should probably be avoided as far as possible; (vii) given the very high rate of recurrence of thrombosis that we have demonstrated here despite anticoagulation (Figure 1), treatment with eculizumab in addition to anticoagulation is warranted in any patient who has developed a thrombosis.
Conclusion
We have confirmed the radiologically documented clinical efficacy of tPA in the treatment of serious refractory thromboses in a carefully selected series of patients with PNH. Since unresolved thromboses can lead rapidly to fatal complications or loss of organ function, we believe that the decision not to give tPA can be just as critical as the decision to give it. Most of our patients were treated before eculizumab was available, and we expect that most who present with acute thromboses will now be given anticoagulation and eculizumab: thus, it may be easier in the future to resolve thrombi through endogenous mechanisms without having to administer tPA. Furthermore, since eculizumab decreases the risk of thrombosis,9,38 the rate of this complication will probably decrease. Nevertheless, we expect that thrombolysis will continue to be an important treatment option for previously undiagnosed PNH patients who first present with thrombosis; for patients who develop thrombosis despite modern treatments; and for patients with thrombosis for whom eculizumab has not become available.
Acknowledgments
We are grateful to Dr Karen Brown, Professor Giacomo Laffi, Professor Pasquale Mennonna and Dr Matteo Mazzetti, and Bridget Lane for their role in the management of patients. We thank Professor Tiziano Barbui, and also the Associate Editor and anonymous reviewers for constructive comments on the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Araten D, Thaler H, Luzzatto L. High incidence of thrombosis in African-American and Latin-American patients with paroxysmal nocturnal haemoglobinuria. Thromb Haemost. 2005;93(1):88–91. doi: 10.1160/TH04-06-0391. [DOI] [PubMed] [Google Scholar]
- 2.Hall C, Richards S, Hillmen P. Primary pro-phylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2003;102(10):3587–91. doi: 10.1182/blood-2003-01-0009. [DOI] [PubMed] [Google Scholar]
- 3.Hillmen P, Lewis S, Bessler M, Luzzatto L, Dacie J. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–8. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 4.Socie G, Mary J, deGramont A, Rio B, Leporrier M, Rose C, et al. Paroxysmal nocturnal hemoglobinuria: long-term follow-up and prognostic factors. Lancet. 1996;348(9027):573–7. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura J, Kanakura Y, Ware R, Shichishima T, Nakakuma H, Ninomiya H, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83(3):193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 6.Nafa K, Bessler M, Mason P, Vulliamy T, Hillmen P, Castro-Malaspina H, et al. Factor V Leiden mutation investigated by amplification created restriction enzyme site (ACRES) in PNH patients with and without thrombosis. Haematologica. 1996;81(6):540–2. [PubMed] [Google Scholar]
- 7.Ray J, Burows R, Ginsberg J, Burrows E. Paroxysmal nocturnal hemoglobinuria and the risk of venous thrombosis: review and recommendations for management of the pregnant and nonpregnant patient. Haemostasis. 2000;30(3):103–17. doi: 10.1159/000022532. [DOI] [PubMed] [Google Scholar]
- 8.deLatour R, Mary J, Salanoubat C, Terriou L, Etienne G, Mohty M, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112(8):3099–106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Muus P, Dührsen U, Risitano A, Schubert J, Luzzatto L, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–8. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico E, Villaça P, Gualandro S, Bassitt R, Chamone D. Successful use of Arixtra® in a patient with PNH, Budd–Chiari syndrome and HIT. J Thromb Haemost. 2003;1(11):2452–3. doi: 10.1046/j.1538-7836.2003.0468c.x. [DOI] [PubMed] [Google Scholar]
- 11.Frawley K, MacKechnie S, Taylor K. Thrombolytic therapy in paroxysmal nocturnal haemoglobinuria complicated by hepatic vein thrombosis. Australas Radiol. 1993;37(4):396–8. doi: 10.1111/j.1440-1673.1993.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Hauser A, Brichta A, Pabinger-Fasching I, Jager U. Fibrinolytic therapy with rt-PA in a patient with paroxysmal nocturnal hemoglobinuria and Budd-Chiari syndrome. Ann Hematol. 2003;82(5):299–302. doi: 10.1007/s00277-003-0639-8. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguchi T, Fukatsu H, Itoh S, Shimamoto K, Sakuma S. Budd-Chiari syndrome with long segmental inferior vena cava obstruction: treatment with thrombolysis, angioplasty, and intravascular stents. J Vasc Interv Radiol. 1992;3(2):421–5. doi: 10.1016/s1051-0443(92)72056-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuo G, Brodsky R, Kim H. Catheter-directed thrombolysis and thrombectomy for the Budd-Chiari syndrome in paroxysmal nocturnal hemoglobinuria in three patients. J Vasc Interv Radiol. 2006;17(2 part 1):383–7. doi: 10.1097/01.RVI.0000196338.87954.CE. [DOI] [PubMed] [Google Scholar]
- 15.Kwan T, Hansard P. Recombinant tissue-plasminogen activator for acute Budd-Chiari syndrome secondary to paroxysmal nocturnal hemoglobinuria. NY State J Med. 1992;92(3):109–10. [PubMed] [Google Scholar]
- 16.Magnan H, Kayton M, DiMichele D, Araten D, Kernan N, Boulad F. Splenic Infarction and subsequent splenic rupture in a patient with paroxysmal nocturnal hemoglobinuria and heparin-induced thrombocytopenia. Pediatr Blood Cancer. 2009;53(3):472–4. doi: 10.1002/pbc.22058. [DOI] [PubMed] [Google Scholar]
- 17.McMullin M, Hillmen P, Jackson J, Ganly P, Luzzatto L. Tissue plasminogen activator for hepatic vein thrombosis in paroxysmal nocturnal haemoglobinuria. J Intern Med. 1994;235(1):85–9. doi: 10.1111/j.1365-2796.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 18.Sholar P, Bell W. Thrombolytic therapy for inferior vena cava thrombosis in paroxysmal nocturnal hemoglobinuria. Ann Intern Med. 1985;103(4):539–41. doi: 10.7326/0003-4819-103-4-539. [DOI] [PubMed] [Google Scholar]
- 19.Tsatalas C, Margaritis D, Pantelidou D, Kotsianidis I, Karayiannakis A, Spanoudakis E, et al. Splenectomy for massive splenic infarction unmasks paroxysmal nocturnal hemoglobinuria. Acta Haematol. 2003;110(4):193–6. doi: 10.1159/000074224. [DOI] [PubMed] [Google Scholar]
- 20.Luzzatto L, Gianfaldoni G, Notaro R. Management of paroxysmal nocturnal haemoglobinuria: a personal view. Br J Haematol. 2011;153(6):709–20. doi: 10.1111/j.1365-2141.2011.08690.x. [DOI] [PubMed] [Google Scholar]
- 21.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 22.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 23.DeLoughery T. Venous thrombotic emergencies. Emerg Med Clin North Am. 2009;27(3):445–58. doi: 10.1016/j.emc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Frey I, Muro G, McDougall C, Dean B, Jahnke H. Cerebral venous thrombosis. Combined intrathrombus rtPA and intravenous heparin. Stroke. 1999;30(3):489–94. doi: 10.1161/01.str.30.3.489. [DOI] [PubMed] [Google Scholar]
- 25.Martinelli I, Stefano VD. Rare thrombosis of cerebral, splanchnic and upper extremity veins. Thromb Haemost. 2010;103(6):1136–44. doi: 10.1160/TH09-12-0873. [DOI] [PubMed] [Google Scholar]
- 26.Menon K, Shah V, Kamath P. The Budd Chirari syndrome. N Engl J Med. 2004;350(6):578–85. doi: 10.1056/NEJMra020282. [DOI] [PubMed] [Google Scholar]
- 27.Schafer C, Zundler J, Bode J. Thrombolytic therapy in patients with portal vein thrombosis: case report and review of the literature. Eur J Gastroenterol Hepatol. 2000;12(10):1141–5. doi: 10.1097/00042737-200012100-00012. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Texeira A, Texeira P, Elias E, Wilde J, Olliff S. Pharmacological thrombolysis in Budd Chiari syndrome: a single center experience and review of the literature. J Hepatol. 2004;40(1):172–80. doi: 10.1016/j.jhep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Alesh I, Kayal F, Stein P. Catheter-directed thrombolysis (intrathrombus injection) in treatment of deep venous thrombosis: a systematic review. Catheter Cardiovasc Interv. 2007;70(1):143–8. doi: 10.1002/ccd.21079. [DOI] [PubMed] [Google Scholar]
- 30.Patterson B, Hinchliffe R, Loftus I, Thompson M, Holt P. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol. 2010;30(4):669–74. doi: 10.1161/ATVBAHA.109.200766. [DOI] [PubMed] [Google Scholar]
- 31.Shindo S, Motohashi S, Kaga S, Inoue H, Matsumoto M, Shindo H. Paroxysmal nocturnal hemoglobinuria: complete resolution of an occluding inferior vena caval thrombus. Abdom Imaging. 2007;32(6):754–7. doi: 10.1007/s00261-007-9184-6. [DOI] [PubMed] [Google Scholar]
- 32.Yin D, Liu L, Zhang S, Tian L, Lu Z, Jiang H. Portal hypertension resulted from paroxysmal nocturnal hemoglobinuria: a case report and review of the literature. International Int J Hematol. 2009;89(3):302–4. doi: 10.1007/s12185-009-0287-4. [DOI] [PubMed] [Google Scholar]
- 33.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, et al. for the International PNH Interest Group, Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillmen P, Young N, Schubert J, Brodsky R, Socié G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 35.Huehn C, Righini M, Starobinski M, Angiello-Scherrer A, Moerlosse PD. Are patients with paroxysmal nocturnal hemoglobinuria at risk for heparin induced thrombocytopenia? J Thromb Haemost. 2003;1(2):389–90. doi: 10.1046/j.1538-7836.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoylaerts M, Rijken D, Lijnen H, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. J Biol Chem. 1982;257(6):2912–9. [PubMed] [Google Scholar]
- 37.Urano T, Nagai N, Matsuura M, Ihara H, Takada Y, Takada A. Human thrombin and calcium bound factor Xa significantly shorten tPA-induced fibrin clot lysis time via neutralization of plasminogen activator inhibitor type 1activity. Thromb Haemost. 1998;80(1):161–6. [PubMed] [Google Scholar]
- 38.Helley D, deLatour R, Porcher R, Rodrigues C, Galy-Fauroux I, Matheron J, et al. Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica. 2010;95(4):574–81. doi: 10.3324/haematol.2009.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]