The prognosis of patients with chronic myeloid leukemia (CML) has improved considerably over the last ten years with the introduction of ABL tyrosine kinase inhibitors (TKI) into clinical practice. TKIs induce complete cytogenetic remissions (CCyR) in 10–45% of patients who are treated with these drugs in advanced phase with minimal toxicity; unfortunately, these remissions are typically short lasting.1,2 Dasatinib alone induces CCyR in 20–40% of patients.2,3 However, the majority relapse within one year and the median survival is eight months.2 Conventional chemotherapy regimens, such as FLAG-IDA, can induce CCyR in 30–40% of patients who have progressed to blastic phase, but again most patients relapse within six months and survival is poor.4 A logical approach might be to combine both strategies in order to improve the outcome. The proposed schedule of combination TKI and chemotherapy is supported by two clinical observations: i) a number of chemotherapeutic agents commonly used in the management of blastic phase CML have been shown to have synergism with a TKI (e.g. cytarabine);5–8 and ii) TKIs have been used successfully in combination with conventional chemotherapy for the treatment of Ph+ ALL.9 We report our experience of dasatinib/FLAG-IDA chemotherapy in 4 CML patients who progressed to blastic phase while on imatinib.
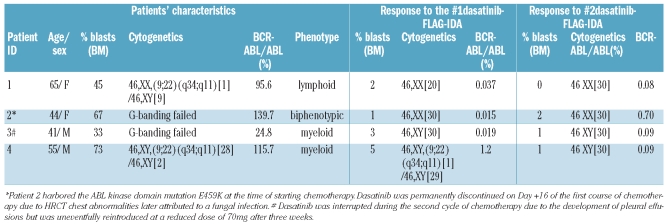
The 4 patients received first-line imatinib for 11, 7, 26 and 10 months, respectively, before progressing to blastic phase. Table 1 shows patients’ characteristics. Patients received two courses of FLAG-IDA (G-CSF 300 μg/day sc Days 0 to 6, fludarabine 30mg/m2 iv Days 1 to 5, cytarabine 2g/m2 iv Days 1 to 5, and idarubicin 12 mg/m2 iv Days 1 to 3) together with dasatinib 100 mg daily. Dasatinib was administered continuously from Day 0 of the first course of chemotherapy. The combination was fairly well tolerated (Table 1) and all patients recovered a neutrophil count over 1×109/L within 30 days of the start of combination therapy.
Table 1.
Patients’ characteristics and responses.
After the first course of chemotherapy, all patients achieved morphological remission (95CI 40–100%); one achieved major cytogenetic response and the remaining 3 achieved both CCyR and major molecular response (MMR). The patients who failed to achieve CCyR after the first course of chemotherapy achieved CCyR and MMR after the second cycle. Currently all 4 patients are alive; Patients 1, 2 and 3 have undergone allogeneic stem cell transplantation (continuing in remission 3, 12 and 13 months post transplant) and patient 4 is scheduled to do so shortly. Dasatinib was reintroduced on Day +30 after transplant in all 3 patients with good tolerance. We have shown that dasatinib can be safely combined with conventional chemotherapy, and although this approach should be tested in a larger number of patients, the combination seems to induce deep remissions in patients with CML in blastic phase, allowing for further therapeutic strategies to enable a continuing response.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 2.Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852–61. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–13. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 4.Wadhwa J, Szydlo RM, Apperley JF, Chase A, Bua M, Marin D, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304–9. doi: 10.1182/blood.v99.7.2304. [DOI] [PubMed] [Google Scholar]
- 5.Tipping AJ, Mahon FX, Zafirides G, Lagarde V, Goldman JM, Melo JV. Drug responses of imatinib mesylate-resistant cells: synergism of imatinib with other chemotherapeutic drugs. Leukemia. 2002;16(12):2349–57. doi: 10.1038/sj.leu.2402775. [DOI] [PubMed] [Google Scholar]
- 6.Kano Y, Akutsu M, Tsunoda S, Mano H, Sato Y, Honma Y, et al. In vitro cytotoxic effects of a tyrosine kinase inhibitor STI571 in combination with commonly used antileukemic agents. Blood. 2001;97(7):1999–2007. doi: 10.1182/blood.v97.7.1999. [DOI] [PubMed] [Google Scholar]
- 7.Scappini B, Onida F, Kantarjian HM, Dong L, Verstovsek S, Keating MJ, et al. In vitro effects of STI 571-containing drug combinations on the growth of Philadelphia-positive chronic myelogenous leukemia cells. Cancer. 2002;94(10):2653–62. doi: 10.1002/cncr.10543. [DOI] [PubMed] [Google Scholar]
- 8.Deau B, Nicolini FE, Guilhot J, Huguet F, Guerci A, Legros L, et al. The addition of daunorubicin to imatinib mesylate in combination with cytarabine improves the response rate and the survival of patients with myeloid blast crisis chronic myelogenous leukemia (AFR01 study) Leuk Res. 2010;35(6):777–82. doi: 10.1016/j.leukres.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Rea D, Legros L, Raffoux E, Thomas X, Turlure P, Maury S, et al. High-dose imatinib mesylate combined with vincristine and dexam-ethasone (DIV regimen) as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia. 2006;20(3):400–3. doi: 10.1038/sj.leu.2404115. [DOI] [PubMed] [Google Scholar]