Abstract
Background
Thalidomide is active in multiple myeloma and is associated with minimal myelosuppression, making it a good candidate for induction therapy prior to high-dose therapy with autologous stem-cell transplantation.
Design and Methods
Oral cyclophosphamide, thalidomide, and dexamethasone was compared with infusional cyclophosphamide, vincristine, doxorubicin, and dexamethasone in patients with newly diagnosed multiple myeloma.
Results
The post-induction overall response rate (≥ partial response) for the intent-to-treat population was significantly higher with cyclophosphamide-thalidomide-dexamethasone (n=555) versus cyclophosphamide-vincristine-doxorubicin-dexamethasone (n=556); 82.5% versus 71.2%; odds ratio 1.91; 95% confidence interval 1.44–2.55; P<0.0001. The complete response rates were 13.0% with cyclophosphamide-thalidomide-dexamethasone and 8.1% with cyclophos-phamide-vincristine-doxorubicin-dexamethasone (P=0.0083), with this differential response being maintained in patients who received autologous stem-cell transplantation (post-transplant complete response 50.0% versus 37.2%, respectively; P=0.00052). Cyclophosphamide-thalidomide-dexamethasone was non-inferior to cyclophosphamide-vincristine-doxorubicin-dexamethasone for progression-free and overall survival, and there was a trend toward a late survival benefit with cyclophosphamide-thalidomide-dexamethasone in responders. A trend toward an overall survival advantage for cyclophosphamide-thalidomide-dexamethasone over cyclophosphamide-vincristine-doxorubicin-dexamethasone was also observed in a subgroup of patients with favorable interphase fluorescence in situ hybridization. Compared with cyclophosphamide-vincristine-doxorubicin-dexamethasone, cyclophosphamide-thalidomide-dexamethasone was associated with more constipation and somnolence, but a lower incidence of cytopenias.
Conclusions
The cyclophosphamide-thalidomide-dexamethasone regimen showed improved response rates and was not inferior in terms of survival outcomes to the standard infusional regimen of cyclophosphamide-vincristine-doxorubicin-dexamethasone. Based on its oral administration and the reduced incidence of infection and cytopenia, cyclophosphamide-thalidomide-dexa-methasone may be considered an effective induction therapy option for patients with newly diagnosed multiple myeloma. (ISRCTN: 68454111)
Keywords: multiple myeloma, thalidomide, induction therapy, CTD, CVAD
Introduction
High-dose therapy (HDT) combined with autologous stem-cell transplantation (ASCT) provides superior response and survival outcomes versus standard chemotherapy in patients with newly diagnosed (ND) multiple myeloma (MM).1,2 Standard induction chemotherapy regimens, however, are often associated with hematologic stem-cell toxicity, which may compromise the collection of stem cells for ASCT. Thalidomide is active in MM3–7 and produces little hematologic toxicity, indicating that it may be preferred for use as induction therapy.8 Previous studies evaluating thalidomide, as a component of induction therapy, have shown that it improves response rates3,4,6,9–12 and progression-free survival (PFS),4,6,10,12,13 and provides similar10,12,13 or improved6 overall survival (OS) rates versus non-thalidomide-containing regimens. Thalidomide also appears to be well tolerated in this setting and is associated with an acceptable rate of adverse events.6,9 The demonstrated efficacy, lack of myelosuppression, and overall tolerability of thalidomide provide a strong rationale for its incorporation in standard induction regimens for patients presenting with NDMM who may be eligible for ASCT. Combination vincristine, doxorubicin, and dexamethasone (VAD) is considered a standard infusional induction therapy for younger MM patients prior to HDT and ASCT.14,15 It has previously been shown that the addition of cyclophosphamide to VAMP (vincristine, doxorubicin, and methylprednisone), a regimen very similar to VAD, increases response rates.2,16 Having developed an oral induction regimen comprising cyclophosphamide, thalidomide, and dexamethasone (CTD),17,18 we compared its efficacy and safety to that of cyclophosphamide plus VAD (CVAD) in a large randomized trial setting whereby, apart from the difference in the mode of administration, thalidomide was in effect an alternative to vincristine plus doxorubicin.
Design and Methods
The MRC Myeloma IX trial was a multicenter, randomized phase III trial. The protocol was approved by the relevant institutional review boards and ethics committees. All patients gave written informed consent.
Patients
Eligible patients were aged 18 years or more and had symptomatic MM. Exclusion criteria were pregnancy, asymptomatic myeloma, solitary bone plasmacytoma or extramedullary plasmacytoma, previous or concurrent active malignancies, and acute renal failure (defined by a serum creatinine level of >500 μmol/L, unresponsive to 72 h of rehydration, urine output <400 mL/day, or need for dialysis).
Samples for fluorescence in situ hybridization (FISH) analysis were collected at study entry to determine the cytogenetic makeup of the patients. As the number of patients in each subgroup was too small to analyze individually, patients were classified as having either a ‘favorable’ or ‘adverse’ interphase FISH. Adverse interphase FISH was defined as t(4;14), t(14;20), t(14;16), gain(1q), del(1p32), and del(17p). Favorable interphase FISH was defined as the absence of any of these abnormalities and included hyperdiploidy, t(11;14), and t(6;14).
Treatments
Patients were assigned to either an intensive or non-intensive treatment pathway19 based on performance status, clinician judgment, and patient preference. In the HDT pathway, presented here, patients were randomized to 21-day cycles of CTD (oral cyclophosphamide 500 mg/week, oral thalidomide 100 mg/day increasing to 200 mg/day if tolerated, and oral dexamethasone 40 mg/day on days 1–4 and 12–15) or CVAD (oral cyclophosphamide 500 mg/week, vincristine 0.4 mg/day, doxorubicin 9 mg/m2/day as a continuous intravenous infusion for 4 days, and oral dexamethasone 40 mg/day on days 1–4 and 12–15), given for up to 6 cycles until maximum response, after which patients in both groups received high-dose melphalan (200 mg/m2) and ASCT (CONSORT flowchart; Figure 1). Randomization was on a 1:1 basis and open-labeled, using minimization based on treatment center, hemoglobin (males: <11.5 g/dL vs. ≥11.5 g/dL; females: <9.5 g/dL vs. ≥9.5 g/dL), serum calcium (<2.6 mmol/L vs. ≥2.6 mmol/L), serum creatinine (<140 μmol/L vs. ≥140 μmol/L), and platelet levels (<150×109/L vs. ≥150×109/L). All patients were randomized at study entry to receive sodium clodronate (1600 mg/day) or zoledronic acid (4 mg every 21–28 days) until progression.
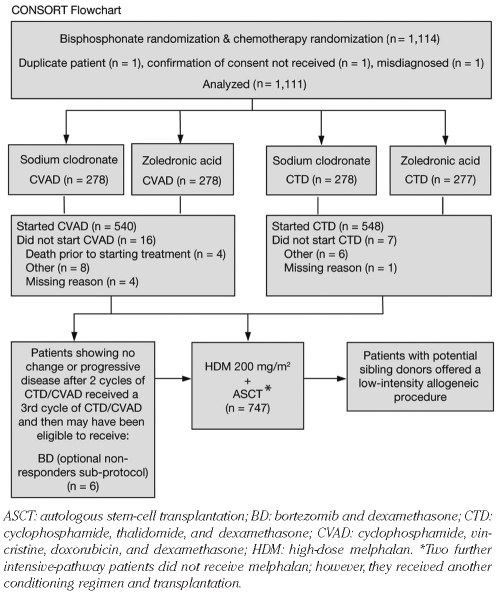
Figure 1.
CONSORT flowchart showing disposition of patients assigned to the intensive-therapy pathway.
Cyclophosphamide and granulocyte colony-stimulating factor were recommended as priming therapy prior to stem-cell collection, and it was assumed that the impact of mobilization was the same in both arms. Venous thromboembolism (VTE) prophylaxis was given at the discretion of the individual clinician and was not otherwise specified in the protocol. The advice given suggested that low-risk patients should receive aspirin prophylaxis and high-risk patients should receive warfarin or a low molecular weight heparin. Risk status was defined according to Palumbo et al.20
Outcome measures
During the running of this protocol, the criteria for assessing response to treatment were updated from the European Group for Bone and Marrow Transplantation (EBMT) criteria to the International Myeloma Working Group uniform response criteria.21,22 To reflect this, a modification of the International uniform response criteria was used retrospectively to assess response in this manuscript. The modification included the use of the minimal response (MR) category defined as 25–49% reduction in serum M-protein level and 50–89% reduction in 24-h urinary light chain excretion, which still exceeds 200 mg/24 h.21 Complete response was defined as negative serum/urine immunofixation, no soft tissue plasmacytomas, and less than 5% plasma cells in the bone marrow. Immunofixation-negative cases without available bone marrow samples were reported as very good partial response. Overall response rate was defined as achieving at least a partial response (PR). Response was assessed after induction and 100 days after ASCT. All assessments were reviewed centrally.
PFS was defined as the time from initial randomization to documented progression or death. Patients with missing follow-up data or those not known to have progressed or to have died at the time of analysis were censored at the last date they were known to be alive and progression-free. OS was calculated similarly as the time from initial randomization to the date of death. VTE and acute renal failure were required to be reported for all patients if they occurred during the study period, or until death or disease progression.
Statistical analysis
The trial was designed to demonstrate that CTD was non-inferior to CVAD with a calculated sample size of 1,080 patients, based on an assumed 40% 5-year OS for CVAD, a non-inferiority margin of 7% (i.e. 33% OS for CTD; hazard ratio [HR], 1.2), a one-sided modified log rank test, at 5% significance and 80% power. If 366 patients were entered, the trial would be powered to detect an increase in the number achieving a CR 100 days after ASCT from 50 to 65% (80% power at 5% significance). The planned number would provide more than 80% power.
For the per-protocol population analysis, patients were analyzed according to the treatment they received. As the intent-to-treat was likely to be less conservative when testing non-inferiority, the per-protocol analysis is presented for the results overall and the intent-to-treat population is used for response assessment after induction and the subgroup analyses. The analysis of response post-HDT was based on the population that received HDT followed by ASCT. In patients who did not receive HDT, response was not assessed at a time that would be directly comparable to 100 days after ASCT.
The assumption of no interaction (chemotherapy effect depending on bisphosphonate and vice versa) between induction chemotherapy and bisphosphonates was prospectively tested before end point assessment in Cox’s regression models not adjusting for the minimization factors. Primary end points were response, PFS, and OS, with quality of life (to be reported separately) and toxicity as secondary end points. For PFS and OS, Cox’s proportional hazards models were used to compare chemotherapy treatment groups while adjusting for bisphosphonate treatment group and the minimization factors. Proportional hazards were assessed by plotting the hazards over time for each arm, and were found to hold. For response, groups were compared with respect to the proportion achieving PR, VGPR, or CR using logistic regression to account for bisphosphonate and the minimization factors (excluding center post hoc because numbers of patients were low at some centers). Post hoc analyses used Fisher’s exact test. Statistical analysis was performed using the SAS 9.0 (SAS Institute Inc., Cary, NC, USA) or Fortran software. All hypothesis tests and CIs are two-sided and at the 5% significance level. The primary end points were ranked and no claims on a rank lower than or equal to the first whose hypothesis could not be rejected were made; in this, PFS and OS have equal ranking, and response a lower ranking.
Results
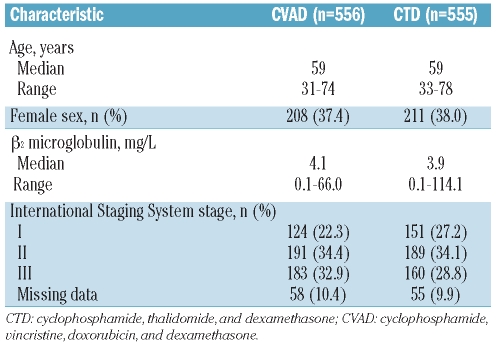
The trial commenced in 2003 and terminated in 2007, recruiting 1,970 patients from 121 centers. A total of 1,114 patients were assigned to intensive therapy with 1,111 evaluable (Figure 1); 556 were randomized to CVAD and 555 to CTD. However, 540 patients actually received CVAD and 553 received CTD (including 5 patients initially randomized to CVAD), and these patients formed the per-protocol population (n=1,093). The median follow up for patients in this study was 47 months (range 0–74 months). On January 20, 2009, the Trial Steering Committee agreed to unblind the chemotherapy results. Here, a cut-off date of October 5, 2009, is used. Patients’ characteristics at baseline were well balanced between the two treatment groups (Table 1). The median patient age was 59 years (range 31–78 years). Overall, 62% of patients were male, 25% had stage I disease, 34% had stage II disease, and 31% had stage III disease according to the International Staging System. The median β2-microglobulin level was 4.0 mg/L (range 0.1–114.1 mg/L). A small proportion of patients aged over 70 years (n=17, 1.5%) were included in the study; 9 patients were randomized to receive CTD induction and 8 to CVAD. Of these 17 patients, only 3 patients (2 CTD and 1 CVAD) subsequently received HDT.
Table 1.
Patients’ characteristics (n=1,111).
A total of 749 patients (67.4%) received HDT followed by ASCT. The median age of patients who received HDT was 58 years (range 31–72 years) and the median age for those who did not receive HDT was 61 years (range 31–78 years).
Response
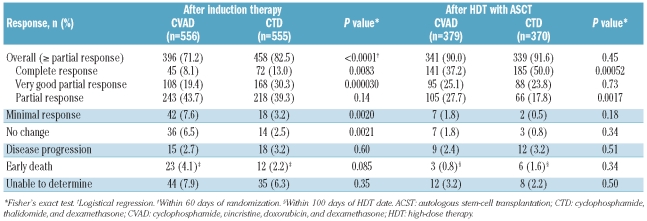
The overall response rate for the intent-to-treat population after induction therapy was significantly higher with CTD than CVAD (82.5% vs. 71.2%; P<0.0001) (analysis performed on the intent-to-treat population, Table 2). The post-induction CR rate was 13.0% in patients assigned to CTD and 8.1% in patients assigned to CVAD (P=0.0083). Response rates at day 100 in patients who received HDT with ASCT were similar in both treatment groups (91.6% CTD vs. 90.0% CVAD; P=0.45), but the CR rates were statistically significantly higher in the CTD group (50.0% vs. 37.2%, respectively; P=0.00052). It should be noted that only 67% of patients included in the trial actually received HDT. Logistical regression analysis revealed that CTD as an induction regimen was a significant predictor of post-induction response (odds ratio 1.91; 95% CI 1.44–2.55; P<0.0001) when controlled for bisphosphonate regimen and minimization factor.
Table 2.
Response after induction therapy (intent-to-treat population) and following high-dose therapy with ASCT (patients receiving ASCT).
Survival
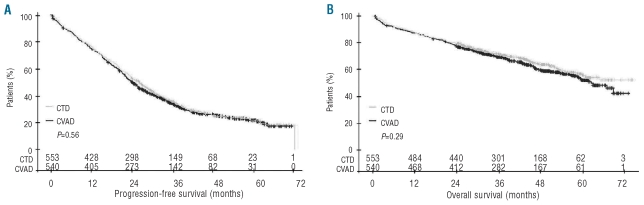
Within this study, in which there were no formal age criteria for exclusion, we saw a significant impact of age on OS where, according to a cut-point analysis, the median OS [95% CI] differed for patients aged 55 years or younger (not reached [NR]; [63-NR]; range 0–74 months), those aged 56–64 years (63 months; [59-NR]; range 0–73 months), and those aged older than 64 years (53 months; [45–69]; range 0–69 months) (Online Supplementary Figure S1). The assumption of no treatment interaction for both PFS (P=0.71) and OS end points was valid (P=0.74), and CTD was non-inferior to CVAD for PFS and OS. In the per-protocol analysis, the median PFS [95% CI] was 27 months ([24–29]; range 0–73 months) in the CTD group and 25 months ([23–26]; range 0–72 months) in the CVAD group (HR 0.94; [0.81–1.09]; P=0.56) (Figure 2A). OS was comparable in both treatment groups (median NR; [61-NR]; range 0–74 months vs. median 63 months; [59-NR]; range 1–73 months; HR 0.92 [0.75–1.13]; P=0.29) (Figure 2B).
Figure 2.
Impact of induction therapy on survival: (A) progression-free survival and (B) overall survival (P values from unadjusted log rank tests; per-protocol population).
In the subset of patients who received ASCT and were treated per protocol, the median PFS (95% CI) was 34 months (31–37) in the CTD group and 33 months (29–36) in the CVAD group (HR 0.90; [0.74–1.10]; P=0.56). OS was comparable in both treatment groups (median NR; [NR-NR] vs. median NR; [67-NR]; HR 0.82 [0.61–1.12]; P=0.20). The 33% of patients in the study who did not proceed to HDT/ASCT and who were progression-free after one year had a median PFS of 25 months (95% CI 21–29); those who were alive at one year had a median OS of 50 months (95% CI 46–63). After adjustment for age, these outcomes were identical to those for elderly patients treated in the non-intensive pathway of the MRC Myeloma IX study.19
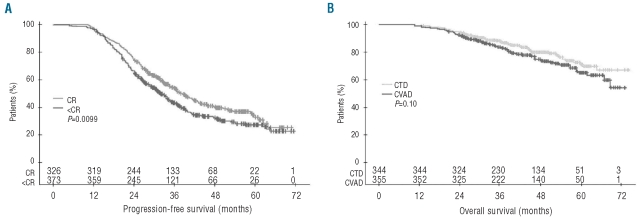
To determine the influence of response on outcome, we compared PFS in patients who achieved a CR following ASCT with that in patients who achieved less than a CR (VGPR, PR, MR, or no change) regardless of treatment group. As expected, PFS was greater in patients who achieved a CR (median 39 months; [95% CI 35–43] vs. 32 months [95% CI 29–35]; P=0.0099) (Figure 3A). Analysis of OS in responding patients only showed a borderline benefit for the CTD group (P=0.10; Figure 3B). Given this substantial, and highly significant, improvement in CR rates in the CTD arm, we hypothesized that, with prolonged follow up, this would inevitably translate into improved OS. We modeled this by concatenating and combining PFS durations and survival after relapse for the different response subgroups to produce predicted OS curves (data not shown). From these it became clear that, based on the improved CR rate and its associated PFS benefit in the CTD-treated patients and with further follow up, the survival benefit would likely increase, equating to a 3% improvement in survival at nine years. This 3% improvement, although small, is clearcut as it results from the longer-term improvement in CR rates combined with the longer PFS duration in patients achieving a CR, and is an approach that could be used in other studies to understand the impact of improved response rates on survival.
Figure 3.
(A) Progression-free survival in patients who achieved a CR following HDT with ASCT compared with those who achieved less than CR. (B) Overall survival according to induction therapy in patients who achieved a response (including no change) following HDT with ASCT (intent-to-treat analysis). ASCT: autologous stem-cell transplantation; CR: complete response; HDT: high-dose therapy.
Cytogenetics
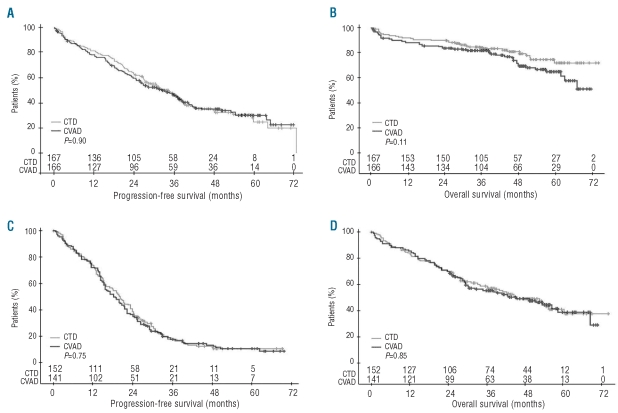
Results from FISH were available for approximately half of all patients (Online Supplementary Table S1). Overall, 293 patients had “adverse” interphase FISH (152 CTD and 141 CVAD) and 333 patients had “favorable” interphase FISH (167 CTD and 166 CVAD). In patients with favorable interphase FISH, median PFS was 34 months with CTD (95% CI 27–38; range 0–73 months) and 32 months with CVAD (95% CI 25–38; range 0–72 months; P=0.90) (Figure 4A). Median OS was NR with CTD (95% CI NR-NR; range 0–74 months) and NR with CVAD (95% CI 62-NR; range 0–72 months), but there was a trend for emerging survival benefit (P=0.11) with CTD (Figure 4B). In patients with adverse interphase FISH, PFS and OS outcomes were similar regardless of the type of induction therapy used (PFS 20 months with CTD vs. 18 months with CVAD, and OS 45 months with CTD vs. 46 months with CVAD) (Figure 4C and D). Among patients who achieved CR after induction therapy (regardless of regimen), patients with favorable interphase FISH had significantly better PFS (P<0.0001) and OS (P=0.0017) than those with adverse interphase FISH (Online Supplementary Figure S2).
Figure 4.
(A) Progression-free survival and (B) overall survival according to induction therapy regimen in patients with a favorable interphase FISH. (C) Progression-free survival and (D) overall survival according to induction therapy regimen in patients with an adverse interphase FISH (intent-to-treat analysis). FISH: fluorescence in situ hybridization.
Safety
The median number of cycles of induction therapy delivered was 5 (range 0–9) for CTD and 4 (range 0–8) for CVAD. The dose of thalidomide was reduced in 29% of patients. Although 749 patients (67%) went on to receive HDT with ASCT, 362 patients did not receive any part of the HDT-with-ASCT regimen. Reasons for this included: patient not fit/clinician’s decision (129 patients), death (93 patients), patient’s decision (29 patients), and inadequate stem-cell collection (15 patients).
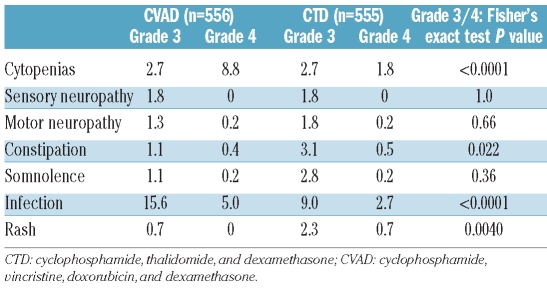
The incidence of severe cytopenias and infection was higher in the CVAD group than the CTD group, whereas grade 3 constipation and somnolence were more common in the CTD group (Table 3). Increased alkaline phosphatase levels were recorded in 18% of patients in the CVAD group and 16% of patients in the CTD group. The incidence of renal insufficiency or failure was similar in both treatment groups (3.2% CVAD and 3.6% CTD), and the incidence of hypothyroidism was low in both treatment groups (<1%).
Table 3.
Grade 3 or higher adverse events.
A total of 208 thromboembolic events were recorded: 116 events occurred in 100 patients randomized to CVAD and 92 events occurred in 86 patients randomized to CTD (P=0.30; Online Supplementary Table S2). As expected, central-line events were more common with the infusional CVAD regimen than the orally administered CTD regimen (39 vs. 5 events). No other pattern was discernible, in particular regarding the incidence of deep-vein thrombosis and pulmonary embolism. There was an early-mortality rate of 8.5% which did not differ between the CVAD and CTD arms (9.4% vs. 7.4%, respectively; P=0.28 Fisher’s exact test). Overall, 217 patients in the CVAD group died versus 195 in the CTD group (P=0.19). The most common causes of mortality related to myeloma or its treatment were: disease progression, infection, renal failure, and transplant-related mortality. Three deaths in the CVAD group and 4 in the CTD group were attributed to VTE. The increased number of deaths in the CVAD group was attributable mainly to an increase in fatal episodes of renal failure and myeloma-related infections. During HDT and the peri-transplantation period, a transplant-related mortality rate of 1.5% was seen that was independent of the induction pathway followed (1.6% [6/379] CVAD vs. 1.4% [5/370] CTD; P=1.0 Fisher’s exact test).
Discussion
This study shows that CTD induction therapy improves both overall response rates and CR rates, compared with CVAD, and provides comparable PFS and OS. The post-induction overall response rate (≥ PR) of 82.5% (CR rate of 13.0%; ≥VGPR rate of 43.2%) achieved with CTD compares favorably with previous studies, in which thalidomide-dexamethasone induction regimens (with or without doxorubicin) produced overall response rates of 63–79% and CR rates of 3–10% (≥VGPR 19–44%).3,4,9,11–14,23 The results are also similar to those achieved with bortezomib-or lenalidomide-containing regimens, which have produced post-induction response rates of approximately 79–93% and ≥VGPR rates of 32–62%.23–26 Although of interest, it must be taken into consideration that these are not direct, randomized comparisons and will reflect differences in methodology, such as response assessment, number of ASCTs, number of treatment cycles given, and characteristics of patients entered. In this study, 10.4% of CVAD patients and 16% of CTD patients became immunofixation negative after induction therapy, and 40.9% and 55.1%, respectively, were immunofixation negative post high-dose therapy. CR was confirmed by bone marrow aspirate in 92.1% of immunofixation-negative patients after HDT and 98.8% of immunofixation negative patients post-transplantation where a bone marrow sample was available. These data indicate a strong correlation between negative immunofixation and less than 5% of plasma cells on bone marrow analysis.
In the CTD treatment group, CR rates increased from 13% post-induction to 50% among patients who received HDT with ASCT. It should be noted that only 67% of patients included in the trial actually received HDT. Although response rates may vary if the intent-to-treat population was used, this increase in the CR rate is particularly encouraging as we show in our subsequent analyses that patients achieving CR have an improved survival outcome. CTD seems to correlate with improved responses compared with thalidomide plus dexamethasone, and with reduced toxicity compared with thalidomide plus dexamethasone and adriamycin. The IFM has shown that bortezomib and dexamethasone (VD) is associated with better responses than VAD, with CR/near-CR rates of 15% vs. 6%, respectively.25 The addition of a third agent to VD improves the post-HDT response rates, with CR rates of 21% with bortezomib, doxorubicin, and dexamethasone (PAD), 38% with bortezomib, thalidomide, and dexamethasone (VTD), and 22% with cyclophosphamide, bortezomib, and dexamethasone (CVD) regimens.23,27,28 The CR rates were 50% with CTD, which is in the same range as those obtained with those regimens; however, exact comparisons will require formal randomized studies.
An improvement in CR rate was noted after ASCT in both arms of the study, with an increase in CR rate of 29.1% in the CVAD arm and 37.0% in the CTD arm. This observation illustrates the importance of high-dose melphalan in improving response rates even when the induction is given to maximum effect.
Overall, the CTD regimen was associated with median PFS and OS values that were not statistically significantly different to those achieved with the CVAD regimen, although the CTD curve was above the CVAD curve. Despite not using an age cut off, and having a high median age (59 vs. 55 years in the prior MRC Myeloma VII study), the PFS and OS results between arms are comparable.2 Survival analyses indicate a trend toward a late improvement in OS favoring the CTD group, and this may become more obvious with further follow up, as has been seen in a previous study.29 In the MRC Myeloma IX study, the principal analyses have been planned to include a further analysis at the 6-year median follow-up time point.
Cytogenetic classifiers can be used to define groups of patients with distinct biology and clinical outcomes; currently, however, only limited data are available on the impact of cytogenetic abnormalities on outcomes in patients undergoing induction therapy for MM. An important result of this study is the significant difference between treatment outcomes depending on the biological subgroup, as defined by FISH, into which the patient falls. Consistent with previous work, we have defined adverse FISH as gain(1q), del(17p), t(4;14), t(14;16) and t(14;20), and favorable FISH as the remainder; in addition we have incorporated del(1p32) as a high-risk factor in younger patients.30 We show that response rates are very similar in the favorable and adverse FISH groups but despite this, there are clear differences between the two risk groups. Median PFS was 33 months in patients with favorable interphase FISH (95% CI 27–37; range 0–73 months) and 19 months in patients with adverse interphase FISH (95% CI 17–21; range 0–70 months; P<0.0001). Median OS was NR in patients with favorable interphase FISH (95% CI NR-NR; range 0–74 months) and 45 months in patients with adverse interphase FISH (95% CI 38–56; range 1–74 months; P<0.0001). Furthermore, we show that, in the group with favorable interphase FISH, there was a trend for improvement in OS for patients receiving thalidomide induction, where there were no effects for those with adverse cytogenetics. In addition, for patients in CR, adverse interphase FISH remains a predictor of poor PFS and OS. These results suggest that the group benefiting from the introduction of thalidomide into the induction therapy of younger patients with NDMM is defined by favorable biological characteristics, identified by their FISH subgroup. Altogether, these findings underscore the need for the incorporation of FISH analysis into the assessment of patients for entry into trials and for the routine management of these patients.
Although the induction regimens evaluated in this study were associated with similar rates of adverse events, the spectrum of events differed. Compared with CVAD, CTD was associated with a higher incidence of severe constipation and somnolence, which is consistent with the known toxicity profile of thalidomide. Notably, the CTD regimen was associated with less myelosuppression than CVAD and this may have contributed to the reduction in fatal cases of infection, facilitated stem-cell collection, and reduced hospital admissions.31
In summary, we found that CTD achieves better response rates and is not inferior compared with CVAD in terms of OS and PFS. In subgroup analyses, a significant survival benefit was observed in patients who achieved a CR and had favorable interphase FISH. Based on these results and comparing an oral to infusional regimen with significant negative impacts on patient lifestyle, which has a substantial risk of myelopsuppression, CTD offers an effective alternative induction regimen associated with improved response rates that is not inferior to CVAD and may have additional benefits in favorable biological subgroups.
Acknowledgments
The authors would like to thank all the patients, investigators, and staff at the participating centers who made this study possible. We are indebted to staff at the Clinical Trials Research Unit, University of Leeds, for trial coordination, data management and analysis; the Department of Immunology, University of Birmingham; Wessex Regional Genetics Laboratory, University of Southampton; Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust; and Institute of Cancer Research, London, for central laboratory investigations. The authors also thank the MRC Leukaemia Data Monitoring and Ethics Committee, the MRC Leukaemia Trial Steering Committee, the NCRI Haematological Oncology Clinical Studies Group, and Myeloma UK.
Appendix
The following Principal Investigators (listed in alphabetical order according to city) participated in the study: Aberdeen – J. Tighe (Aberdeen Royal Infirmary); Abergavenny – N. Parry-Jones (Nevill Hall Hospital); Airdrie – I. Singer (Monklands District General Hospital); Aylesbury – R. Aitchison (Stoke Mandeville Hospital); Bangor – J. Seale (Ysbyty Gwynedd); Barnsley – D. Chan-Lam (Barnsley District Hospital NHS Trust); Barnstaple –C. Rudin (North Devon District Hospital); Basildon – P. Cervi (Basildon Hospital); Bath – C. Singer (Royal United Hospital NHS Trust); Birmingham – M. Cook (Queen Elizabeth Hospital), Y. Hasan (City Hospital), D.W. Milligan* (Heart of England Foundation Trust); Blackpool – M.P. Macheta (Blackpool Victoria Hospital); Bodelwyddan – C. Hoyle (Glan Clwyd Hospital); Bolton – M. Grey (Royal Bolton Hospital); Boston – V. Tringham (Pilgrim Hospital); Bournemouth – R. Hall (Royal Bournemouth Hospital); Bradford – L.J. Newton (Bradford Royal Infirmary); Bristol – R. Evely (Bristol Haematology and Oncology Centre); Bury St Edmonds – M. Karanth (West Suffolk Hospital); Cambridge – J. Craig (Addenbrooke’s Hospital), A. Whiteway (Southmead Hospital); Canterbury – C. Pocock (Kent & Canterbury Hospital); Cardiff – H. Jackson (University Hospital of Wales); Carshalton – J. Behrens (St Helier Hospital); Cheltenham – S. Chown (Cheltenham General Hospital); Chester – E. Lee (Countess of Chester Hospital); Chesterfield – R. Collin (Chesterfield Royal Hospital); Chichester – P. Bevan (St Richard’s Hospital); Colchester – M. Hamblin (Colchester General Hospital); Coventry – B. Harrison (University Hospital); Croydon – H. Lumley (Mayday University Hospital); Darlington – M. Mahmoud (Darlington Memorial Hospital); Dartford – A. Kamat (Darent Valley Hospital); Derby – A. McKernan (Royal Derby Hospital); Doncaster – B. Paul (Doncaster Royal Infirmary); Dorchester – A. Moosa (Dorset County Hospital); Dudley – J. Neilson (Russell’s Hall Hospital); Dundee – K. Gelly (Ninewells Hospital); Eastbourne – J. Beard (Eastbourne District General Hospital); Edinburgh – H. Roddie (Western General Hospital); Epsom – L. Jones (Epsom General Hospital); Exeter –C. Rudin (Royal Devon and Exeter Hospital); Falkirk – R. Neilson (Falkirk and District Royal Infirmary); Gateshead – G. Summerfield (Queen Elizabeth Hospital); Gillingham – M. Aldouri (Medway Maritime Hospital); Glasgow – G. McQuaker (Glasgow Royal Infirmary), A. Morrison (Southern General Hospital), R. Soutar (Western Infirmary), P. Tansey (Victoria Infirmary); Gloucester – S. Chown (Gloucestershire Royal Hospital); Grantham – V. Tringham (Grantham and District Hospital); Great Yarmouth – S. Sadullah (James Paget Hospital); Grimsby – K. Speed (Diana Princess of Wales Hospital); Guildford – G. Robbins (Royal Surrey County Hospital); Harrogate – G. Bynoe (Harrogate District Hospital); Harrow – N. Panoskaltsis (Northwick Park Hospital); Hereford – L. Robinson (Hereford County Hospital); High Wycombe – R. Aitchison (Wycombe General Hospital); Hull – H. Sayala (Hull and East Yorkshire Hospitals NHS Trust); Ilford – I. Grant (King George Hospital); Ipswich – J.A. Ademokun (Ipswich Hospital NHS Trust); King’s Lynn – P. Coates (Queen Elizabeth Hospital); Kingston upon Thames – Z. Abboudi (Kingston Hospital); Leeds – G. Cook* (Leeds Teaching Hospitals NHS Trust); Lincoln – K. Saravanamuttu (Lincoln County Hospital); Liverpool – P. Chu (Royal Liverpool University Hospital), B. Hammer (University Hospital Aintree); Llanelli – P. Cumber (Prince Philip Hospital); London – J.D. Cavenagh* (St Bartholomew’s Hospital), N. Panoskaltsis (Central Middlesex Hospital); Manchester – D. Alderson (Trafford General Hospital), J. Cavet (Christie Hospital), J. Houghton (Hope Hospital), J. Yin (Manchester Royal Infirmary); Melrose – J. Tucker (Borders General Hospital); Merthyr Tydfil – W. Bashi (Prince Charles Hospital); Middlesbrough – A. Wood (James Cook University Hospital); Milton Keynes – D. White (Milton Keynes Hospital); Newcastle upon Tyne – G. Jackson (Freeman Hospital); North Shields – C. Tiplady (North Tyneside General Hospital); Norwich – M. Auger (Norfolk and Norwich University Hospital); Nottingham – N.H. Russell* (Nottingham University Hospitals); Nuneaton – M. Narayanan (George Eliot Hospital); Orpington – A. Lakhani (Princess Royal University Hospital); Paisley – A. Sefcick (Royal Alexandra Hospital); Plymouth – S. Rule (Derriford Hospital); Poole – A. Bell (Poole General Hospital); Prescot – J. Nicholson (Whiston Hospital); Romford – A. Brownell (Oldchurch Hospital); Rotherham – H.F. Barker (Rotherham District General); St Leonards on Sea – J. Beard (Conquest Hospital); Salisbury – J. Cullis (Salisbury District Hospital); Scarborough – A. Zaheer (Scarborough Hospital); Sheffield – J. Snowden (Royal Hallamshire Hospital); Sidcup – S. Bowcock (Queen Mary’s Sidcup NHS Trust); Southampton – A. Smith (Southampton General Hospital); Southport – D. O’Brien (Southport and Formby District General Hospital); South Shields – M. Galloway (South Tyneside District Hospital); Stafford – P. Revell (Mid-Staffordshire General Hospital); Stockport – M. Haj (Stepping Hill Hospital); Stockton-on-Tees – Z. Maung (University Hospital of North Tees); Sutton – G. Morgan (Royal Marsden Hospital); Sutton-in-Ashfield – R. Faulkner (King’s Mill Hospital); Swansea– S. Al-Ismail (Singleton Hospital); Swindon – N. Blesing (The Great Western Hospital); Taunton – S. Bolam (Musgrove Park Hospital ); Torquay – D. Turner (Torbay Hospital); Truro – A. Kruger (Royal Cornwall Hospital); Tunbridge Wells – D. Gillett (Kent & Sussex Hospital); Uxbridge – R. Kaczmarski (Hillingdon Hospital); Wakefield – D. Wright (Mid Yorkshire NHS Trust); Warwick – A. Borg (Warwick Hospital); West Bromwich – Y. Hasan (Sandwell General Hospital); Westcliff-on-Sea – P. Cervi (Southend General Hospital); Wirral – G. Galvani (Arrowe Park Hospital); Wolverhampton – S. Basu (New Cross Hospital); Worcester – S. Shafeek (Worcestershire Royal Hospital); Worthing – A. O’Driscoll (Worthing Hospital). *Member of the National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group.
Footnotes
A complete list of the members of the NCRI Haematological Oncology Clinical Studies Group appears in the Appendix
Funding: financial support for the MRC Myeloma IX trial was obtained from the NHS, NIH, UK Biomedical Research Centre at the Royal Marsden NHS Trust, Myeloma UK, and the Medical Research Council. Unrestricted educational grants from Novartis, Schering Health Care Ltd, Chugai, Pharmion, Celgene, and Ortho Biotech supported the trial coordination and the laboratory studies. None of the funding sources was involved in the study design, data collection, analyses, or interpretation. The authors received editorial support from Excerpta Medica in the preparation of this manuscript, funded by Celgene Corporation. Anna Georgieva, MD, PhD, provided editorial support. The authors were fully responsible for content and editorial decisions for this manuscript.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–6. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Rosiñol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–7. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 6.Zervas K, Mihou D, Katodritou E, Pouli A, Mitsouli CH, Anagnostopoulos A, et al. VAD-doxil versus VAD-doxil plus thalidomide as initial treatment for multiple myeloma: results of a multicenter randomized trial of the Greek Myeloma Study Group. Ann Oncol. 2007;18(8):1369–75. doi: 10.1093/annonc/mdm178. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–14. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Facon T, Sonneveld P, Bladé J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111(8):3968–77. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 9.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, van der Holt B, Martin H, Barge R, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93(1):124–7. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 10.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell trans-plantation for multiple myeloma. N Engl J Med. 2006;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 11.Macro M, Divine M, Uzunhan Y, Jaccard A, Bouscary D, Leblond V, et al. Dexamethasone+thalidomide (Dex/Thal) compared to VAD as a pre-transplant treatment in newly diagnosed multiple myeloma (MM): a randomized trial [abstract] Blood. 2006;108:Abstract 57. [Google Scholar]
- 12.Lokhorst HM, van der Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115(6):1113–20. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 13.Vogl DT, Liu SV, Chong EA, Luger SM, Porter DL, Schuster SJ, et al. Post-transplant outcomes of induction therapy for myeloma: thalidomide and dexamethasone versus doxorubicin, vincristine, and dexamethasone prior to high-dose melphalan with autologous stem cell support. Am J Hematol. 2007;82(12):1071–5. doi: 10.1002/ajh.21038. [DOI] [PubMed] [Google Scholar]
- 14.Cavo M, Zamagni E, Tosi P, Tacchetti P, Cellini C, Cangini D, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicin dexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106(1):35–9. doi: 10.1182/blood-2005-02-0522. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar SV. Multiple myeloma: the death of VAD as initial therapy. Blood. 2005;106:2–3. doi: 10.1182/blood-2005-07-2610. [DOI] [PubMed] [Google Scholar]
- 16.Raje N, Powles R, Kulkarni S, Milan S, Middleton G, Singhal S, et al. A comparison of vincristine and doxorubicin infusional chemotherapy with methylprednisolone (VAMP) with the addition of weekly cyclophosphamide (C-VAMP) as induction treatment followed by autografting in previously untreated myeloma. Br J Haematol. 1997;97(1):153–60. doi: 10.1046/j.1365-2141.1997.d01-2122.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Davies FE, Horton C, Jenner MW, Krishnan B, Alvares CL, et al. The combination of cyclophosphamide, thalidomide and dexamethasone is an effective alternative to cyclophosphamide - vincristine -doxorubicin - methylprednisolone as induction chemotherapy prior to autologous transplantation for multiple myeloma: a case-matched analysis. Leuk Lymphoma. 2006;47(11):2335–8. doi: 10.1080/10428190600821955. [DOI] [PubMed] [Google Scholar]
- 18.Sidra G, Williams CD, Russell NH, Zaman S, Myers B, Byrne JL. Combination chemotherapy with cyclophosphamide, thalidomide and dexamethasone for patients with refractory, newly diagnosed or relapsed myeloma. Haematologica. 2006;91(6):862–3. [PubMed] [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, Russell NH, Bell SE, Szubert AJ, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–8. doi: 10.1182/blood-2011-02-338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 21.Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 22.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 23.Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo A, Gay F, Falco P, Crippa C, Montefusco V, Patriarca F, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28(5):800–7. doi: 10.1200/JCO.2009.22.7561. [DOI] [PubMed] [Google Scholar]
- 25.Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–9. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo A, Cavallo F, Ben Yehuda D, Omedè P, Siniscalchi A, Cavalli M, et al. A prospective, randomized study of melphalan, prednisone, lenalidomide (MPR) versus melphalan (200 mg/m2) and autologous transplantation (Mel200) on newly diagnosed myeloma patients: an interim analysis [abstract] Blood. 2009;114:Abstract 350. [Google Scholar]
- 27.Sonneveld P, Schmidt-Wolf I, van der Holt B, el Jarari L, Bertsch U, Salwender H, et al. HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, doxorubicin, dexamethasone (PAD) vs VAD followed by high-dose melphalan (HDM) and maintenance with bortezomib or thalidomide in patients with newly diagnosed multiple myeloma (MM) [abstract] Blood. 2010;116:Abstract 40. [Google Scholar]
- 28.Kumar S, Flinn IW, Richardson PG, Hari P, Callander NS, Noga SJ, et al. Novel three-and four-drug combination regimens of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for previously untreated multiple myeloma: results from the multi-center, randomized, phase 2 EVOLUTION study [abstract] Blood. 2010;116:Abstract 621. [Google Scholar]
- 29.Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112(8):3115–21. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd KD, Ross FM, Chiecchio L, Dagrada GP, Konn ZJ, Tapper WJ, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2011 Aug 12; doi: 10.1038/leu.2011.204.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–35. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]