Abstract
Older chronic lymphocytic leukemia patients have poor outcomes with standard treatments and are underrepresented in clinical trials. We retrospectively reviewed outcomes of refractory chronic lymphocytic leukemia patients in two age categories (≥70 and <70 years) treated with single-agent flavopiridol, a drug active in genomically high-risk patients, during two trials. No significant difference between older and younger patients was observed in response rates (43 vs. 47%) or progression-free survival (median 8.7 vs. 9.9 months, P>0.80). Although overall survival was worse in older patients (median 2.1 vs. 2.4 years, P=0.02); when adjusted for other factors this difference was no longer significant (P≥0.10). With the exception of infections (older 29% vs. younger 62%) no significant association with toxicity was observed. These data demonstrate that flavopiridol administration to older chronic lymphocytic leukemia patients is feasible, tolerable, and may have similar efficacy to that in younger patients. Development of treatment approaches including flavopiridol should be considered for these older patients.
Keywords: chronic lymphocytic leukemia, elderly, flavopiridol, refractory, relapsed
Introduction
Although the most rapidly growing portion of the United States population is the elderly, they are consistently underrepresented in clinical cancer trials.1 The incidence of chronic lymphocytic leukemia (CLL) is markedly increased in older people, with a median age at diagnosis of 72 years. In fact, 75% of CLL patients in the United States are over the age of 65 years and 50% are over the age of 75 years.2 In contrast, key trials to evaluate the first-line treatment for CLL enrolled patients with a median age between 58 and 66 years.3 Studies focused on older CLL patients have been limited. For example, whereas first-line fludarabine was shown to be a superior therapy to chlorambucil,4 its benefit to patients aged 65 and older was not observed in a recently reported randomized phase III study.5 The benefit of first-line chemoimmunotherapy with the addition of rituximab has improved outcome of CLL patients but this benefit was less appreciated in patients over the age of 70 (n=30 of 224).6 A phase III clinical trial demonstrated that among 245 patients aged older than 65 (n=81, ≥70), response rate was improved and time to progression was extended in those that received first-line chemoimmunotherapy with rituximab versus those who received front-line chemotherapy, although no significant improvement in overall survival was observed. These older patients had higher incidences of at least one grade 3 or 4 toxicity, notably hematologic toxicity and bacterial infections, when compared with the patients under the age of 65.7 In the therapy of relapsed CLL, even less is known about the impact of age. However, one study in relapsed CLL with fludarabine, cyclophosphamide, and rituximab (n=177, median age=59 years, range=36–81 years) demonstrated a significant association between younger age and complete response rates, indicating less benefit of this treatment regimen in elderly patients.8 Collectively, these studies emphasize the importance of considering age and independent assessment of the feasibility of administration of new therapeutic agents in older patients.
Flavopiridol, is a pan cyclin-dependent kinase inhibitor9 which has demonstrated noteworthy clinical activity in relapsed and refractory CLL patients including those with del(17p13.1).10–11 The common side effects observed with flavopiridol, including hyperacute tumor lysis syndrome (TLS), cytokine release syndrome (CRS), neutropenia, diarrhea and fatigue, are often seen but are manageable. Given the activity of single-agent flavopiridol in relapsed CLL, we sought to determine the feasibility and impact of treating patients aged 70 or older in this setting by retrospectively reviewing outcome of such patients enrolled on 2 clinical trials at our institution.
Design and Methods
Patients and treatment
Patients included in this study were enrolled on two National Cancer Institute (NCI)-sponsored and Ohio State University (OSU) institutional review board (IRB)-approved trials. All patients provided written informed consent. Patient enrollment criteria included: age older than 17 years; diagnosis of CLL, prolymphocytic leukemia arising from CLL, or small lymphocytic lymphoma; Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less; and serum creatinine and total bilirubin levels lower than 2 times the normal value. The combined total of 116 patients was then divided by age into 2 categories (≥70 years and <70 years) for comparison. Traditionally, most studies define elderly as over the age of 65. However, as mentioned previously, CLL is diagnosed at a median age of 72 years and 50% of CLL patients in the United States are over the age of 75.2 Thus, we chose to use 70 as the cutoff point, better defining the elderly population of CLL patients. The distinct treatment and prophylactic treatment regimens have been summarized previously.10–12
Toxicity and response assessment
Toxicity was assessed by the NCI Common Toxicity Criteria of Adverse Events (version 3) and modified NCI Common Toxicity Criteria guidelines for evaluating hematologic toxicity in leukemia. Patients were assessed for clinical response by the 1996 NCI Working Group response criteria13 after two, four, and six cycles. Progression-free survival (PFS) was calculated from the date of registration to time of disease progression or death, whichever occurred first. Patients were censored for PFS at the time last known to be progression-free, next treatment, or stem cell transplantation. Overall survival (OS) was calculated from the date of registration to the date of death, censoring patients alive at last follow up.
Statistical analysis
The primary aims were to assess differences in pre-treatment characteristics, treatment tolerability, and clinical outcome between patients aged at least 70 and younger patients. Associations between age groups and categorical or continuous variables were described using Fisher’s exact or Wilcoxon’s rank sum tests, respectively. Estimated probabilities of PFS and OS were calculated by the Kaplan-Meier method, and the log rank test evaluated differences between survival distributions. Logistical regression and proportional hazards models were used to evaluate the impact of age group on response or PFS and OS, respectively, when controlling for variables deemed a priori to be most important; the models were not developed using an automated selection procedure. Hence, all models were controlled for treatment dose/schedule and Rai stage, as well as presence of complex karyotype, a variable associated with both increased age and clinical outcome. Statistical significance was set at alpha = 0.05.
Results and Discussion
Patients’ characteristics
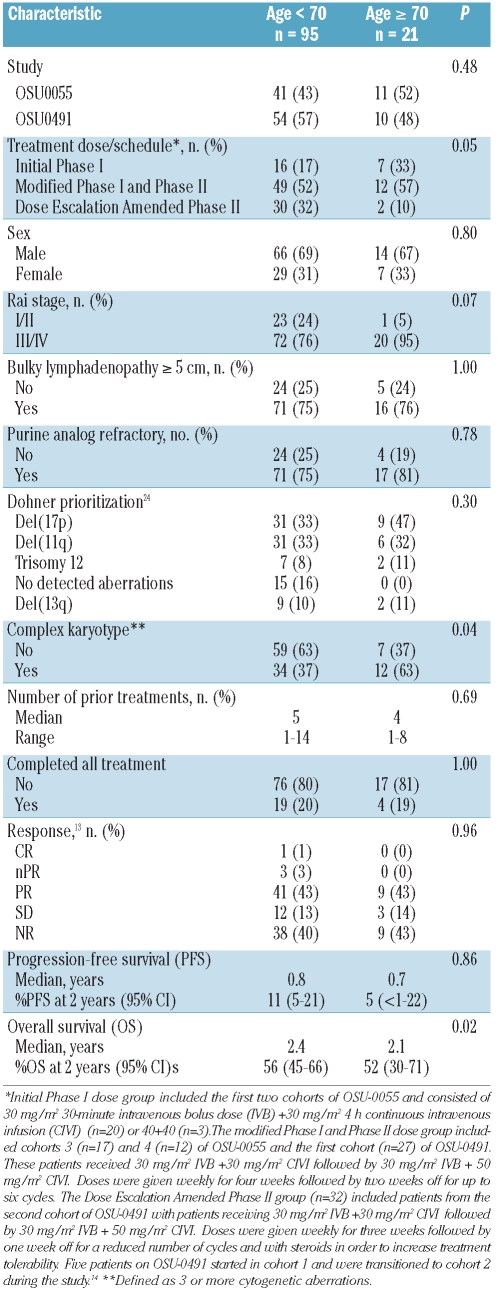
A total of 116 patients were enrolled in the two studies, with a median age of 60 years (range 31–84). Of those, 95 (82%), were younger than 70 years of age and 21 (18%) were aged 70 or older. For most pre-treatment characteristics as summarized in Table 1, there was no great difference between older and younger patients. However, older patients presented more frequently (63 vs. 37%, P=0.04) with complex karyotype (three or more cytogenetic aberrations on stimulated metaphase karyotype), and with advanced Rai stage III/IV (95 vs. 76%, P=0.07) compared to younger patients. Lastly, the two groups of patients were non-randomly distributed among the treatment doses/schedules of flavopiridol (P=0.05); just over half of younger and older patients received 30+30 mg/m2 of flavopiridol followed by 30+50 mg/m2, whereas a smaller proportion of older patients received this dose with an amended schedule aimed at improving tolerability, and a higher proportion of older patients received either 30+30 mg/m2 or 40+40 mg/m2 without the intrapatient dose escalation. However, even with this discrepancy, there was no significant difference in the median number of cycles of flavopiridol received (P=0.69) or the proportion of patients who completed all therapy (P=1.00; Table 1).
Table 1.
Toxicity
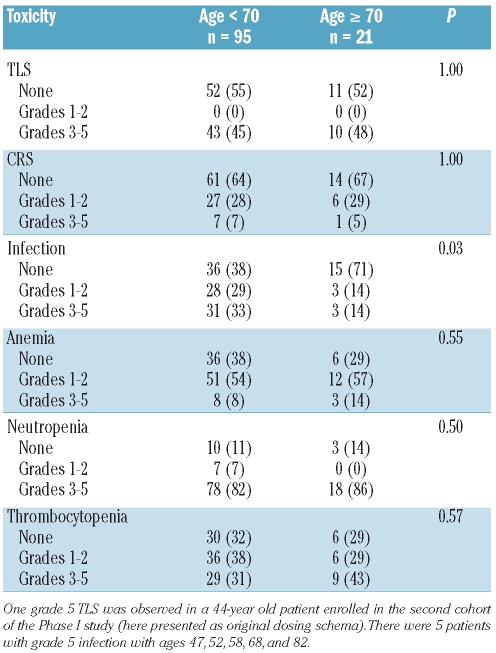
Common toxicities including TLS, CRS, infection, and hematologic toxicities, anemia, neutropenia, and thrombocytopenia were examined between the groups and are summarized in Table 2. We noted no significant associations between age group and grade of toxicity, with the exception of infection (P=0.03). Only 29% of older patients experienced infection with one grade 5 (patient age=82 years), 2 grade 3, and 3 grade 2 infections, as compared with 62% of younger patients.
Table 2.
Toxicity of 116 CLL patients according to age.
Response, progression-free survival (PFS), overall survival (OS)
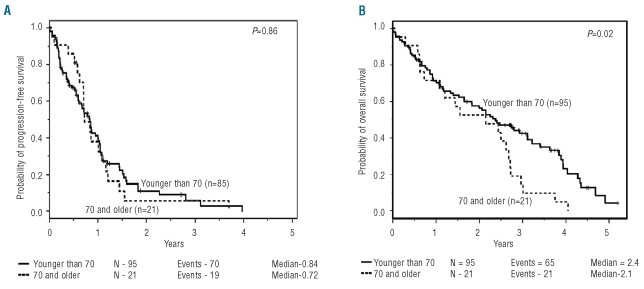
No significant difference was observed in response rates, with 43% of older patients achieving response versus 47% of younger patients (odds ratio=0.8, 95% CI: 0.3–2.2) (Table 1). All responses were partial or nodular partial remissions, except for a 67-year old patient who achieved a complete remission. The estimated median PFS for older and younger patients was 8.7 and 9.9 months, respectively (hazard ratio (HR)=1.0 [95%CI: 0.6–1.7]; Table 1; Figure 1A). There remained no significant differences in response rates or PFS when controlling for treatment dose/schedule, Rai stage and presence of complex karyotype (P=0.76 and P=0.93, respectively). Although OS was shorter in older patients compared with younger patients (HR=1.8 [95%CI: 1.1–2.9]; Figure 1B), following adjustment for Rai stage, the higher risk due to older age was lessened (HR=1.5 [95%CI: 0.9–2.5], P=0.10) and diminished further when also controlling for treatment dose/schedule and presence of complex karyotype (HR=1.2 [95% CI: 0.7–2.1], P=0.54).
Figure 1.
(A) Progression-free survival following treatment with flavopiridol dichotomized by age <70 or ≥70 years and (B) overall survival following treatment with flavopiridol dichotomized by age <70 or ≥70 years.
Discussion and Results
We demonstrate that the cyclin-dependent kinase inhibitor flavopiridol is highly active in the treatment of older CLL patients (≥70 years). We demonstrate no significant difference in toxicity, response, or PFS in older patients compared to younger patients enrolled on two sequential phase I/II flavopiridol trials performed at our institution.10, 11 Of 27 patients censored for PFS (n=2 aged 70 or older and n=25 younger than 70 years), 7 went off-study to receive transplant and one patient remains progression-free and alive at 2.7 years after going on study (age=39 years). Most of the remaining censored patients had stable disease with continued symptoms and went off-study to receive other therapies. With respect to OS, there have been 86 deaths with 30 patients younger than 70 years of age alive at last follow up. Although OS was shorter in older patients, older age tended to be associated with adverse risk factors Rai stage III/IV (P=0.07) and complex karyotype (P=0.04). Furthermore, only 2 of the older patients were treated on the amended dose escalation schema of the phase II clinical trial, where number of weekly treatments per cycle was reduced from four to three and prophylactic dexamethasone was added to improve treatment tolerability and delivery. When controlling for these three factors, older age no longer provided a significant amount of information in explaining survival (P=0.54), albeit the wide confidence interval on the risk of death included hazard ratios as little as 0.7 but as high as 2.1.
The older population is the most rapidly growing population in the United States and the incidence of CLL is markedly increased in these patients2 with a disproportionate lack of representation of these patients in clinical trials.3 Given that flavopiridol has demonstrated class-specific dose limiting toxicities of hyperacute TLS and CRS, concern for feasibility in older patients might be raised. However, in this study, there was no difference in the number of flavopiridol cycles received and the proportion of patients who completed all cycles of therapy between older and younger patients. In addition, in this study there were no significant associations between age group and grade of common toxicities, with the exception of infection (P=0.03), where older patients experienced less infection than younger patients (29% vs. 62%). We considered that older patients may have experienced fewer infections due to the pharmacokinetics (PK) of flavopiridol or its glucuronide metabolite (flavo G) (sample preparation and metabolite quantification as previously described).12,15 However, a review of the PK data showed no significant differences in PK parameter estimates between the two age groups (data not shown). Thus, it is unclear why older patients had fewer infections with flavopiridol and this is currently being investigated at our institution. Nevertheless, these data collectively provide support that flavopiridol can be administered in older patients just as safely as in younger patients.
Other trials and reports of new therapeutic approaches have only paid minimal attention to the impact of age on outcome. For example, several recent trials studying lenalidomide, an immunomodulatory analog of thalidomide, have shown promising results for this drug in relapsed CLL with high-risk cytogenetic features. Two phase II studies evaluating lenalidomide in 45 and 44 relapsed CLL patients demonstrated overall response rates of 47 and 32%, respectively, with complete remission rates of 9 and 7%, respectively.16–17 Unfortunately, the former study only included 12 patients over 70 years of age and the second study reported a median age of 64, but did not specifically detail the ages of the patients or the impact of age on outcome. The pivotal study of ofatumumab included 138 patients with CLL who were either refractory to both fludarabine and alemtuzumab or fludarabine-refractory with bulky lymphadenopathy (>5cm) and demonstrated OR rates of 58 and 47%, respectively;18 only 10 patients (7%) were over the age of 70. Furthermore, the interim analysis of a large multicenter phase II study of flavopiridol in patients with fludarabine-refractory CLL, which demonstrated a 31% OR rate, included patients with a median age of 61 years;19 much below our targeted population of patients over the age of 70. Similarly, recent phase I and II trials targeting Bcl-2 with oblimersen,20 obatoclax,21 and ABT-263,22 have also included few older patients and have not examined the impact of age on outcome in any depth.
Our retrospective study is also limited by its small number of patients seen at a single institution, meaning that definitive conclusions cannot be drawn from the data. In addition, the enrollment criteria required the patients to have an ECOG performance status of 2 or less, which may add bias by including patients who are fitter than those seen in the general CLL population. Previous analysis has shown a trend towards inferior survival in non-Hodgkin’s lymphoma patients with a higher comorbid disease burden.23 As our study was not designed to identify differences between the older and younger groups, a comorbidity index, such as the Sorror version of the Charlson Index,24 was not calculated as a part of the patients’ baseline characteristics. The use of a comorbidity index would be required to provide more information about the safety of flavopiridol administration to patients with a higher comorbid disease burden.
In summary, our study demonstrates that flavopiridol administration to older CLL patients is both feasible and acceptably tolerated relative to younger patients. When controlling for the fact that older patients presented with more aggressive disease, increased age in itself did not appear to be associated with inferior efficacy of flavopiridol. Although our results are based on a relatively small cohort of patients aged 70 and older, there is currently no strong evidence that these older patients cannot tolerate or respond favorably to treatment with flavopiridol. Hence, future development of treatment approaches with both single-agent and combination strategies of flavopiridol should be considered for older CLL patients.
Footnotes
Funding: the authors would like to thank the Leukemia and Lymphoma Society SCOR grant, the D Warren Brown Foundation, NIH/NCI; P01 CA81534, and 5KL2RR025754-02, 5K12 CA133250-03, N01-CM-62207, and U01 CA 076576. AJ is a Paul Calabresi Scholar.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Melbert D, Krapcho M, Stinchcomb DF, Howlader N, Horner MJ. Report. Bethesda, MD: 2008. SEER Clinical Statistics Review, 1975–2005. [Google Scholar]
- 3.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(2):171–8. doi: 10.1080/10428190802688517. [DOI] [PubMed] [Google Scholar]
- 4.Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1750–7. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–91. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 6.Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 8.Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4070–8. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 9.de Azevedo WF, Jr, Canduri F, da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Bioche Biophys Res Commun. 2002;293(1):566–71. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 10.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27(35):6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109(2):399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113(12):2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–7. [PubMed] [Google Scholar]
- 14.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni W, Albanese KA, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95(7):1098–105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–9. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 16.Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–7. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–55. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanasa MC, Andritsos L, Brown JR, Gabrilove J, Caligaris-Cappio F, Larson R, et al. Interim analysis of EFC6663, a multicenter phase 2 study of alvocidib (flavopiridol), demonstrates clinical responses among patients with fludarabine refractory CLL. Blood (ASH Annual Meeting Abstracts) 116:Abstract 58. [Google Scholar]
- 19.O'Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(30):7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson WH, O'Connor OA, Roberts AW. ABT-263 activity and safety in patients with relapsed or refractory lymphoid malignancies in particular chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(Suppl):Abstract 8574. [Google Scholar]
- 22.Janssen-Heijnen ML, van Spronsen DJ, Lemmens VE, Houterman S, Verheij KD, Coebergh JW. A population-based study of severity of comorbidity among patients with non-Hodgkin's lymphoma: prognostic impact independent of International Prognostic Index. Br J Haematol. 2005;129(5):597–606. doi: 10.1111/j.1365-2141.2005.05508.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]