Abstract
Background
The treatment of acute myeloid leukemia of older, medically non-fit patients still poses a highly unmet clinical need, and only few large, prospective studies have been performed in this setting. Given the established activity of hypomethylating agents such as 5-aza-2'-deoxycytidine (decitabine) in myelodysplastic syndromes and acute myeloid leukemia with 20–30% bone marrow blasts, we investigated whether this drug is also active in patients with more than 30% blasts.
Design and Methods
To evaluate the efficacy and toxicity of decitabine in patients over 60 years old with untreated acute myeloid leukemia ineligible for induction chemotherapy, 227 patients (median age, 72 years), many with comorbidities, adverse cytogenetics and/or preceding myelodysplastic syndrome were treated with this hypomethylating agent. During the initial decitabine treatment (135 mg/m2 total dose infused intravenously over 72 hours every 6 weeks), a median of two cycles was administered (range, 1–4). All-trans retinoic acid was administered to 100 patients during course 2. Fifty-two patients who completed four cycles of treatment subsequently received a median of five maintenance courses (range, 1–19) with a lower dose of decitabine (20 mg/m2) infused over 1 hour on 3 consecutive days every 4–6 weeks.
Results
The complete and partial remission rate was 26%, 95% CI (20%, 32%), and an antileukemic effect was noted in 26% of patients. Response rates did not differ between patients with or without adverse cytogenetics; patients with monosomal karyotypes also responded. The median overall survival from the start of decitabine treatment was 5.5 months (range, 0–57.5+) and the 1-year survival rate was 28%, 95%CI (22%,34%). Toxicities were predominantly hematologic.
Conclusions
Decitabine is well tolerated by older, medically non-fit patients with acute myeloid leukemia; myelosuppression is the major toxicity. The response rate and overall survival were not adversely influenced by poor-risk cytogenetics or myelodysplastic syndrome. Because of these encouraging results, randomized studies evaluating single-agent decitabine versus conventional treatment are warranted. The study is registered with the German Clinical Trials Registry, number DRKS00000069.
Keywords: DNA hypomethylation, medically non-fit patients, comorbidities, decitabine, DAC
Introduction
Among patients with acute myeloid leukemia (AML), older patients have a worse outcome than younger patients, because of a higher degree of resistance to conventional chemotherapy, a higher frequency of adverse cytogenetics, an often decreased performance status and comorbid conditions.1–4 Possibly half or more of newly diagnosed AML patients above the age of 70 will not be considered eligible for induction chemotherapy due to one or more of these factors, but selection criteria applied by the treating physicians are not yet well understood.5,6 The treatment of older AML patients is, therefore, a highly unmet clinical need.7 The DNA hypomethylating azanucleoside drug 5-aza-2'-deoxycytidine (DAC, DACogen®)8 has significant single-agent activity in myelodysplastic syndromes (MDS)9 and AML,10,11 even in patients with poor-risk karyotypes.12–14 Higher-risk MDS and AML of the elderly constitute a biological continuum. We, therefore, performed a phase II multicenter trial of low-dose DAC in older AML patients deemed ineligible for standard chemotherapy because of comorbidities, reduced performance status, adverse cytogenetics, and secondary or treatment-related AML. The schedule used (DAC infusions over 72 h, repeated every 6 weeks), was identical to that developed in Europe and approved by the Food and Drug Administration for MDS treatment.
The DNA hypomethylating agents 5-azacytidine and DAC are known to reactivate multiple genes in concert with gene demethylation. This has been demonstrated in both in vitro models, using AML cell lines,15,16 and in vivo, when primary blasts from MDS or AML patients treated with these drugs were subjected to DNA methylation and expression analyses.17–19 Therefore, like valproic acid and other histone deacetylase inhibitors, they may have the potential to (re)sensitize malignant cells to the antileukemic activity of low-dose chemotherapy20 or the differentiating activity of retinoids.21–23 All-trans retinoic acid (ATRA) is not highly effective in AML except in the presence of the PML-RARA fusion protein. However, in vitro studies of the combination of a DNA hypomethylating agent with ATRA suggest that AML cell lines lacking PML-RARA may become sensitized to the antileukemic activity of the retinoid.24,25 We hypothesized that in the subgroup of AML patients who do not have an objective response to a single course of DAC, but only an antileukemic effect or stable disease, the addition of ATRA during the second course of DAC treatment might improve their response and, therefore, planned combination treatment for these patients (see below).
DNA hypomethylating drugs, when given as single agents, need to be administered over a prolonged period in order to optimize the response; furthermore, since the relapse rate of MDS patients is very high once treatment is stopped, it may be a better strategy to continue therapy as long as the disease is controlled rather than administer a limited number of cycles, and then re-treat the patient at the time of relapse.26 Therefore, in the present trial patients had the option of continuing with prolonged, maintenance outpatient treatment with DAC, at a dose that was substantially lower than the dose administered in the first four courses.
Design and Methods
Eligibility and selection of patients
Patients aged 61 years or older (no upper age limit) with previously untreated de novo or secondary AML following MDS (by French-American-British classification, i.e. with >30% bone marrow blasts) were eligible, provided they were not scored as eligible for, or not consenting to, induction chemotherapy (see the Results section for reasons given for ineligibility for induction). Inclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance status 0–2, total bilirubin concentration less than twice the upper limit of normal and serum creatinine less than 1.5 mg/dL unless leukemia-related. Patients with white blood counts (WBC) greater than 20×109/L were eligible provided that after a short course of hydroxyurea, followed by a 2–3 day observation period, the WBC fell below 20×109/L (the indication for hydroxyurea cytoreduction was changed to a WBC >50×109/L in an amendment in January 2006, to make a larger cohort of patients eligible for the study). Exclusion criteria were acute promyelocytic leukemia, treatment with cytokines within the previous 4 weeks, another malignancy not in remission, NYHA grade IV heart failure, uncontrolled active infection, a psychiatric disorder interfering with treatment, or known allergy to imidazoles. Presence of translocation (8;21) or inversion 16 was not an exclusion criterion. The study was approved by the institutional review board of each treating center, and informed consent was given according to the Declaration of Helsinki. The trial was initiated in April 2003. Results were analyzed as of August 31, 2009.
Comorbidity scoring
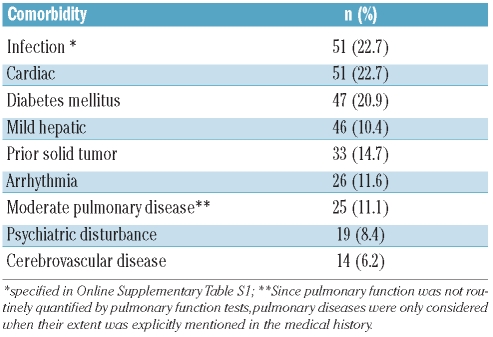
Comorbidities were scored retrospectively by screening the patients’ medical history records (Table 1B). The definitions for comorbidities were used as suggested by Sorror et al.27. For infections this implied “infections, requiring continuation of antimicrobial treatment” (specified in Online Supplementary Table S1).
Table 1B.
Quantification of comorbidities at baseline. Prevalence of the most frequent comorbidities (occuring in >5% of patients) according to HCT-CI27 in 225 evaluable patients at treatment initiation.
Treatment regimens
The initially Food and Drug Administration-approved DAC schedule developed in Europe for MDS treatment, i.e. 15 mg/m2, three times daily on 3 consecutive days (total dose 135 mg/m2, repeated every 6 weeks), was administered intravenously over 3 h. The study drug was kindly provided by SuperGen, Inc. (Dublin, CA, USA), and MGI Pharma (Minneapolis, MN, USA). In the case of an antileukemic effect or stable disease (both defined below) after course 1, administration of the second course of DAC was to be followed from day 4 by ATRA (45 mg/m2/day per os) for a total of 28 days. ATRA dosing was not to be repeated during all subsequent courses. This was based on the rationale that in patients not having achieved complete or partial remission, the (subsequent) response might be enhanced by the addition of ATRA, as has been described for the addition of valproic acid to DAC,28 and the addition of valproic acid and ATRA to 5-azacytidine.22 After successful application of a total of four courses, patients in complete or partial remission or who had had an antileukemic effect at evaluation of course 4 were eligible for maintenance treatment with DAC at 20 mg/m2 (given intravenously for 1 h on 3 consecutive days, repeated every 6–8 weeks). In case of progressive disease at any time during study, patients were taken off treatment. Following a protocol amendment in January 2006, DAC dosing over 3 days was to be repeated (only during the initial course of treatment, not at any point thereafter) after 5 days of rest, i.e. on days 8 – 10 of course 1, in patients with a WBC greater than 20×109/L, resulting in a total dose of 270 mg/m2 during course 1. In the case of excess toxicity and/or severe hypoplasia/cytopenia beyond 6 weeks after administration of DAC, the dose in subsequent courses was reduced to 50% of the previous dose level.
Response
Bone marrow aspiration was performed after cycles 1, 2 and 4. A complete remission was defined as a non-blastic marrow aspirate (blast cells <5%), platelet count greater than 100×109/L, WBC greater than 1.5×109/L, and no extramedullary leukemia. A partial remission was defined as a cellular marrow aspirate with 5% to 25% blasts, with a platelet count greater than 100×109/L, WBC greater than 1.5×109/L, and no clinical or imaging evidence of leukemia; or a cellular marrow aspirate with less than 5% blasts, platelets less than 100×109/L and WBC less than 1.5×109/L. An antileukemic effect was defined as a greater than 25% reduction of bone marrow blasts relative to the initial blast percentage but not enough to fulfill the criteria for a partial remission. Progressive disease was defined as a greater than 25% relative increase in blasts in the peripheral blood or bone marrow compared to before start of treatment. Stable disease was defined by the absence of a complete or partial response, or antileukemic effect, and no progressive disease. Death from any cause occurring within 6 weeks after initiation of DAC treatment was defined as early death.
Central hematopathology and cytogenetic review, molecular diagnostics
A central morphological review of peripheral blood and bone marrow smears before and during treatment was performed by Dr. Pierre W. Wijermans, and implemented in a central response review of all responses as reported by the local cytologists. Karyotypes were centrally reviewed by Prof. Anne Hagemeijer according to ISCN criteria and to the presence or absence of complex karyotype29 and monosomal karyotype.30 If less than ten metaphases with a normal karyotype were analyzed, then the cytogenetic analysis was classified as a failure. Patients' bone marrow DNA was genotyped for mutational status of FLT3 and NPM1, as described elsewhere.31
Statistics
The primary endpoint of the study was best response, defined as complete remission, partial remission or antileukemic effect. Secondary endpoints were an objective response (complete remission, partial remission), overall survival, defined as time from start of treatment to death, and safety, assessed as toxicity of laboratory parameters over courses 1 to 4, and adverse events over the entire study duration. Treatment with DAC was to be considered to be effective if the best response rate was 20% or higher and to be ineffective if the best response rate was 5% or lower, which is expected in patients receiving only best supportive care.5 The error probability of regarding DAC as effective when it is ineffective was set at 15%. The error probability of regarding DAC as ineffective when it is effective was set at 10%. The required sample size was calculated according to the optimal two-stage design by Simon.32 In the first stage, 12 patients were to be treated in the study; if at least one patient had a response, the recruitment was to be continued until 29 patients had been included, otherwise recruitment was to be stopped. If three or more of these 29 patients had a response, the treatment was to be considered effective and would be studied further. Since this criterion was achieved after treating 29 patients, the study was extended to a multicenter trial in the meantime and was then opened to recruit patients for an additional 2.5 years, to increase the precision in the assessment of response rate, overall survival rate, safety, and to be able to investigate the impact of prognostic factors.
All AML patients receiving at least one dose of the study drug were included in the analysis. The rate of patients with best response (complete remission, partial remission, or antileukemic effect) and the rate of patients with an objective response (complete remission or partial remission) was calculated with 95% confidence intervals (95%CI). The overall survival rate was estimated by the Kaplan-Meier method (patients alive at last follow-up were censored). Logistic regression was used for analyses of best response and objective response, and the odds ratios with 95%CI were estimated. For analyses with respect to overall survival, Cox regression was used, and the hazard ratios with 95%CI were estimated. Two-sided P-values were calculated from Wald tests. Patients receiving ATRA during course 2 were compared with patients not receiving ATRA during course 2 with respect to the overall survival time, defined as time from start of course 2 until death. Further details are given in the Online Supplementary Appendix.
Results
Patients’ characteristics and treatment feasibility
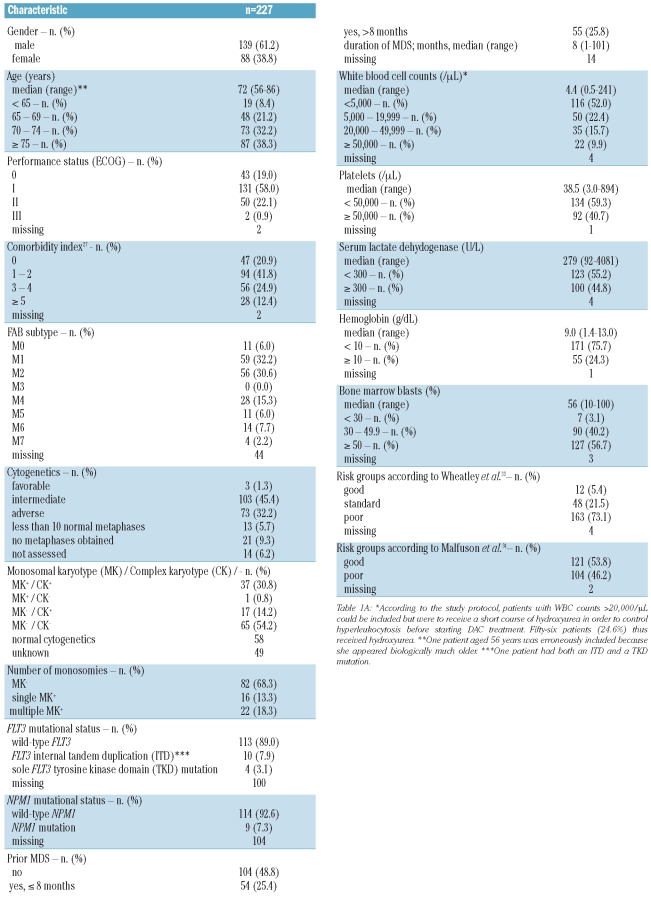
Between April 2, 2003 and October 1, 2007, 227 evaluable patients were recruited and treated at nine study centers. Eight additional patients enrolled on the study had to be excluded from the analysis because they were not evaluable: in seven patients treatment with DAC was not started; in one additional patient the diagnosis was revised to non-Hodgkin's lymphoma during application of the first course of DAC, and the patient proceeded to CHOP treatment. Patients had a median age of 72 years (the oldest patient was 86 years old), and many presented with poor-risk features (Table 1, Online Supplementary Table S1): frequent comorbidities [median 2, 25% with 3–4 and 12% with >4 comorbidity points according to the Hematopoietic Cell Transplant Comorbidity Index (HCT-CI)], secondary AML evolving from MDS (51%), adverse cytogenetics (32%), ECOG performance status of 1 or more (81%), WBC greater than 20×109/L (26%), and a very low rate of NPM1 mutations (7.3%). Notably, the rate of flt-3 mutations was less than 10%. The main reasons for ineligibility for standard induction chemotherapy reported by the treating physicians were poor performance status, comorbidities and refusal by the patient (Online Supplementary Table S2). Overall, 227 patients received a total of 577 courses of DAC at the dose level of 135 mg/m2 per course (median 2, range 1 – 4). As regards the feasibility of the 6-week treatment interval, when determining the time interval between the first day of DAC and the first day of the next course for all 350 fully applied treatment courses 1, 2 and 3, 195 (56%) were initiated within day 43 of the preceding course, whereas the remaining 155 courses had to be delayed for different reasons. Thus 91 courses (26%) were initiated between day 44 and day 53, 38 (11%) between day 54 and day 63, and 26 (7%) between day 64 and day 100.
Table 1A.
Baseline clinical characteristics of the patients and their diseases.
Responses
Objective responses were attained in 59 patients [26%, 95%CI (20%, 32%)]: complete remission, n=30, partial remission, n=29. Another 60 patients (26.4%) attained an antileukemic effect as best response, leading to a best response rate of 52%, 95%CI (46%, 59%). Fifty-seven patients (25.1%) had stable disease and 19 (8.4%) had progressive disease. The 6-week death rate was 12.8% (29 patients), three patients (1.3%) were not evaluable for response because they were lost to follow-up. The median number of courses until best response was two for complete or partial remission and one for attainment of an antileukemic effect.
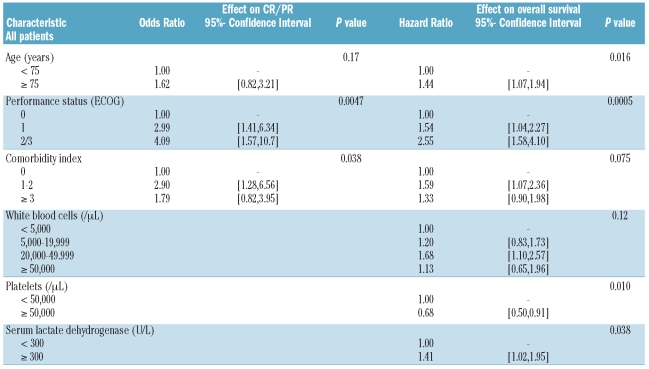
Although the primary endpoint of our study was best response, we present results primarily with regard to objective response, because of the broader acceptance of this endpoint. In univariate analyses, age, performance status, and the number of comorbidities (coded by HCT-CI) were significantly associated with an objective response, whereas preceding MDS and cytogenetics were not (Online Supplementary Table S3). Objective response rates were 29% and 27% for patients with adverse and non-adverse cytogenetics,4 respectively. In multivariate analysis, only performance status and comorbidities retained their prognostic effect on objective response (Table 2): patients with one or more comorbidity points received a median of two treatment courses, compared to four administered to patients without comorbidities. The Wheatley score showed a good separation of the patients with respect to objective response, whereas the Malfuson score had no prognostic effect (Online Supplementary Table S3). Similar overall results were obtained in uni- and multivariate analyses of the effects of patients’ characteristics on the rate of best response (not shown). Online Supplementary Table S4 presents the clinical features and outcome of the 30 patients attaining a complete remission.
Table 2.
Effect of patient and disease characteristics on best response to treatment (complete and partial remissions), and on overall survival, multivariate analyses (prognostic factors with P<0.1 in univariate analysis were included).
Survival
The median survival time of all 227 patients was 5.5 months, with a 1-year overall survival rate of 28%, 95%CI (22%, 34%) and a 2-year overall survival rate of 13%, 95%CI (8%, 17%) (Online Supplementary Figure S1). In univariate analyses (Online Supplementary Table S5), patients aged 75 years or older had a shorter overall survival, with a 1-year overall survival rate of 17%, hazard ratio (HR) 1.52, 95%CI (1.15, 2.02), than those under 75 years who had a 1-year overall survival rate of 34% (P=0.0038; Online Supplementary Figure S2). Overall survival decreased significantly with reduced performance status (P<0.0001; Online Supplementary Figure S3). Interestingly, while patients without comorbidity (as assessed by HCT-CI) had a higher 1-year overall survival rate (45%) than patients with comorbidities, the outcomes of those with a comorbidity index of 1–2 [1-year overall survival rate of 24%, HR 1.57, 95%CI (1.07, 2.30)] and those with a comorbidity index of ≥3 [1-year overall survival rate of 23%, HR 1.52, 95% CI (1.04, 2.24)] were similar (Online Supplementary Figure S4). The 1-year overall survival rate of patients with adverse cytogenetics was 21%, while that of patients with non-adverse cytogenetics was 30% [HR 1.33, 95% CI (0.98,1.82), Online Supplementary Figure S5]. The following parameters were associated with a worse outcome: increased WBC (P=0.0041, Online Supplementary Figure S6), platelet count less than 50×109/L (P=0.068, Online Supplementary Figure S7) and serum lactate dehydrogenase of 300 U/L or higher (P=0.008, Online Supplementary Figure S8). The Wheatley score (Online Supplementary Figure S9) separated the three risk groups more effectively than did the Malfuson score (Online Supplementary Figure S10). In multivariate analysis (Table 2), patient’s age, performance status, platelet count, and lactate hydrogenase level were independent predictors for overall survival (P<0.05).
Duration of myelodysplastic syndrome prior to decitabine treatment
One hundred and nine patients (51%) had prior MDS of known duration, with a median duration of 8 months (25% quartile 3, 75% quartile 25; range, 1–101). A comparison of the objective response rates according to MDS duration revealed a trend to an increase in objective response rates with longer duration of MDS [<3 months: 4/25 (16%), 3–8 months: 5/29 (17%), 8–25 months: 7/27 months (26%), ≥25 months: 10/28 (36%) (test for trend P=0.066, as continuous covariate P=0.087)]. The results were similar when this analysis was adjusted for other prognostic factors showing an effect on objective response in this population of secondary AML patients (comorbidity index, lactate dehydrogenase) (test for trend P=0.085, as continuous covariate P=0.10).
There was also a trend to a better overall survival in patients with previous MDS of longer duration (<3 months: 1-year overall survival rate 23%, 3–8 months: 28%, 8–25 months: 26%, ≥25 months: 46%, test for trend P=0.16, Online Supplementary Figure S11, as continuous covariate P=0.17). When this analysis was adjusted for other prognostic factors showing an effect on overall survival in this population of secondary AML patients (performance status, comorbidity index, and WBC), the results were similar (test for trend P=0.11, as continuous covariate P=0.036).
Outcome in patients with monosomal and/or complex karyotype
Of 178 patients with successful assessment of monosomal and/or complex karyotype, 58 showed a normal karyotype, 120 had clonal abnormalities. Fifty-four of these had a complex karyotype (i.e. 3 or more aberrations) and 66 had non-complex changes. Among the cytogenetically abnormal cases, 38 fulfilled the criteria for monosomal karyotype (MK+) described by Breems et al.30 All MK+ cases but one belonged to the complex karyotype group, with monosomies 7, 17 and 5 being most frequent. Twenty-two MK+ patients had two or more autosomal monosomies, 16 had one autosomal monosomy and at least one structural aberration (Table 1A).
In AML patients receiving standard chemotherapy, patients with MK+ had the worst outcome, particularly when more than one monosomy was present.30,35 In the present trial, MK+ patients did not do worse than MK–cases with abnormal cytogenetics. Indeed, the MK+ patients had a higher rate of objective responses (37% complete/partial remissions) than the MK– patients with abnormal cytogenetics, whether with a complex karyotype (12%) or without (20%) (Online Supplementary Table S3). Of note, the objective response rate was higher in patients with multiple monosomies compared to those with a single monosomy (45% versus 25%).
The overall survival of MK+ patients was comparable to that of both groups of MK– patients (Online Supplementary Figure S13). Considering the overall survival of MK+ patients divided according to whether they had one versus two or more monosomies, the latter group appeared to derive more benefit (1-year overall survival rate 13% versus 27%, respectively; Online Supplementary Figure S14), however the numbers of patients are quite limited.
Feasibility of the combined treatment with decitabine and all-trans retinoic acid
After administration of the first DAC course to 227 patients, 59 (26%) did not receive the second treatment course: 29 patients had early death, 15 had progressive disease, 12 did not proceed to further treatment within the study despite having attained at least stable disease (stable disease, 7; antileukemic effect, 4; partial remission, 1). Three additional patients were lost to follow-up.
Of the 168 patients who received a second treatment course during the study, 68 received DAC alone. Of these, 34% had a complete remission (n=6) or partial remission (n=17), 19 patients (28%) had an antileukemic effect, 24 had stable disease (35%), and two had progressive disease (3%) after the first course. The other 100 patients received DAC followed by ATRA, based on the rationale described in the Design and Methods section. Thirty-eight patients had an antileukemic effect, 54 had stable disease and two had progressive disease. Six additional patients in partial remission (according to the central response review) also received ATRA because response evaluation by the local cytologist was an antileukemic effect (n=4) or stable disease (n=2). Survival from start of course 2 was similar in both groups, also after adjusting for age, performance status and platelet count [HR 1.07, 95%CI (0.76,1.51), P=0.49].
To further dissect the effect of the ATRA add-on, we decided to compare survival from the start of course 2 only in patients with an antileukemic effect or stable disease who either did (n=92, 38 antileukemic effect, 54 stable disease) or did not receive ATRA (n=43, 19 antileukemic effect, 24 stable disease). The survival of patients receiving DAC+ATRA [HR 0.84, 95%CI (0.56,1.26), P=0.39] was comparable to that of patients receiving DAC alone (Online Supplementary Figure S12).
Outpatient maintenance treatment of patients with acute myeloid leukemia using a 3-day schedule of low-dose decitabine
Eighty-one of 227 patients completed all four courses of DAC treatment. Of these, 43 patients had obtained a complete remission (n=20), partial remission (n=10) or antileukemic effect (n=13), and were, therefore, eligible for continuation with DAC (20 mg/m2) administered intravenously over 1 h on 3 consecutive days, and continued with this maintenance treatment. Two additional patients with stable disease, five patients whose remission status was not fully evaluable, and two who had lost their initial response but were judged to benefit from continuation of DAC also received this treatment. The other 29 patients did not receive continued treatment, 17 per protocol (after course 4 evaluated as having progressive disease, n=16, or stable disease, n=1) and 12 – despite the patients having been evaluated as having had an antileukemic effect (n=8), partial remission (n=2) or complete remission (n=2) after course 4 – because of death (n=5), intercurrent medical conditions or loss to follow-up. Overall, 306 courses of DAC were given (median 5; range, 1–19). Further improvement of the response status of the patients on maintenance treatment was not systematically scored, and relapse after complete remission was not systematically recorded.
Safety
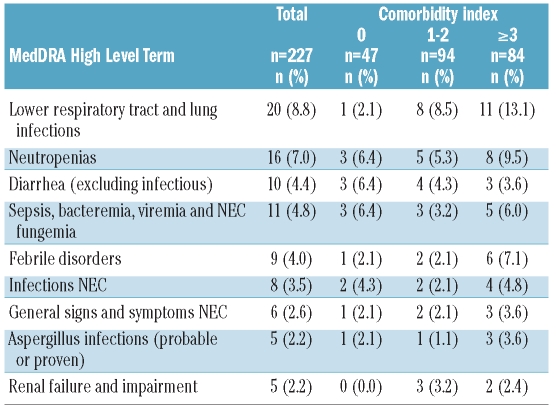
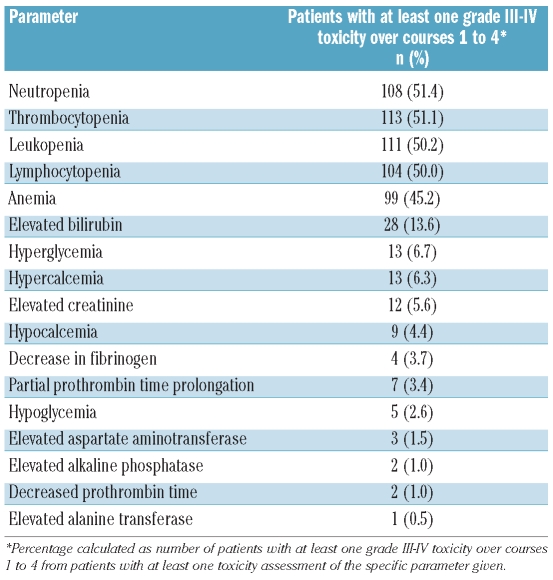
Seventy-six patients (33.5%) experienced at least one grade III–IV adverse event (coded by MedDRA high-level terms), listed in Table 3A (incidence ≥2%). Most adverse events were related to aggravation of pre-existing cytopenias and infectious complications, whereas gastrointestinal (diarrhea) or renal events were much less frequent. Hematologic toxicities, captured by laboratory parameters, were the most frequent side effects (Table 3B; during maintenance treatment, no systematic laboratory toxicities were captured between the DAC administrations). Only one patient (1.9%) discontinued maintenance treatment because of hematologic toxicity. Interestingly, when looking at the comorbidities in patients experiencing adverse events, lower respiratory tract and lung infections occurred much more frequently in patients with comorbidities, but this was not seen to the same degree for other events (Table 3A). This may indicate that the presence of comorbidities does not prohibit this treatment.
Table 3A.
Toxicity: grade III–IV adverse events (MedDRA high level term) occurring with an incidence of at least 2%.
Table 3B.
Maximum toxicity of laboratory parameters over courses 1 to 4.
Discussion
Older patients with AML able to tolerate standard chemotherapy have a median survival of 6–8 months,1,36 with a 5-year survival rate of 5–15%, while medically non-fit older AML patients receiving low-dose cytarabine or hydroxyurea have a median survival of approximately 4 and 3 months, respectively,7 with 2-year survival rates of about 8% and 0%, respectively. Novel approaches, such as clofarabine, lenalidomide, hypomethylating agents and low-dose cytarabine combined with valproic acid are, therefore, being actively studied as alternatives.37–40 We conducted a phase II trial of low-dose DAC in this population. Despite accumulated poor-risk patient features, the objective response rate was 26%, and an additional 26% of patients achieved an antileukemic effect. The toxicity profile of DAC was similar to that seen in MDS, i.e. primarily hematologic. Interestingly, similar response rates were obtained in patients with adverse and non-adverse cytogenetics, and in patients with AML preceded by MDS and de novo AML. Indeed, patients with MDS lasting 8 or more months had a somewhat better outcome than those with MDS of less than 8 months’ duration or de novo AML. Whether this is due to slower kinetics of the disease and/or features germane to secondary AML such as the hypermethylator phenotype (not found to such a degree in de novo AML) is unclear. Notably, improved survival following DAC treatment has previously been described in MDS patients with longer disease duration,13 and could be confirmed in the phase III trial comparing DAC treatment to best supportive care in higher risk MDS.41
The clinical needs of older AML patients with complex karyotype are still largely unmet because, even with standard chemotherapy, the risk of relapse is exceedingly high.42–44 We had previously noted an encouraging response rate and relative improvement of survival in MDS patients with complex karyotype treated with decitabine.12 Extending our observation to patients with or without monosomy of chromosome 7, we then found a tendency to better outcome when this monosomy was present, either as a single chromosomal abnormality or when part of a complex karyotype.45 Since then the striking, negative predictive impact of a monosomal karyotype (irrespectively of the chromosome involved) on the outcome of AML patients treated with standard chemotherapy has been uncovered30 and confirmed.35 In an extension of our previous observation of an encouraging outcome in patients with monosomy 7 (alone or in combination with other aberrations) treated with decitabine, we now looked at the outcome of patients with the MK+ genotype (which is defined irrespectively of the monosomal autosome). Indeed, MK+ patients did not have a worse outcome than the MK– patients, and patients with multiple monosomies tended to have a higher remission rate and longer overall survival than those with a single monosomy. This preliminary observation needs confirmation, but might suggest a positive effect of decitabine, possibly by reactivating haplo-insufficient tumor suppressor genes mapping on monosomal autosomes.
Prolonged treatment with DAC is superior to administration of a fixed number of treatment courses and subsequent termination of treatment, since the relapse rate is exceedingly high.26 We, therefore, implemented the option of low-dose DAC maintenance treatment in the study. The schedule chosen was based on the efficacy of the 3-day DAC dosing, and the positive experience with the 20 mg/m2 daily dose.46 Two-thirds of the patients completing the four cycles of DAC received this type of maintenance which was easily applicable, and associated with negligible non-hematologic toxicity. This schedule may also be beneficial to patients in need of maintenance after other types of AML treatment, and was implemented in the AML trial E2906 of the ECOG (NCT01041703).
DAC has shown activity in AML not only at the dose and schedule applied in the present trial, but also at an alternative schedule of 1-hour infusions given on 5 consecutive days, repeated every 28 days.11 This phase II study (from which patients with a WBC >50×109/L were excluded), performed at three centers, also resulted in an encouraging response rate, and a median survival of 7.7 months. In a phase I study of DAC (given over 10 days) alone or in combination with valproic acid in AML patients (median age, 70 years), the rate of complete remissions + complete remissions with incomplete blood count recovery was 38%, even though more than half of the patients had relapsed disease.10 A randomized phase III trial (NCT00260832) comparing low-dose DAC with standard care of older AML patients has completed recruitment, and showed improvement in overall survival with DAC treatment from 5 to 7.7 months (Thomas et al. ASCO 2011).
While ATRA is a very potent differentiation inducer in acute promyelocytic leukemia, the conundrum of its apparent inactivity in other AML subtypes has driven efforts to delineate other subgroups of AML which may be responsive to this compound (in one study shown for patients with NPM1 mutations and wild-type FLT347). Based on the preclinical results that treatment of AML blasts with DNA hypomethylating agents can render cells sensitive to ATRA, the present study allowed that patients with a modest response to one course of DAC were eligible for subsequent ATRA during the second course. The toxicity profile of this combination treatment was not different from that of DAC alone (data not shown), and differentiation syndrome impeding this approach was not observed. A survival analysis of patients who did or did not receive ATRA during course 2 did not reveal a superior outcome for patients who received ATRA. To investigate this issue more rigorously and with ATRA given during all treatment courses, we have embarked on a randomized prospective phase II AML trial (DECIDER/AMLSG14-09, NCT00867672) addressing the potential benefit of ATRA and/or valproic acid as add-on drugs to DAC (20 mg/m2 given on 5 consecutive days, repeated every 28 days).
In conclusion, in this study we have demonstrated that DAC administered intravenously over 72 h, followed by subsequent prolonged outpatient maintenance, is well tolerated even by older and medically non-fit AML patients, with myelosuppression being the only major toxicity. Interestingly, response rate and overall survival were not influenced by adverse cytogenetics and/or preceding MDS.
Acknowledgements
We would like to express our gratitude to the dedicated clinical study teams of all participating Centers, with special thanks to Christine Kalkhof, Cordula Heers, Petra Kinzel, Jessica Baumer, Ljudmila Bogatyreva and Dr. Roland Weis in Freiburg. We thank Guillermo Garcia-Manero for sharing unpublished data, and Drs. Hartmut Henß, Roland Mertelsmann and Michael Cullen for continued helpful discussions. This work was supported in part by the German José Carreras Leukemia Foundation (grant F06/04 to BD), and the European LeukemiaNet (WP5, WP8).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61–9. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 2.Estey EH. Adaptive randomization in a treatment study of patients with adverse karyotype acute myeloid leukemia. Curr Oncol Rep. 2003;5(5):390. doi: 10.1007/s11912-003-0024-8. [DOI] [PubMed] [Google Scholar]
- 3.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Deschler B, de Witte T, Mertelsmann R, Lübbert M. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: problems and approaches. Haematologica. 2006;91(11):1513–22. [PubMed] [Google Scholar]
- 6.Schiffer CA. "I am older, not elderly," said the patient with acute myeloid leukemia. J Clin Oncol. 2010;28(4):521–3. doi: 10.1200/JCO.2009.25.8616. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–24. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 8.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Issa JP, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112(11):2341–51. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25(25):3884–91. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 11.Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 12.Lübbert M, Wijermans P, Kunzmann R, Verhoef G, Bosly A, Ravoet C, et al. Cytogenetic responses in high-risk myelodysplastic syndrome following low-dose treatment with the DNA methylation inhibitor 5-aza-2'-deoxycytidine. Br J Haematol. 2001;114(2):349–57. doi: 10.1046/j.1365-2141.2001.02933.x. [DOI] [PubMed] [Google Scholar]
- 13.Wijermans PW, Lübbert M, Verhoef G, Klimek V, Bosly A. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2'-deoxycytidine (decitabine) in 177 patients. Ann Hematol. 2005;84(Suppl 1):9–17. doi: 10.1007/s00277-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 14.Wijermans P, Lübbert M, Verhoef G, Bosly A, Ravoet C, Andre M, et al. Low-dose 5-aza-2'-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18(5):956–62. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 15.Flotho C, Claus R, Batz C, Schneider M, Sandrock I, Ihde S, et al. The DNA methyl-transferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia. 2009;23(6):1019–28. doi: 10.1038/leu.2008.397. [DOI] [PubMed] [Google Scholar]
- 16.Schmelz K, Sattler N, Wagner M, Lübbert M, Dörken B, Tamm I. Induction of gene expression by 5-aza-2'-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanisms. Leukemia. 2005;19(1):103–11. doi: 10.1038/sj.leu.2403552. [DOI] [PubMed] [Google Scholar]
- 17.Daskalakis M, Nguyen TT, Nguyen C, Guldberg P, Köhler G, Wijermans P, et al. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-aza-2'-deoxycytidine (decitabine) treatment. Blood. 2002;100(8):2957–64. doi: 10.1182/blood.V100.8.2957. [DOI] [PubMed] [Google Scholar]
- 18.Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH, et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114(13):2764–73. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stresemann C, Bokelmann I, Mahlknecht U, Lyko F. Azacytidine causes complex DNA methylation responses in myeloid leukemia. Mol Cancer Ther. 2008;7(9):2998–3005. doi: 10.1158/1535-7163.MCT-08-0411. [DOI] [PubMed] [Google Scholar]
- 20.Qin T, Youssef EM, Jelinek J, Chen R, Yang AS, Garcia-Manero G, et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clin Cancer Res. 2007;13(14):4225–32. doi: 10.1158/1078-0432.CCR-06-2762. [DOI] [PubMed] [Google Scholar]
- 21.Cimino G, Lo-Coco F, Fenu S, Travaglini L, Finolezzi E, Mancini M, et al. Sequential valproic acid/all-trans retinoic acid treatment reprograms differentiation in refractory and high-risk acute myeloid leukemia. Cancer Res. 2006;66(17):8903–11. doi: 10.1158/0008-5472.CAN-05-2726. [DOI] [PubMed] [Google Scholar]
- 22.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–8. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 23.Trus MR, Yang L, Suarez Saiz F, Bordeleau L, Jurisica I, Minden MD. The histone deacetylase inhibitor valproic acid alters sensitivity towards all trans retinoic acid in acute myeloblastic leukemia cells. Leukemia. 2005;19(7):1161–8. doi: 10.1038/sj.leu.2403773. [DOI] [PubMed] [Google Scholar]
- 24.Momparler RL, Dore BT, Momparler LF. Effect of 5-aza-2'-deoxycytidine and retinoic acid on differentiation and c-myc expression in HL-60 myeloid leukemic cells. Cancer Lett. 1990;54(1–2):21–8. doi: 10.1016/0304-3835(90)90086-d. [DOI] [PubMed] [Google Scholar]
- 25.Niitsu N, Hayashi Y, Sugita K, Honma Y. Sensitization by 5-aza-2'-deoxycytidine of leukaemia cells with MLL abnormalities to induction of differentiation by all-trans retinoic acid and 1alpha,25-dihydroxyvitamin D3. Br J Haematol. 2001;112(2):315–26. doi: 10.1046/j.1365-2141.2001.02523.x. [DOI] [PubMed] [Google Scholar]
- 26.Rüter B, Wijermans PW, Lübbert M. Superiority of prolonged low-dose azanucleoside administration? Results of 5-aza-2'-deoxycytidine retreatment in high-risk myelodysplasia patients. Cancer. 2006;106(8):1744–50. doi: 10.1002/cncr.21796. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–9. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chun K, Hagemeijer A, Iqbal A, Slovak ML. Implementation of standardized international karyotype scoring practices is needed to provide uniform and systematic evaluation for patients with myelodysplastic syndrome using IPSS criteria: an International Working Group on MDS Cytogenetics Study. Leuk Res. 2010;34(2):160–5. doi: 10.1016/j.leukres.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–7. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 31.Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 32.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 33.Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 34.Malfuson JV, Etienne A, Turlure P, de Revel T, Thomas X, Contentin N, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93(12):1806–13. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 35.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116(13):2224–8. doi: 10.1182/blood-2010-02-270330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziogas DC, Voulgarelis M, Zintzaras E. A network meta-analysis of randomized controlled trials of induction treatments in acute myeloid leukemia in the elderly. Clin Ther. 2011;33(3):254–79. doi: 10.1016/j.clinthera.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Burnett AK, Russell NH, Kell J, Dennis M, Milligan D, Paolini S, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28(14):2389–95. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 38.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 39.Sekeres MA, Gundacker H, Lancet J, Advani A, Petersdorf S, Liesveld J, et al. A phase 2 study of lenalidomide monotherapy in patients with deletion 5q acute myeloid leukemia: Southwest Oncology Group Study S0605. Blood. 2011;118(3):523–8. doi: 10.1182/blood-2011-02-337303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corsetti MT, Salvi F, Perticone S, Baraldi A, De Paoli L, Gatto S, et al. Hematologic improvement and response in elderly AML/RAEB patients treated with valproic acid and low-dose Ara-C. Leuk Res. 2011;35(8):991–7. doi: 10.1016/j.leukres.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Lübbert M, Suciu S, Baila L, Rüter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–96. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 42.Fröhling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Döhner K, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108(10):3280–8. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 43.Knipp S, Hildebrand B, Kündgen A, Giagounidis A, Kobbe G, Haas R, et al. Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer. 2007;110(2):345–52. doi: 10.1002/cncr.22779. [DOI] [PubMed] [Google Scholar]
- 44.Bello C, Yu D, Komrokji RS, Zhu W, Wetzstein GA, List AF, et al. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. 2011;117(7):1463–9. doi: 10.1002/cncr.25598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rüter B, Wijermans P, Claus R, Kunzmann R, Lübbert M. Preferential cytogenetic response to continuous intravenous low-dose decitabine (DAC) administration in myelodysplastic syndrome with monosomy 7. Blood. 2007;110(3):1080–2. doi: 10.1182/blood-2007-03-080630. [DOI] [PubMed] [Google Scholar]
- 46.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 47.Schlenk RF, Döhner K, Kneba M, Götze K, Hartmann F, Del Valle F, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica. 2009;94(1):54–60. doi: 10.3324/haematol.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]