Abstract
Background
Dyskeratosis congenita is a cancer-prone bone marrow failure syndrome caused by aberrations in telomere biology.
Design and Methods
We studied 65 patients with dyskeratosis congenita and 127 unaffected relatives. Telomere length was measured by automated multicolor flow fluorescence in situ hybridization in peripheral blood leukocyte subsets. We age-adjusted telomere length using Z-scores (standard deviations from the mean for age).
Results
We confirmed that telomere lengths below the first percentile for age are very sensitive and specific for the diagnosis of dyskeratosis congenita. We provide evidence that lymphocytes alone and not granulocytes may suffice for clinical screening, while lymphocyte subsets may be required for challenging cases, including identification of silent carriers. We show for the first time using flow fluorescence in situ hybridization that the shortest telomeres are associated with severe variants (Hoyeraal-Hreidarsson and Revesz syndromes), mutations in DKC1, TINF2, or unknown genes, and moderate or severe aplastic anemia. In the first longitudinal follow up of dyskeratosis congenita patients, we demonstrate that telomere lengths decline with age, in contrast to the apparent stable telomere length observed in cross-sectional data.
Conclusions
Telomere length by flow fluorescence in situ hybridization is an important diagnostic test for dyskeratosis congenita; age-adjusted values provide a quantitative measure of disease severity (clinical subset, mutated gene, and degree of bone marrow failure). Patients with dyskeratosis congenita have accelerated telomere shortening. This study is registered at www.clinicaltrials.gov (identifier: NCT00027274).
Keywords: bone marrow failure, dyskeratosis congenita, telomeres, longitudinal study
Introduction
Dyskeratosis congenita (DC, MIM 30500, 127550, 224230) is an inherited bone marrow failure syndrome in which abnormalities in telomere biology lead to very short telomere lengths.1 Telomeres are long nucleotide repeats (TTAGGG)n at the ends of chromosomes which protect chromosomal integrity.2–3 More than half of the patients with DC have mutations identified in a gene required for telomere maintenance (DKC1, MIM 30126; TERC, MIM 602322; TERT, MIM 187270; TINF2, MIM 604319; NHP2, MIM 606470; NOP10, MIM 606471, or WRAP53 [protein TCAB1], MIM 612661). Inheritance is X-linked recessive, autosomal dominant, or autosomal recessive. In addition to the diagnostic triad of nail dystrophy, lacey reticular pigmentation, and oral leukoplakia, patients with DC are at very high risk of bone marrow failure (BMF), cancer, pulmonary and liver disease, and multiple other medical problems.4–5 The diagnostic triad and other DC-related physical findings and complications frequently develop over time and at different rates, which can make the diagnosis challenging.6 Correct diagnosis is important for medical management, genetic counseling, and decisions regarding hematopoietic stem cell transplantation (HSCT). Identification of DC in apparently healthy family members who may be HSCT donors is especially important because stem cells from an individual with undiagnosed DC fail to rescue the DC patient’s bone marrow.7
In our previous report of 26 patients with DC and 54 relatives, we showed that telomere length was diagnostic for DC. Specifically, we found that telomeres below the first percentile for age measured in peripheral blood leukocyte subsets using automated multicolor flow fluorescence in situ hybridization (flow FISH) clearly discriminated between patients with DC and unrelated normal controls or unaffected relatives.8
We now have a much larger cohort, with more than double the numbers of participants: 65 patients and 127 relatives. The new data replicate our original results, and the combined cohort provides a more robust assessment of which blood cell types are most informative for clinical decision-making. Furthermore, the association between telomere length and genotype, phenotype, and clinical complications has not previously been adequately described, because the earlier studies by us and others lacked statistical power,8–9 although we and others did observe very short telomeres in several patients with mutations in TINF2.10–12 In the current large study, we show that baseline telomere length measured by automated multicolor flow FISH is highly associated with clinical DC subtypes, genotypes, and hematologic status. In addition, prior studies using cross-sectional data suggested that telomere lengths in patients with DC were stable over time.8–9 We now present the first longitudinal data that provide clear evidence that telomeres shorten significantly more rapidly with age in patients with DC than in unaffected individuals.
Design and Methods
Participants
This study was approved by the Institutional Review Board of the National Cancer Institute, is a component of NCI protocol 02-C-0052, and is registered in www.clinicaltrials.gov (NCT00027274). The original cohort enrolled between January 2000 and July 2006,8 while the new cohort enrolled from August 2006 through the end of December 2010. All analyses were made with the combined data.
Individuals with “classic DC” often had a component of BMF, and always had at least one feature of the diagnostic triad (dyskeratotic fingernails, lacey reticular pigmentation, and oral leukoplakia) or other physical abnormalities.6 A silent carrier of DC was a clinically unaffected first degree relative who shared the proband’s germline mutation in a DC gene. Family members who had the familial mutation, with a history of BMF, myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), or a DC-type cancer (primarily head and neck or anogenital squamous cell carcinoma) were included as “classic DC”.13 Severe subtypes of DC included Hoyeraal-Hreidarsson (HH) and Revesz syndromes (RS). HH is characterized by cerebellar hypoplasia, microcephaly, developmental delay, immunodeficiency, intrauterine growth retardation, and BMF. Features defining RS are bilateral exudative retinopathy (similar to acquired unilateral Coats’ retinopathy), intrauterine growth retardation, BMF, sparse fine hair, and central nervous system (CNS) calcifications.5 Patients with an inherited BMF syndrome other than DC14 were excluded. Probands with aplastic anemia and very short telomeres, but no physical features of DC and wild-type DC genes, were also excluded, as were their relatives, despite the caveat that some of them may turn out to have DC once all DC genes have been identified.
To be conservative and avoid potential overclassification of DC, family members of bona fide DC patients who had very short telomeres, lacked any physical abnormalities, were hematologically normal, and did not have a mutated DC gene were called “DC Relatives”, along with family members whose telomeres were within the normal range.8 “DC Rel 1” included members of families where the proband did not have a mutation in a known DC gene, while “DC Rel 2” was the classification for family members in whom the proband’s mutated gene was identified, but the relative lacked the familial mutation. Relatives were primarily parents and siblings, with a few grandparents included. The classification of “DC patient” versus “DC relative” may change as new genes are found for DC, or if signs of DC develop in those individuals.
Laboratory methods
Gene sequencing was performed in CLIA-certified laboratories. Patients designated as “unknown genotype” were wild-type for all known DC genes. Bone marrow status was classified as severe aplastic anemia (SAA) when Hb was less than 8 g/dL, neutrophils less than 500/μL, and platelets less than 20×109/L;15 moderate aplastic anemia (MAA) when values were below normal but not SAA, and normal when values were within the normal range for age.
The method used for automated multicolor flow FISH measurement of telomere lengths has been described previously.8,16 We analyzed six leukocyte subsets: granulocytes, total lymphocytes, CD45RA-positive/CD20-negative naïve T cells, CD45RA-negative memory T cells, CD20-positive B cells, and CD57-positive NK/NKT cells. Normal telomere lengths in kilobases (kb) were determined from approximately 400 normal individuals ranging from birth to 100 years of age. “Very short” telomeres were defined as below the first percentile of the age-matched controls. Denominators for analyses included only the number of patients for whom a given lineage was available.
Statistical analysis
Since normal telomere length decreases with age, age-adjustment was provided by conversion of individual data into Z-scores using the formula:
where X = the telomere length for the patient;
μ = the mean telomere length for age-matched controls;
and σ = the standard deviation (SD) of age-matched controls.
The Z-score compared the telomere measurement in each individual with the age-matched mean and SD of the values in the normal controls, and thus accounted for the known wide inter-individual telomere length variability. Z-scores are more precise than delta TEL that compares telomere length of one case to the value in a single age-matched control or to the mean of such controls. The Z-score below the first percentile of a normal distribution (−2.33 SD) was considered diagnostic for DC in these analyses; a normal Z-score would be zero. Use of the Z-score allowed comparisons of telomere length to be made between patients with DC and normal controls, and within diverse subtypes of patients with DC, as well as patients with DC with their relatives. All comparisons were adjusted for possible differences in age.
Analyses were performed with Microsoft Excel (Microsoft Office Excel 2007) and Stata11 (StataCorp Release 11.1, College Station, TX, USA). P values were two-sided; P<0.05 was considered statistically significant. Performance characteristics included odds ratios (OR) in favor of DC, 95% confidence intervals (CI), sensitivity (sens), specificity (spec), and positive and negative predictive values (PPV and NPV), based on the frequency of telomeres below the first percentile of normal in comparisons between patients with DC and their unaffected relatives.
Results
Diagnostic utility of leukocyte subsets
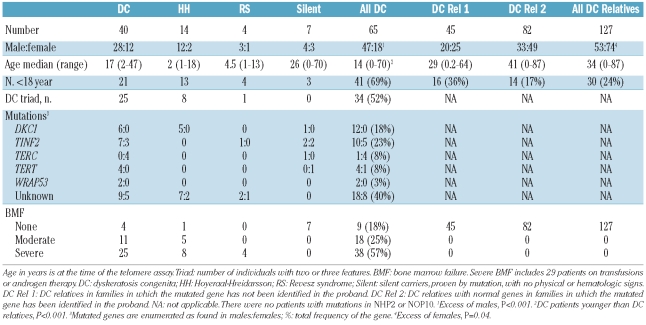
The total cohort (Table 1) consists of 65 patients with DC and 127 unaffected relatives (45 in families without known genes, and 82 in families in which the gene is known). Detailed information about the original dataset (26 patients and 54 relatives) was published previously.8 Twenty-four patients were members of nine families, while 41 were the only affected person in their family. Patients were significantly younger than their relatives, and twice as many patients were under 18 years of age. There were many more males in the DC patients. Twelve males had mutations in DKC1, and 17 in other DC genes; 18 males had unknown genotypes. Ten females had mutations identified, while 8 were unknown. Overall, about one quarter of the patients had mutations in TINF2, while 40% did not have mutations in any of the known genes. Unlike findings reported by another group in which the patients with TINF2 mutations were predominantly children,11–12 our 15 patients with these mutations included 8 children (0–13 years) and 7 adults (21–70 years). The diagnostic triad was represented by at least two features in about half the DC patients. One-third of the DC patients had no marrow failure at the time of diagnosis; this includes the 7 silent carriers as well as 2 patients with some non-triad DC physical findings.
Table 1.
Subjects in cohort.
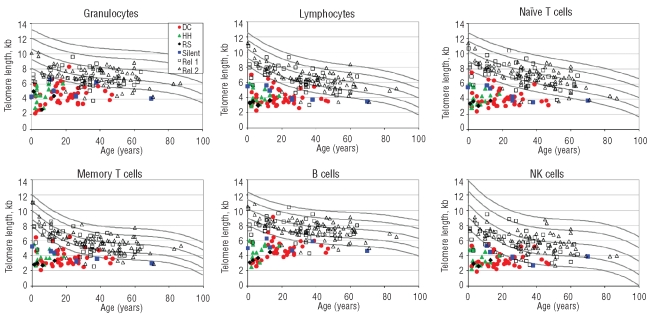
The results for the telomere lengths are shown in Figure 1 for the six leukocyte subsets that were analyzed. In general, the telomeres from the DC patients were significantly shorter than the normal controls (below the first percentile of normals for age), and shorter than the DC relatives. There was some variation across leukocyte subsets. In particular, 22 relatives had very short telomeres in granulocytes compared with 11 or less in other lineages. The relatives with very short telomeres in multiple lineages may be silent carriers in families in which the DC gene is not yet known. Conversely, 2 DC patients (mutation positive) had telomeres that were clearly in the normal range in granulocytes but were very short in several other lineages.
Figure 1.
Telomere length according to age in patients with DC and their relatives. The vertical axis represents telomere length in kb. The curved lines in the figures indicate the 1st, 10th, 50th, 90th, and 99th percentiles of results from 400 normal controls. Colored symbols represent patients with DC and their relatives. Red circles: classical DC patients; green triangles: Hoyeraal-Hreidarsson; black diamonds: revesz syndrome; blue squares: silent carriers; open black squares: DC relatives in families with unknown genes; open black triangles: DC relatives without mutations in the probands’ genes. Top panels show granulocytes, lymphocytes, and CD45RA-positive/CD20-negative naïve T cells. Bottom panels show CD45RA-negative memory T cells, CD20-positive B cells, and total NK/NKT cells.
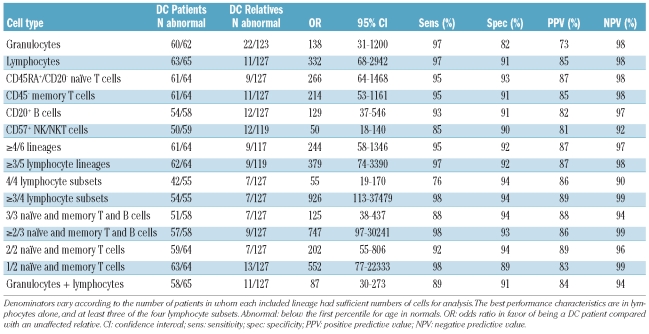
We compared the numbers of DC patients with the number of unaffected relatives with very short telomeres (Table 2). Granulocytes were very sensitive but not very specific, leading to a low PPV (73%). In contrast, total lymphocytes had sensitivity and specificity of 97% and 91% respectively, and 85% PPV. The data suggest that total lymphocytes perform better than granulocytes to diagnose DC if only a single lineage is studied. Several combinations of lymphocyte subsets or individual lymphocyte lineages all had sensitivities and specificities above 90%. Since CD57+ NK/NKT cells are a very heterogenous group of cells, the telomere length of this category had a greater variability (SD was higher), and the yield and sensitivity were poor compared with other lymphocyte subsets. The best performance characteristics appear to be in the group in which three of the four lymphocyte subsets were very short, with sensitivity and specificity of 98 and 94%, and the highest PPV 89%. This group had the highest odds ratio in favor of a DC patient versus a relative: 926 compared with 332 for total lymphocytes.
Table 2.
Telomere lengths in DC patients compared with DC relatives.
Misclassification was rare. Only 2 patients (silent carriers, with mutations in DC genes) would have been classified as normal using lymphocytes alone, or using three of four lymphocyte subsets. However, 11 relatives would be classified as DC using lymphocytes, compared with 7 (36% fewer) using the three of four lymphocyte subsets strategy. The 7 relatives with very short telomeres in the lymphocyte subsets were all included among the 11 with very short telomeres in total lymphocytes. These relatives were asymptomatic, and were primarily in families in which the mutated gene in the proband was unknown.
Association of telomere length with disease severity
Transformation into Z-scores was used to adjust for age, and allowed comparisons of several groups. As a combined group, telomere Z-scores in DC patients were well below the range of their relatives (P<0.001) (Figure 2A shows data for lymphocytes). Patients with the severe HH and RS phenotypes had much lower age-adjusted Z-scores than patients with classic DC or silent carriers (P=0.001 by rank sum). The HH and RS subtypes typically develop medical problems consistent with DC at very young ages, which could be a consequence of the exceedingly short telomeres in these patients. The relatives of patients in whom the mutated gene had not been identified (Rel 1) had lower telomere Z-scores than the unaffected relatives (Rel 2) in whose family the gene was known; this could reflect inclusion of currently unidentified silent carriers.
Figure 2.
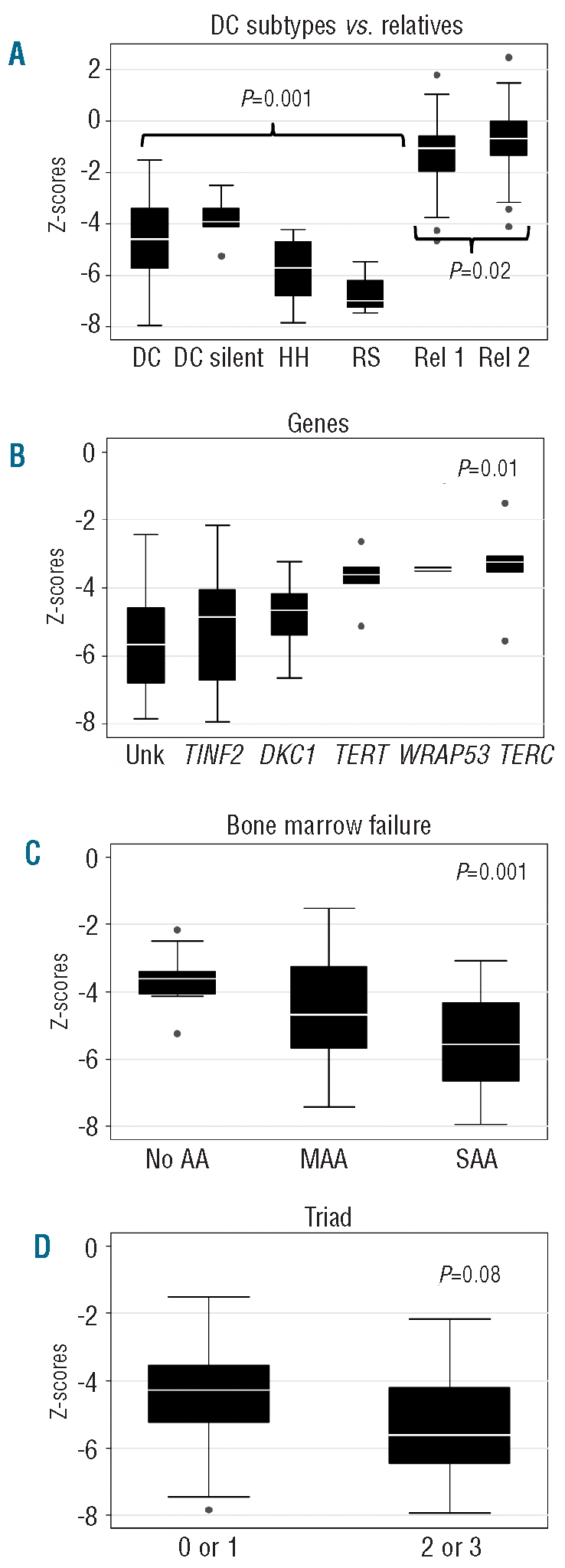
Telomere length Z-scores in DC patients according to various categories, and relatives. (A) Typical DC patients, silent carriers with mutations in DC genes, Hoyeraal-Hreidarsson (HH), and Revesz Syndrome (RS). Telomeres for those with HH and RS are much shorter than in other DC patients and silent carriers after age-adjustment by Z-scores; P=0.002 by rank sum. All DC patients have lower Z-scores when compared with relatives, P<0.0001 by Student’s t-test. Outliers among relatives with low Z-scores may include silent carriers of DC in families in which genes have not been identified. Telomere Z-scores in relatives in families without known genes are lower than in those in families with known mutations, P=0.02 by Student’s t-test. (B) Telomere Z-scores are lower in those with mutations in unknown genes, DKC1, and TINF2 than in those with mutations in TERC, TERT, and WRAP53; P=0.01 by rank sum. (C) The Z-scores are lower in those with increased severity of bone marrow failure (MAA: moderate aplastic anemia; SAA: severe aplastic anemia); P for trend=0.001. Z-scores in patients without aplastic anemia are similar to those with MAA, P=0.1; SAA are lower than MAA, P=0.05; and SAA are much lower than those with no AA, P<0.001. (D) Telomere Z-scores are not significantly different in those with two or three features of the clinical triad than in those with none or only one such feature; P=0.08.
There was a significant effect of genotype on lymphocyte telomere length Z-scores (Figure 2B). Patients with mutations in unknown genes, in TINF2 or in DKC1 had shorter telomeres than those with TERT, WRAP53, or TERC mutations (P=0.01). The Z-scores for the first three categories listed were around minus 5 SD, while they were around minus 4 for the second set of three genes. Lower Z-scores were also associated with more severe BMF (Figure 2C). The patients with normal hematopoiesis had Z-scores of around minus 3.6 SD, while those with MAA or SAA had respective Z-scores of around minus 4.6 and minus 5.4 (P for trend = 0.001). There was also a trend towards lower Z-scores with more features of the diagnostic triad. Specifically, the average Z-score was minus 4.6 for patients with none or only one feature of the triad, compared with minus 4.6 and minus 5.3 in those with two or three such features, respectively (P=0.08) (Figure 2D). Results for leukocyte subsets other than lymphocytes showed similar associations (data not shown).
Telomeres shorten with age in patients with DC
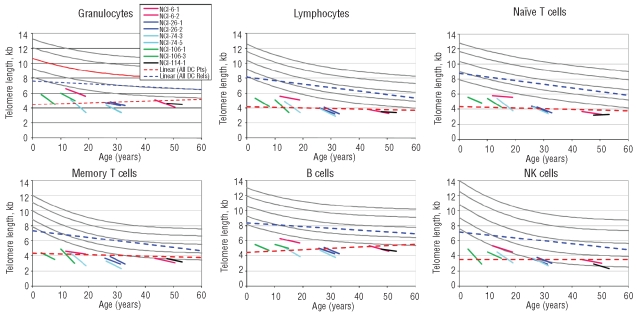
We evaluated longitudinal changes in telomere length in 9 patients with DC with measurements repeated once at a median of 5.5 years (range 4.9–7.0) after the first measurement. Data for all leukocyte subsets are shown in Figure 3; details are provided here for lymphocytes. The slopes for lymphocytes in the patients with DC averaged minus 0.17 kb ± 0.1 (range −0.02 to 0.34) per year. After exclusion of the 3 patients with TERC mutations (NCI 6-1, NCI 6-2, and NCI 114-1), the slopes averaged −0.22 kb ± 0.07. The normal slope (based on the cross-sectional decline in the normal controls shown in Figure 3) was −0.05–0.1 kb, i.e. an annual loss of 0.05–0.1 kb and less than the value in the DC patients. The mean decline in Z-score in the patients from the first to the second lymphocyte sample over the five years was significant, minus 0.5 SD (P=0.02 by Wilcoxon’s signed rank test, and 0.01 by paired t-test; data not shown). If the DC patients’ telomeres were tracking the normal age-associated decline, there should have been no change in the Z-score.
Figure 3.
Regression lines for telomere length according to age. The short lines indicate the change in telomere lengths in 9 DC patients over 5-year intervals. Red: NCI-6-1 and NCI-6-2 are mother and son with a mutation in TERC; Blue: NCI-26-1 and NCI-26-2 are identical twins with a mutation in TERT; Teal blue: NCI-74-3 and NCI-74-5 are brothers with a mutation in TINF2; Green: NCI-106-1 and NCI-106-3 are brothers with a mutation in DKC1; Black: NCI-114-1 with a mutation in TERC. The slope of the cross-sectional data for telomere length vs. age for the DC patients (_ _ _) is not significantly different from 0 in any lineage. In contrast, the slope for the cross-sectional results in DC relatives (_ _ _) is significantly negative (P<0.001) in all lineages.
The dashed lines represent the summary linear trends in the cross-sectional data from the patients with DC and their relatives (Figure 3). Consistent with previous observations by us and by Du et al.,8–9 the cross-sectional results from all DC patients were essentially flat (for lymphocytes, P=0.4 by F-test compared with zero slope; for all leukocyte subsets P=0.1–0.9). As expected, the cross-sectional telomere lengths from the DC relatives demonstrated a significant decline with age, similar to the cross-sectional control data (P<0.001 for all leukocyte subsets). The longitudinal measurements thus demonstrate a significant decline with age in individual DC patients, in contrast to the lack of decline observed in the cross-sectional analyses.
Discussion
DC is characterized by exceedingly short telomeres and mutations in telomere biology genes. In our first report, we found that very short telomeres in leukocyte subsets measured by automated multicolor flow FISH were diagnostic of DC. Our new data confirm that telomere length less than the 1st percentile for age in normal subjects in leukocyte subsets is highly sensitive and specific for DC, and suggest that telomere length in patients with DC is a fundamental measure of clinical severity. The precision of the automated method provides highly reproducible results with a very low standard deviation.
Our study suggests that flow FISH telomere length in granulocytes is not a useful diagnostic tool, despite the accuracy of the measurements, because of the low specificity and PPV. Rather, the most useful single lineage screening test for DC is total lymphocytes, with a PPV of 85%. We agree that relatives who do not carry mutations in DC genes have essentially normal lymphocyte telomere lengths.17 However, in cases in which the diagnosis is not clear, such as in a relative who may or may not be a silent carrier, the additional information gleaned from telomere length measurements in lymphocyte subsets (i.e. below the first percentile in 3 of the 4 subsets) may be critical. This is especially important in clinically normal relatives of a patient with an unknown gene mutation who are being considered as hematopoietic stem cell donors.7 Indeed in our cohort, one silent carrier has subsequently developed mild thrombocytopenia and a hypocellular marrow.
The size of our cohort, analysis by flow FISH, and age-adjustment with Z-scores allowed detailed comparisons of flow FISH telomere length to be made in DC subgroups. We found that extremely short telomeres were strongly associated with the more severe clinical phenotypes, HH and RS, even though the gene associated with these phenotypes has not yet been discovered in many of these patients (5 of our 15 subjects with HH were due to DKC1 mutations, and one of the 4 with RS had a mutation in TINF2). There was also a trend toward shorter telomeres in those patients with more features of the diagnostic triad. Ongoing longitudinal studies of the clinical complications in these patients are required to better understand these associations.
Patients with mutations in DKC1, TINF2, and unknown genes had shorter telomeres than those with mutations in TERC, TERT, and WRAP53. The former group was younger than the latter, but the use of Z-scores permitted us to conclude for the first time that the association of telomere length with genotype was independent of age. Walne et al. also found that telomeres were shortest in patients with mutations in TINF2, followed by DKC1, compared with controls, using Southern blots and delta TEL rather than flow FISH.11 These findings suggest that sequencing of TINF2 and DKC1 (the latter in males) should precede examination of the other known DC genes in patients with extremely short telomeres.
Patients with more severe hematologic disease, such as severe aplastic anemia (SAA) or moderate aplastic anemia (MAA), had significantly shorter telomeres than patients without AA. This may reflect the fact that in patients with the shortest telomeres in peripheral blood cells, telomeres in the hematopoietic stem cells are too short to support adequate differentiation. Alternatively, this could be related to the severity of bone marrow stress, with subsequent acceleration of the shortening of telomeres.
Our longitudinal study of 9 patients with DC shows that not only does telomere length decline with age, but that the decline may be accelerated when compared with the predicted normal rate (approximately 0.2 kb per year compared with 0.05).18 This is in contrast with the flat cross-sectional results, which may be due to a “survival bias” reflecting that older patients in the cohort had to survive complications of DC and hence tended to be those with a milder phenotype.8–9 Younger patients with a more severe phenotype may be identified as DC by their clinical presentation, while older patients whose course is milder may be recognized only after they develop age-associated complications.
Our cohort has several strengths, which include detailed characterization of the physical and laboratory features of children and adults, using identical criteria, as well as personal examination of the majority of the participants. Automated flow FISH of leukocyte subsets is precise, reproducible, sensitive and specific. The limitations of our study include the fact that family members may currently be misclassified as “unaffected relatives”, and may later be reclassified as “silent carriers” only when mutated DC genes are identified in their families. Many DC-associated clinical and laboratory findings are age-dependent. The NCI cohort may be subject to biased referrals or volunteerism of unknown magnitudes. Our dataset is not sufficiently large to determine whether some of the interesting associations we noted are independent or correlated, such as DC subtype, age, features of the triad, genes, and BMF. In addition, we do not have longitudinal measurements of telomere length from relatives, patients with acquired aplastic anemia, or normal controls.
Taken together, our new data suggest that, when telomere lengths in patients with DC are measured precisely and age is accounted for, the values provide a fundamental measure of disease severity. Furthermore, our results also stress the potential clinical importance of obtaining longitudinal data to determine the rate of telomere loss in individuals with DC. It is possible that patients with excessive telomere attrition rates (e.g. >0.2 kb/year) may be on a more rapid trajectory towards a serious complication of this disorder. Understanding the interplay of all the features discussed above requires longer follow up of a larger cohort. These findings will help identify patients who are most likely to have DC, and who thus require syndrome-specific management and medical and genetic counseling.
Acknowledgments
The authors would like to thank all the subjects for their enthusiastic participation in the IBMFS study. We also thank Mark H Greene MD for helpful discussions, Irma Vulto for excellent technical assistance and Lisa Leathwood RN and members of the IBMFS team at Westat for their extensive efforts.
Footnotes
Funding: this research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (BPA, NG, SAS, PSR), and by contracts N02-CP-91026, N02-CP-11019 and HHSN261200655001C with Westat, Incorporated. GMB was supported by the Swiss National Foundation and the Bernese Cancer League. Work in the Lansdorp laboratory was supported by grants from the Canadian Institutes of Health Research (RMF-92093), the NIH (R01GM094146), the Canadian Cancer Society and the Terry Fox Foundation (018006 and 105265).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Molecules and Diseases. 2001;27:353–7. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 2.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 3.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326(5955):948–52. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–79. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 5.Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23(2):215–31. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107(7):2680–5. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362(9396):1628–30. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, et al. Very short telomere length by flow FISH identifies patients with Dyskeratosis Congenita. Blood. 2007;110(5):1439–47. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113(2):309–16. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a Component of the Shelterin Telomere Protection Complex, is Mutated in Dyskeratosis Congenita. Am J Human Genetics. 2008;82:501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walne AJ, Vulliamy TJ, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: Analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vulliamy T, Beswick R, Kirwan M, Hossain U, Walne A, Dokal I. Telomere length measurement can distinguish pathogenic from non-pathogenic variants in the shelterin component, TIN2. Clin Genet. 2010 Dec 6; doi: 10.1111/j.1399-0004.2010.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24(3):101–22. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: A prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 16.Baerlocher GM, Vulto I, de jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1(5):2365–76. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 17.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]